Abstract
A peptide composed of 9 amino acids, 7 residues from N-terminus of human erythrocytic Band 3 protein (AcMEELQDD) followed by cysteine and glutamic acids, was conjugated to hemoglobin (Hb) serving as an allosteric effector for oxygen release. The activated polyethylene glycol (PEG), maleimide-PEG-N-hydroxysuccinimidyl, was used to crosslink Hb with the peptide. The putative conjugation site on Hb for effective enhancement of oxygen release was characterized as Lys-β95 by liquid chromatography-tandem mass spectrometry. In addition, the conjugated peptide causes a rightward shift of the oxygen dissociation curve as compared to that of its parent Hb when the degree of oxygen saturation is higher than 50%. Furthermore, this conjugated peptide remains effective on lowering Hb's oxygen affinity after Hb polymerization by another PEG crosslinker. The allosteric properties of the peptide-conjugated Hb may provide a new aspect of Hb-based oxygen carriers.
INTRODUCTION
Free hemoglobin (Hb) has the function of carrying oxygen molecules, but it still cannot directly be put into the human body. The reason lies in that Hb in plasma easily tends to dissociate from tetramer into dimers. The excessive amount of Hb dimers will be transferred to kidney, precipitate in the loop of Henle, and result in acute renal toxicity Citation[1]. In addition, Hb solution will cause vasoconstriction due to its excess oxygen delivery to tissues Citation[2] or its extravasation into the spaces between endothelial and smooth muscle cells, reacting with nitric oxide (NO) therein Citation[3], Citation[4]. Moreover, free Hb exhibits a high oxygen affinity in the absence of allosteric effector 2,3- diphosphoglycerate (2,3-DPG) and would result in inappropriate oxygen release in vivo Citation[5].
To overcome the dimer formation of unmodified Hb, crosslinking of Hb has been performed either chemically or by genetically engineered α-α fused Hb Citation[6]. Regarding the oxygen affinity, the modification of Hb with pyridoxal derivatives Citation[7], Citation[8] was used to mimic the action of 2,3-DPG to lower the oxygen affinity. To further prevent the formation of Hb dimers, bis(3,5-dibromosalicyl) fumarate was used to crosslink between Hb α chains under anaerobic condition Citation[9]. These modifications would, however, still have a short in vivo half life or lack an appropriate oxygen release due to the limited dynamic behavior of Hb during the oxy-deoxy transition.
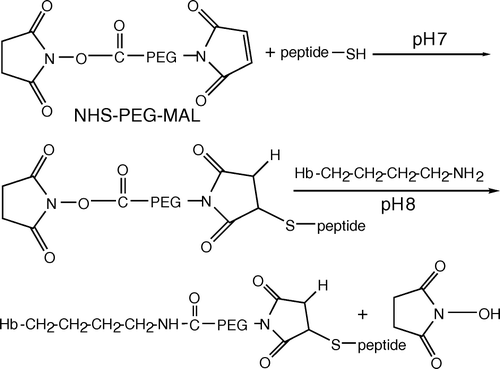
The N-terminal 11 residues of band 3 have been shown to bind to Hb and shift the oxygen dissociation curve for Hb to the right Citation[10], which prompted us to have Hb conjugated with the band 3 derived peptide as a dynamic modulator for oxygen release. To create the site for conjugation, the band 3-N terminal derived 7 residues were added sequentially with cysteine and glutamic acid at the C terminus. In this study, the activated polyethylene glycol (PEG), maleimide-PEG-N-hydroxysuccinimidyl (MAL-PEG-NHS) was used as a crosslinker and spacer for Hb conjugation. Schematic representation of this conjugation is shown in . MAL is the functional group to crosslink with cysteine of the peptide, whereas NHS crosslinks with lysine exhibited on the outer surface of Hb. This conjugated peptide was demonstrated to be able to increase the oxygen release even after Hb is polymerized with another activated PEG.
Figure 1. Schematic representation of peptide-PEG-Hb synthesis. Peptide in HEPES buffer (5 mM HEPES, pH 7) reacted to MAL-PEG-NHS for 3 min at room temperature. The molar ratio is 1:1 in anaerobic conditions. Using a 1.2:1 molar ratio of peptide-PEG solution to deoxyhemoglobin in HEPES buffer (8.3 g/L NaCl and 20 mM HEPES, pH 8) was incubated in room temperature for 1 h. MAL and NHS are the functional groups in PEG for crosslinking with cysteine in the peptide and lysine in the outer surface of hemoglobin, respectively.
