Abstract
In sustained severe ischemia, reperfusion with oxygen carriers may result in ischemia-reperfusion injuries because of the release of damaging oxygen radicals. A nanobiotechnology-based polyhemogloin-calatase-superoxide dismutase can prevent this because the oxygen carrier, polyhemoglobin, is linked to antioxidant enzymes, catalase and superoxide dismutase. However, these antioxidant enzymes come from nonhuman sources and recombinant human enzymes are expensive. This paper describes our study on extracting these enzymes from red blood cells and analyzing the amount of enzymes needed for adequate protection from ischemia-reperfusion.
INTRODUCTION
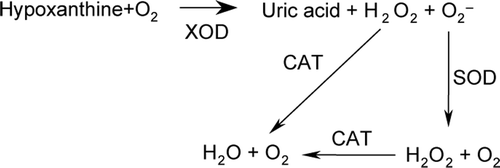
Glutaraldehyde can crosslink hemoglobin molecules into polyhemoglobin (PolyHb) Citation[4–6]. A number of groups have carried out independent developments, extensive studies, and clinical trials and PolyHb continues to show much promise when used in conditions with no sustained severe ischemia, as in surgery Citation[12], Citation[14] and in early prehospital emergency situations Citation[13]. PolyHb is useful for those conditions with no sustained severe ischemia; however, in other clinical situations, as in sustained severe hemorrhagic shock, stroke, myocardial infarction and organ transplantation, the use of PolyHb may result in ischemia–reperfusion injury because of the formation of oxygen radicals Citation[1], Citation[5], Citation[9]. In prolonged severe ischemia (60 minutes or more), abnormal ATP metabolism results in accumulating amounts of hypoxanthine. When the tissues are reperfused with oxygen, xanthine dehydrogenase is converted into xanthine oxidase and hypoxanthine is converted into highly reactive superoxide and hydroxyl radicals. Free radicals could damage cellular proteins, DNA, and the plasma membrane. Damage to the cell's membrane may in turn cause the release of more free radicals. Such reactive species may also act indirectly in redox signaling to turn on apoptosis. Catalase and superoxide dismutase in red blood cells can prevent this to some extent. Superoxide radical (O2−) is dismutated by superoxide dismutase into oxygen and hydrogen peroxide. The latter is in turn transformed by catalase into water and oxygen ().
Scheme 1 Relationship between xanthine oxidase (XOD), superoxide dismutase (SOD), catalase (CAT), superoxide radical (O2−), and hydrogen peroxide (H2O2).
However, even red blood cells with their content of catalase and superoxide dismutase cannot prevent ischemia-reperfusion injury in severe cases of sustained ischemia, as in sustained severe hemorrhagic shock, stroke, or myocardial infarction. Furthermore, when using hemoglobin with the enzymes extract from red blood cells much of the antioxidant enzymes are lost during polyHb preparation, especially in those cases where highly purified hemoglobin is used. A nanobiotechnological method has been used to prepare a soluble complex of polyhemoglobin-catalase-superoxide dismutase (PolyHb-CAT-SOD) Citation[9]. This way, the resulting PolyHb-CAT-SOD can contain much higher concentration of these antioxidant enzymes than those normally present in red blood cells. Detailed in-vitro and in-vivo studies in this laboratory show the effectiveness of this preparation in preventing the oxidative effects of PolyHb in conditions with sustained oxygen lack Citation[6–8].
Laboratory research in smaller animals only requires small amounts of rather expensive commercial catalase and superoxide dismutase. However, for scaling up towards preclinical and clinical trials, the commercial sources of these antioxidant enzymes can be rather expensive. Furthermore, they come from nonhuman sources; although recombinant human enzymes can be used, they could be rather expensive. This paper describes our study on extracting these enzymes together with hemoglobin from red blood cells and the use of this for the preparation of PolyHb-CAT-SOD. The resulting preparation does not have sufficient antioxidant enzymes for adequate protection from ischemia-reperfusion. However, only small amounts of additional antioxidant enzymes are needed and these can be extracted directly from red blood cells.
MATERIALS AND METHODS
Materials
Hydrogen peroxide (30%), sodium sulfide (Na2S), glucose oxidase, glucose, xanthine oxidase, xanthine, lysine (monohydrochloride, SigmaUltra > 99%), glutaraldehyde (25%), and Drabkin's reagent were purchased from Sigma- Aldrich Chemical Co. Chloroform and ethanol were obtained from Fishersci Inc.
Stroma-free Hemoglobin (SFHb) Preparation
Stroma-free hemoglobin was prepared according to the published laboratory method Citation[6]. Briefly, fresh bovine blood with heparin as an anticoagulant was centrifuged at 4000 X g for 60 minutes at 4°C. The plasma supernatant and the upper layer of the red blood cell pellet that contains the buffy coat were removed. The sedimented red blood cells were washed four times with sterile, ice-cold 0.9% NaCl. Red blood cells were then suspended in 2 packed-cell volumes of potassium phosphate, 12.5 mM, pH 7.4, and mixed thoroughly. After 30 minutes for lysis to occur, the stroma lipid and in suspension was removed with 1/2 volumes of ice-cold reagent-grade toluene two times. Cellular debris was separated by centrifugation at 15000 X g for 2 hour at 4°C. The solution was aliquoted and stored at −80°C.
Superoxide Dismutase, Catalase Activity and Hemoglobin Concentration
Superoxide dismutase activity was determined by superoxide dismutase kit (R&D Systems Inc.). Catalase activity was determined by catalase assay kit (Calbiochem Inc.). Hemoglobin concentration was determined at 540 nm by spectrophotometric analysis with the Drabkin's reagent from Sigma-Aldrich.