MATERIALS AND METHODS
Chemicals
Drugs were obtained from Sigma Chemical Co. (St. Louis, MO), except as specifically stated otherwise. Peptides were synthesized at DigitalGene Biosciences Co. (Taipei, Taiwan). The PEG derivatives, MAL-PEG-NHS and succinimidyl propionate-PEG-succinimidyl propionate (SPA-PEG-SPA), were purchased from NOF Co. (Tokyo, Japan). CM-sepharose was obtained from General Electric Co. (Fairfield, CT). The reagents for isoelectric focusing (IEF) were from Bio-Rad Laboratories, Inc. (Hercules, CA). The sequencing grade modified trypsin was purchased from Promega Co. (Madison, WI).
Preparation of Hb Solutions
Hb solutions were prepared as described earlier Citation[11].
Peptide Design
The first 7 residues of human erythrocytic band 3 protein, AcM-E-E-L-Q-D-D, were used to serve as a dynamic effector for oxygen release of Hb Citation[10]. In order to conjugate the peptide with Hb, the residues were added sequentially with cysteine and glutamate at C terminus. Cysteine in the peptide is the site for crosslinking with maleimide in MAL-PEG-NHS and glutamate is for increasing the surface probability of cysteine according to the Emini method in Seqweb (version 3.1, Accelrys, Madison, WI).
Preparation of Peptide-PEG-Hb
The following procedures were maintained in anaerobic conditions by purging reagents with argon. 0.383 mg of peptide in 0.2 ml HEPES buffer (5 mM HEPES, pH 7) reacted with 1.13 mg MAL-PEG-NHS (molar ratio of peptide to MAL-PEG-NHS = 1:1) for 3 min at room temperature and then cysteine was added to a final concentration of 1 mM to consume the residual MAL function group. The resulting peptide-PEG-NHS solution was immediately injected into 1 ml of 277 µM deoxyHb (molar ratio of peptide-PEG-NHS to deoxyHb = 1.2:1) in HEPES buffer (8.3 g/L NaCl and 20 mM HEPES, pH 8) using a gas-tight syringe and the reaction was performed at room temperature for 1 h. The reaction mixture was dialyzed 3 times with 10 mM pH 6.5 potassium phosphate buffer and fractionated by a CM-sepharose column (0.7×14.5 cm) using 10 ml of 100-190 mM NaCl gradient and 15 ml 200 mM NaCl. The Hb concentration in each fraction (0.5 ml) was measured by a UV/Vis spectrophotometer at 542 nm (Cary 50, Varian, Palo Alto, CA). Similarly, peptide-PEG-cysteine was prepared using this protocol except MAL-PEG-NHS reacting exclusively with cysteine instead of peptide.
Polymerization of Peptide-PEG-Hb with SPA-PEG-SPA
The peptide-PEG-Hb solution was mixed with unmodified Hb in a molar ratio of 1:1 at room temperature for 1 h and then this mixture was dialyzed 3 times with HEPES buffer (8.3 g/L NaCl and 20 mM HEPES, pH 8). 0.342 mg of SPA-PEG-SPA dissolved in 100 µL deoxygenated HEPES buffer (5 mM HEPES, pH 7) was injected into the argon-purged Hb mixture using a gas-tight syringe in a molar ratio 8:1 and the reaction was carried out at room temperature for 1 h. The degree of Hb polymerization was accessed using SDS-polyacrylamide gel electrophoresis.
SDS-polyacrylamide Gel Electrophoresis
Each lane of gel was loaded with 3 µg of Hb and the electrophoresis was carried out using 5 and 15% of polyacrylamide in the stacking and separating gels, respectively.
Mass Spectrometry of Peptide-PEG-Hb
The matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) was equipped with the linear mode of a nitrogen laser (VSL-337, 337 nm, 3 ns pulse) and the accelerating voltage in the positive-ion source was 25 kV. The MS spectra were secured by a Voyager DE-PRO Biospectrometry Workstation (Applied Biosystems). The Hb solutions were dialyzed with deionized water 3 times to remove salts. 1 µL of 14 µM (in heme) Hb solution was mixed with 10 µL of sinapinic acid (44.6 mM in 70% (v/v) acetonitrile buffer) and the mixture was measured by MALDI-TOF MS after air-drying at room temperature Citation[12].
Two Dimensional Gel Electrophoresis (2D-GE)
The first dimensional of 2D-GE was carried out in 7 cm of pH 3-10 linear gradient IEF gel. The gel was loaded with 10 µg of desalted Hb protein and rehydrated in a rehydration buffer (8 M urea, 2% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 50 mM dithiothreitol (DTT) and 0.2% Bio-Lyte ampholytes) at 50 V, 20 °C for 12–16 h. After rehydration, the IEF gel was run with 250 V for 15 min, 250–4000 V for 2 h, and 4000–5500 V-h. After isoelectric focusing, the strip was removed and sequentially equilibrated 10 min with buffer I (6 M urea, 0.375 M Tris, pH 8.8, 2% SDS, 20% glycerol, 2.5% (w/v) DDT) and 10 min with buffer II (6 M urea, 0.375 M Tris, pH 8.8, 2% SDS, 20% glycerol, 2.5% (w/v) iodoacetamide). 15% polyacrylamide gel was used for the second dimension of 2D-GE. After electrophoresis, the gel was stained with Coomassie brilliant blue R-250.
Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS)
50 µL of 3.5 µM (use 11.28 µg) desalted Hb was digested at 37°C for 16 h using 5 µg of modified trypsin in 25 mM ammonium bicarbonate. 20 µL of the resulting peptide was mixed with 20 µL of 5% ACN/0.1% formic acid and provided to MS analysis for peptide identification. Nano-HPLC-ESI-MS/MS was performed to determine the site of peptide cross-linking in Hb. C18 microcapillary column (75 µm×15 cm) was used in the nano-HPLC system (LC Packings, Netherlands) and the mobile phase consisted of buffer A (5% v/v ACN/0.1% v/v formic acid) and buffer B (80% v/v ACN/0.1% v/v formic acid) with 40 min linear gradient from 100% to 40% buffer A and 0% to 60% buffer B, 10 min 0% to 100% buffer A, and 20 min 100% buffer A at 200 nL/min flow rate. An ion trap mass spectrometer (LCQ DECA XP Plus, ThermoFinnigan, San Jose, CA) was coupled to the nano-HPLC system, which was equipped with an ESI source. The performing parameters were 1.3 kV of spraying voltage and 200°C of heating capillary temperature Citation[13].
Oxygen Dissociation Curve
The oxygen dissociation curve was obtained by the protocatechuic acid (PCA)/ protocatechuic acid 3,4-dioxygenase (PCD) system proposed by Vandegriff Citation[14]. Solution conditions were 10 µM (in heme) of various Hbs, 8.3 g/L NaCl, 20 mM HEPES pH 7.4, and 1 mM EDTA. The data were analyzed using singular value decomposition (SVD) algorithm against oxy-, deoxy-, and metHb standard spectra to estimate the amount of oxy-, deoxy-, and metHb in the solution. PCA and PCD were added to the Hb solution at final concentrations of 1.4 mM and 0.06 units/mL, respectively.
RESULTS
Synthesis of Peptide-PEG-Hb
When the N-acetyl peptide MEELQDDYC was synthesized, corresponding to the first 8 residues of band 3 and an added cysteine at the C-terminus for the crosslinking purpose, the cysteine was not able to react with MAL-PEG-NHS, due likely to the lower surface probability of cysteine in solution. To increase the reactivity of cysteine in peptide, we estimated the surface probability of cysteine by the Emini method in Seqweb (version 3.1, Accelrys, Madison, WI). The surface probability of cysteine in peptide can be increased from 0.672 for MEELQDDYC to 0.716, 0.935, and 0.970 for MEELQDDC, MEELQDDCD, and MEELQDDCE, respectively. Therefore, the N-acetyl peptide MEELQDDCE was selected as the Hb oxygen modulator in our following studies due to its higher surface probability.
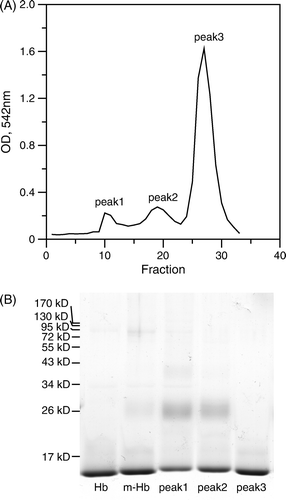
A shows the elution profile of the Hb conjugates from a CM-sepharose column. The peaks were analyzed using SDS-polyacrylamide gel and the first two peaks were due to conjugated Hb and followed by an unmodified Hb peak as shown in B. The ratio of peptide conjugation to Hb was about 10% in the unpurified Hb mixture. The peptide-PEG conjugated Hb α or β chain in Peak 1 and 2 exhibited a diffused band at molecular weight around 26 kD. Notably, the ratios of conjugated to unmodified Hb monomer in Peak 1 and 2 are, respectively, 0.94±0.04 and 0.68±0.02, indicating that the Hbs eluted from the CM-sepharose column were primarily dimers. Here, we denoted Peak 1 and 2 as peptide-PEG-Hb-1 and peptide-PEG-Hb-2.
Figure 2. CM-sepharose elution profile and SDS-polyacrylamide gel electrophoresis of the peptide-PEG-Hb mixture. (A) Peptide-PEG-Hb was separated by ion exchange chromatography on a CM-sepharose column and monitored at 542 nm. The CM-sepharose column was eluted with each 1 mL, 100–190 mM NaCl difference 10 mM gradient, and later was eluted with 15 mL, 200 mM NaCl and collected 0.5 mL in each fraction. The fraction 10–13, fraction 18–21, and fraction 26–28 were collected as peak1, peak2, and peak3, respectively. (B) 15% SDS-polyacrylamide gel was used to characterized various Hbs. Hb: unmodified Hb; m-Hb: unpurified peptide-PEG-Hb; peak1-3: same as A.
Characterization of Peptide-PEG Conjugated Hb
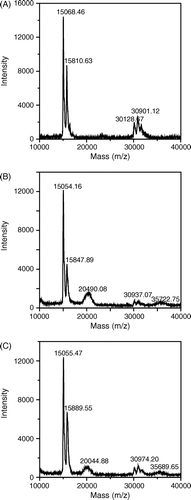
To characterize the conjugation of peptide-PEG to Hb, the molecular weights of Hb fractions were first analyzed using MALDI-TOF MS. The molecular weight of MAL-PEG and the peptide are ∼3,500 Da and 1,153 Da, respectively. Therefore, the theoretical molecular weights of peptide-PEG-conjugated α and β chain become ∼19,710 Da and ∼20,500 Da, respectively. A shows the MS spectrum of unmodified Hb, which contains two major peaks corresponding to the α chain at 15,068.46 Da and the β chain at 15,810.63 Da and three minor peaks around 30,901 Da corresponding to the Hb dimers. Peptide-PEG-Hb-1 has the peaks of unmodified Hb but the relative intensity of β chain with respect to α chain is lower as compared with unmodified Hb (A and B), indicating that the peptide-PEG was primarily linked to the β chain of Hb in peptide-PEG-Hb-1. In addition, there is a significant extra broader peak at 20,490.08 Da, which is close to the theoretical molecular weight of peptide-PEG-conjugated β chain 20,500 Da, further confirming the primary β chain conjugation. On the other hand, the broader peak in peptide-PEG-Hb-2 is at 20044.88 Da, which represents a Hb mixture with a similar amount of α and β chain conjugated with the peptide (C).
Figure 3. MALDI-TOF mass spectra of various Hbs. Various Hbs were dialyzed against deionized water 3 times to remove salt before analyzing by a MALDI-TOF mass spectrometer. (A): unmodified Hb; (B): peptide-PEG-Hb-1; (C): peptide-PEG-Hb-2.
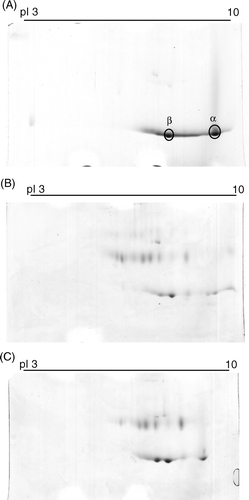
In A, the pI of unmodified Hb α and β chain are 9.5 and 8.0, respectively. The synthetic peptide contains five acidic amino acids. When conjugated to Hb, the peptide shifts the pI of Hb α and β chain to a lower pH range with a greater change in peptide-PEG-Hb-1 than in peptide-PEG-Hb-2 as shown in B and C.
Figure 4. Two dimensional gel electrophoresis of various Hbs. The first dimensional was carried out in 7 cm, pH 3-10 linear gradient IEF gel and the second dimensional was performed using 15% polyacrylamide gels and followed by staining with the Coomassie brilliant blue R-250. (A): unmodified Hb; (B): peptide-PEG-Hb-1; (C): peptide-PEG-Hb-2.
Identification of the Conjugation Site of Hb
To identify the sites of peptide-PEG conjugation on Hb, peptide-PEG-Hb-1 and peptide-PEG-Hb-2 were trypsinized and analyzed by LC-MS/MS equipped with a C18 microcapillary column. Peak 2 and 9 in the LC profile of trypsinized unmodified Hb (A) were shifted to the left as compared to that for peptide-PEG-Hb-1 (B), suggesting their conjugation with hydrophilic peptide-PEG. On the other hand, the chromatographic profile for peptide-PEG-Hb-2 fragments does not show any apparent peak shift (C). The amino acid sequences of the peaks in the LC profiles were determined according to b and y ion series in tandem MS as shown in . In particular, Peak 2 and 9 represent for Hb β83-95 and α61–90, indicating that the sites of peptide-PEG conjugation in peptide-PEG-Hb-1 probably locate, at least, at Lys-β95 and Lys-α61.
Figure 5. LC-MS/MS chromatographic profiles of the digestion products of various Hbs using TPCK-treated trypsin. (A): unmodified Hb; (B): peptide-PEG-Hb-1; (C): peptide-PEG-Hb-2. The peptide sequences for the corresponding peaks are listed in .
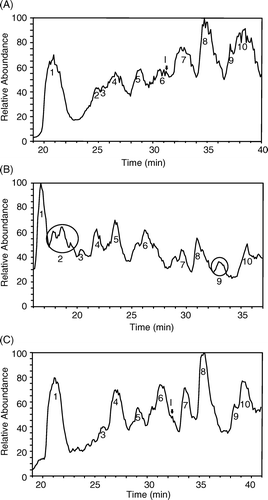
Table 1. The peptide sequences of the chromatographic peaks in LC profiles of trypsinized Hbs
Polymerization of Peptide-PEG-Hb
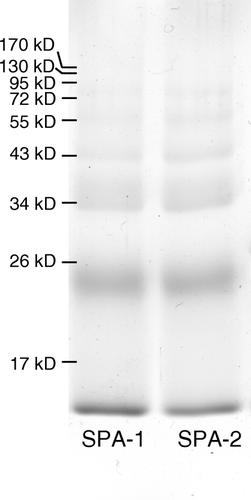
One drawback of Hb solution as an oxygen carrier is the cause of kidney damage by Hb dimers. To lower down the formation of Hb dimer, SPA-PEG-SPA was used to crosslink intra- and inter-Hb molecules. Peptide-PEG-Hb after CM-sepharose purification was primary dimers, so we first incubated peptide-PEG-Hb-1 and peptide-PEG-Hb-2 with unmodified Hb in a molar ratio of 1:1 and then polymerized the Hb mixture with SPA-PEG-SPA, forming SPA-peptide-PEG-Hb-1 and SPA-peptide-PEG-Hb-2, respectively. The degree of inter-molecule crosslink was analyzed by 15% SDS-polyacrylamide gel as shown in . The percentage of proteins with molecular weight higher than Hb dimers in the polymerization mixture in peptide-PEG-Hb-1 and peptide-PEG-Hb-2 are around 22%.
Figure 6. SDS-polyacrylamide gel of polymerization products of peptide-PEG-Hb with SPA-PEG-SPA. Peptide-PEG-Hb and unmodified Hb was mixed in a molar ratio of 1:1 for 1 h. SPA-PEG-SPA in HEPES buffer (5 mM HEPES, pH 7) were then added to the Hb mixture in a molar ratio of 8:1. SPA-1 and SPA-2: polymerization of peptide-PEG-Hb-1/Hb and peptide-PEG-Hb-2/Hb with SPA-PEG-SPA, respectively.
Oxygen Dissociation Curve of Peptide-PEG-Hb
To test the function of the conjugated peptide in the modulation of Hb oxygen release, oxygen dissociation curves for various Hb solutions were obtained by the PCA/PCD system with the simultaneous measurements of the Hb spectra and dissolved oxygen concentration () Citation[14]. Peptide-PEG-Hb-1/unmodified Hb (1:1) and SPA-peptide-PEG-Hb-1 show a significant rightward shift of their oxygen dissociation curves in the range of the oxygen partial pressure higher than 10 mmHg (A) and have a lower Hill coefficient (). On the other hand, peptide-PEG-Hb-2 and SPA-peptide-PEG-Hb-2 do not show any significant change in oxygen affinity and co-operativity (A insert and ). Using cysteine instead of peptide as an allosteric effector can also decrease the oxygen affinity of Hb, but the extent is smaller than peptide-PEG-Hb-1 (B).
Figure 7. Oxygen dissociation curves for various Hbs. The oxygen dissociation curve was obtained using the PCA (1.4 mM)/PCD (0.06 units/ml) enzyme system. 10 M (in heme) of various Hbs was dissolved in the buffer containing 8.3 g/L NaCl, 20 mM HEPES pH 7.4, and 1 mM EDTA. The data were analyzed using SVD algorithm against oxy-, deoxy-, and metHb standard spectra. (A) unmodified Hb (squares), peptide-PEG-Hb-1/Hb (molar ratio 1:1) (circles), SPA-peptide-PEG-Hb-1 (triangles), and unmodified Hb + 5 mM 2,3-DPG (inverse triangles). The inset shows unmodified Hb (squares), peptide-PEG-Hb-2/Hb (molar ratio 1:1) (circles), SPA-peptide-PEG-Hb-2 (triangles). (B) unmodified Hb (squares), peptide-PEG-Hb-1/Hb (molar ratio 1:1) (circles), cysteine-PEG-Hb/Hb (molar ratio 1:1) (triangles).
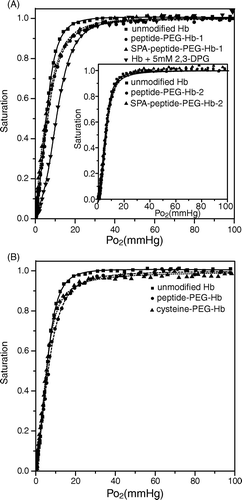
Table 2. Hill coefficients of various Hbs
DISCUSSION
In this study, we described a novel approach to Hb modification by conjugation with a synthetic peptide corresponding to the first N-terminal 7 residues of red cell membrane band 3 protein and two additional amino acids followed, cysteine and glutamine, using a bifunctional PEG crosslinker. The synthetic peptide and PEG are, respectively, served as an allosteric effector and a spacer arm for dynamically modulating Hb's oxygen affinity. The putative conjugation sites on Hb are characterized as Lys-β95 and Lys-α61 by LC-MS/MS. Furthermore, this conjugated 9-residue synthetic peptide remains effective on lowering Hb's oxygen affinity after Hb polymerization by another PEG crosslinker. Taken together, our results demonstrate that the equipment of Hb with a flexible allosteric effector is practicable and may exhibit a better oxygen release behavior.
Previous studies identified that the N-terminal 5 to 7 residues of band 3 being the binding site for deoxyHb can lower Hb oxygen affinity Citation[10]. To study effects of conjugated peptide on Hb oxygen dissociation, we chose cysteine and lysine as the conjugation sites for peptide and Hb, respectively. Notably, cysteine is generally considered to be hydrophobic and is uncommonly found on the surface of a protein or peptide. Therefore, it is often necessary to increase the surface probability of cysteine on the peptide design to enhance its reactivity with the crosslinker. In our study, we found that when monitored by Ellman's reaction, the extent of reaction between the synthetic peptide and MAL-PEG-NHS at room temperature (pH 7.0) could reach 80% within 3 min after optimizing the surface probability of cysteine in the peptide. On the other hand, the potential sites on Hb for conjugation using NHS are the 4 N termini, 11 lysines in α chain, and 11 in β chain. Due to a low pKa relative to the ε-amino group, the N termini have a higher reactivity with crosslinkers than lysine Citation[15]. However, the usage of the N termini of β chain may hinder the binding of allosteric effector and reduce the stabilization of T state. To reduce the reactivity of N termini with NHS in peptide-PEG-NHS, the conjugation reaction was performed under anaerobic condition.
Peptide-PEG-Hb-1 eluted earlier than peptide-PEG-Hb-2 from a CM-sepharose column as shown in A, indicating a higher exposure probability of the negative charges of the conjugated peptide in peptide-PEG-Hb-1 than in peptide-PEG-Hb-2. This result may explain the effectiveness of the conjugated peptide in peptide-PEG-Hb-1 on modulation of Hb's oxygen affinity and is consistent with the lower average pI value for peptide-PEG-Hb-1 as compared to that for peptide-PEG-Hb-2 ().
The ratio of conjugated to unconjugated Hb α or β chain was close to 1 in peptide-PEG-Hb-1 (B), demonstrating that peptide-PEG-Hb-1 exists primarily as dimers. This result is consistent with other PEGylated Hbs Citation[15], Citation[16]. The molecular basis for enhancing the formation of Hb dimers upon purification of PEGylated Hb using a CM sepharose column is still unclear. Hu et al. proposed that the hydrated PEG around Hb may shield the charge of α and β chains, decrease the electrostatic attractions for the α1β2 interface, and result in the formation of Hb dimmers Citation[16]. On the other hand, evidence shows that binding of band 3 to oxyHb promotes dimer formation Citation[17]. In the case of peptide-PEG-Hb-1, the acidic peptide derived from band 3 N terminus may bind to positively charged α subunits Citation[18] in oxy state and in turn increase dimer formation.
Different from free PEG, the conjugated PEG increases the oxygen affinity of Hb Citation[15], Citation[19]. Colombo et al. calculated from the data of p50 and osmotic pressure that the number of bound water molecules in oxyHb is ∼60 more than that in deoxyHb. The existence of free PEG favors the release of water during R→T transition and results in the increase in p50. In contrast, the water molecules liberated during R→T transition in PEGylated Hb may require doing more work on pushing the conjugated PEG away. In the absence of allosteric effector, this work seems greater than the energy released by the osmotic work of conjugated PEG and the formation of salt bridges in T state. However, when additional ionic interactions formed between the acidic peptide and the positively charged group within the 2,3-DPG binding site during R→T transition such as in peptide-PEG-Hb-1, this will overcome the hindrance of conjugated PEG and stabilize the Hb in T state.
The effective length of PEG in the random coil conformation is defined by the Flory dimension, RF=aN3/5, where a=3.5 Å is the length of an oxyethylene unit and N is the degree of polymerization Citation[20]. The molecular weight of the PEG we used is around 3400 Da, which corresponds to N ∼ 77 and RF∼47 Å. The linear distances from Lys 82β2 to Lys 95β2, Lys 95β1, Lys 61α2, and Lys 61α1 are 24.1, 32.5, 37.8, and 42.4 Å, respectively (). As the average diameter of Hb containing the above residues is 52.5 Å, so the arc distances from Lys 82β2 to Lys 95β2, Lys 95β1, Lys 61α2, and Lys 61α1 become 25.0, 35.0, 42.2, and 49.4 Å, respectively. Svergun et al. estimated the length of the PEG chains on the Hb with two PEG grafted as ∼60% of their full Flory dimension Citation[21]. If we apply Svergun's observation, the length of our PEG on the Hb would become ∼28 Å. Therefore, the peptide conjugated to Lys β95 is more likely the allosteric effector for Hb oxygen release than that to Lys α61.
Figure 8. The distances of possible conjugation locations of the peptide to the 2,3-DPG binding site. This graph and measurements were generated using The PyMOL Molecular Graphics System Citation[22] based on the deoxyHb structure (3HHB).
![Figure 8. The distances of possible conjugation locations of the peptide to the 2,3-DPG binding site. This graph and measurements were generated using The PyMOL Molecular Graphics System Citation[22] based on the deoxyHb structure (3HHB).](/cms/asset/cb6bb8de-5b14-4e15-9332-7611c13e8f60/ianb19_a_366638_f0008_b.gif)
In summary, we have demonstrated that when conjugated to Hb by an activated PEG, a synthetic peptide derived from the first N-terminal 7 residues of erythrocytic band 3 protein can serve as an allosteric effector for Hb oxygen release. The putative conjugation site on Hb for effective reduction of oxygen affinity is characterized as Lys-β95 by LC-MS/MS. The conjugated synthetic peptide remains able to help the Hb oxygen release after Hb polymerized by another PEG crosslinker. This study has provided an alternative choice to solve the poor oxygen release from Hb solution and further suggests the potential target site for direct mutagenesis of Hb to specifically conjugate the peptide-derived allosteric effector.
Acknowledgements
We are grateful to the National Center for High-performance Computing, Taiwan, for computer time and facilities. This work was supported by grants NSC 92-2320-B-194-003, NSC 93-2320-B-194-003, and NSC 96-2221-E-194-037 from the National Science Council, Taiwan.
References
- Bunn, H. F. (1993). Am. J. Hematol. 42: 112–117.
- Chang, T. M. (1999). Trends Biotechnol. 17: 61–67.
- Davis, K. L., Martin, E., Turko, I. V., and Murad, F. (2001). Annu. Rev. Pharmacol. Toxicol. 41: 203–236.
- Moncada, S. (1997). Ann. N. Y. Acad. Sci. 811: 60–67.
- Sanders, K. E., Ackers, G., and Sligar, S. (1996). Curr. Opin. Struct. Biol. 6: 534–540.
- Looker, D., Abbott-Brown, D., Cozart, P., Durfee, S., Hoffman, S., Mathews, A. J., Miller-Roehrich, J., Shoemaker, S., Trimble, S., Fermi, G., Komiyama, N. H., Nagai, K., and Stetler, G. L. (1992). Nature. 356: 258–260.
- Benesch, R. E., Yung, S., Suzuki, T., Bauer, C., and Benesch, R. (1973). Proc. Natl. Acad. Sci. U. S. A. 70: 2595–2599.
- Lenz, G., Junger, H., van den, E. R., Brotman, B., and Prince, A. M. (1991). Biomater. Artif. Cells Immobil. Biotechnol. 19: 709–718.
- Chatterjee, R., Welty, E. V., Walder, R. Y., Pruitt, S. L., Rogers, P. H., Arnone, A., and Walder, J. A. (1986). J. Biol. Chem. 261: 9929–9937.
- Walder, J. A., Chatterjee, R., Steck, T. L., Low, P. S., Musso, G. F., Kaiser, E. T., Rogers, P. H., and Arnone, A. (1984). J. Biol. Chem. 259: 10238–10246.
- Yin, C. C., and Huang, K. T. (2007). J. Biomed. Sci. 14: 245–254.
- Tyan, Y. C., Liao, J. D., Jong, S. B., Liao, P. C., Yang, M. H., Chang, Y. W., Klauser, R., Himmelhaus, M., and Grunze, M. (2005). J. Mater. Sci. Mater. Med. 16: 135–142.
- Lee, Y. S., Chen, P. W., Tsai, P. J., Su, S. H., and Liao, P. C. (2006). Proteomics. 6: 2236–2250.
- Vandegriff K. D., Rohlfs R. J., Magde M. D., Jr, Winslow R. M. Anal. Biochem. 1998; 256: 107–116
- Hu T., Prabhakaran M., Acharya S. A., Manjula B. N. Biochem. J. 2005; 392: 555–564
- Hu T., Manjula B. N., Li D., Brenowitz M., Acharya S. A. Biochem. J. 2007; 402: 143–151
- Cassoly R., Salhany J. M. Biochim. Biophys. Acta. 1983; 745: 134–139
- Perutz M. F. Q. Rev. Biophys. 1989; 22: 139–237
- Colombo M. F., Rau D. C., Parsegian V. A. Science. 1992; 256: 655–659
- Kenworthy A. K., Hristova K., Needham D., McIntosh T. J. Biophys. J. 1995; 68: 1921–1936
- Svergun D. I., Ekstrom F., Vandegriff K. D., Malavalli A., Baker D. A., Nilsson C., Winslow R. M. Biophys. J. 2008; 94: 173–181
- DeLano, W. L. (2002). The PyMOL Molecular Graphics System, DeLano Scientific, Palo Alto, CA.