Extraction of Superoxide Dismutase and Catalase from Stroma-free Hemoglobin
Superoxide dismutase was extracted by ethanol/chloroform 62.5/37.5 (v/v), followed by salting out with K2HPO4, (1/3, w/w), and acetone (75%, v/v) precipitation Citation[2]. Catalase was extracted from ammonium sulfate fractionation of SFHb by adding solid ammonium sulfate (between 10 and 50% (w/v) saturation).
Enrichment of SOD and Catalase in Stroma-free Hemoglobin
SOD and catalase extraction were redissolved with fresh stroma-free hemoglobin solution. Enzymes’ content were adjusted with volume of stroma-free hemoglobin solution. The solution was dialyzed against 0.1 M, pH 7.4 potassium phosphate buffers at 4°C overnight.
Preparation of PolyHb, PolySFHb and PolySFHb-SOD-CAT
Polymerization reactions began with 8.2 g/dL hemoglobin in 0.1M potassium phosphate buffer, pH 7.6. Prior to the start of crosslinking, 1.3 M of lysine was added at a molar ratio of 10:1 lysine/hemoglobin. Crosslinking reaction was started with the addition of glutaraldehyde (0.5 M) at molar ratio of 17:1 glutaraldehyde/hemoglobin. Glutaraldehyde was added in four equal aliquots over a period of 15 min. After 20 hours of crosslinking with constant stirring under aerobic conditions at 4°C, the reaction was stopped with the addition of excess lysine at a molar ratio of 100:1 lysine/hemoglobin. Solutions were centrifuged at 15,000 X g for 1 hour. Then the supernatants were dialyzed at 4°C against Ringer's lactate solution overnight and passed through a sterile 0.45 µm micron filter.
Scavenging of Hydrogen Peroxide (H2O2) by Cross-linked Catalase in PolySFHb Solution
PolyHb or PolySFHb or PolySFHb-CAT or PolySFHb-SOD-CAT was mixed with 200 µM hydrogen peroxide. After allowing the mixture to stand for 3 min at 22°C aliquots of sample were taken and mixed with sodium azide, and then reacted with 1 ml color reagent (horseradish peroxidase/4-aminoantipyrine/3,5-dichloro-2-hydroxybenzenesulfonic acid) Citation[10]. The absorbance at 520 nm was recorded. Hydrogen peroxide and the color reagent participated in a peroxidase-catalyzed reaction to form a dye, which can be measured at this wavelength.
ROS (Reactive Oxygen Species) Scavenging by Cross-linked SOD in PolyHb Solution
Reaction mixtures were added 1.0 µM (as heme) of polyhemoglobin, 100 µM xanthine, 100 µM EDTA, and 150 µM nitro blue tetrazolium (NBT) in 50 mM sodium carbonate buffer, pH 10.2. The reaction was initiated by the addition of 10 mU/ml of xanthine oxidase. The absorbance change at 550 nm was monitored at 25°C for 5.5 min by using Ultraspec2100 pro Spectrophotometer.
Polyhemoglobin Oxidation by Hydrogen Peroxide (H2O2)
Reaction volumes were prepared containing 10 uM (as heme) of polyhemoglobin in 50 mM potassium phosphate, pH 7.4. A series of amounts of hydrogen peroxide was added into PolyHb (10 µM), PolySFHb (10 µM), and PolySFHb-SOD-CAT (10 µM), and the absorbance spectra were recorded over time using Ultraspec2100 pro Spectrophotometer.
Polyhemoglobin Oxidation by Glucose/glucose Oxidase System and Xanthine/xanthine Oxidase System
Reaction volumes were prepared containing 10 µM (as heme) of polyhemoglobin in 50 mM potassium phosphate, pH 7.4. Samples also contained either xanthine (100 uM)/xanthine oxidase (10 mU/ml) or glucose (2mM)/glucose oxidase (0.2 U/ml). Reactions (total volume 3 ml) are started with addition of the oxidase enzymes at 22°C. Absorbance spectra (500-700 nm) are recorded at 1 scan/min for 24 min using Ultraspec2100 pro Spectrophotometer.
Ferrylhemoglobin Measurement
Hydrogen peroxide (100 µM, and 500 µM) was added to reaction volumes containing 10 µM PolyHb or PolySFHb or PolyHb-SOD-CAT in 50 mM potassium phosphate, pH 7.4. Either xanthine (100 uM)/xanthine oxidase (10 mU/ml) or glucose (2 mM)/glucose oxidase (0.2 U/ml) were used as the oxidation system in separate experiments. Sodium sulfide (200 µM) was added to mixtures, and absorbance at 620 nm was measured continuously for 10–15 min.
RESULTS AND DISCUSSION
Purified hemoglobin with nearly all of the red blood cell enzymes removed was purchased from Biopure. This was used to prepared PolyHb that shows minimal enzyme activity (). SFHb is the content of red blood cells in the form of hemolysate that contains most of the red blood cell enzymes and the Hb:SOD:CAT ratio was 1g:12000U:33800U (). SFHb was polymerized to form PolySFHb and the Hb:SOD:CAT ratio was 1g:10800U:23000U (Catalase 23.0 U/mgHb, and SOD 10.8 U/mgHb) (). PolyHb-SOD-CAT was prepared with additional enzymes added to SFHb before polymerization, resulting in a Hb:SOD:CAT ratio of 1g:15060U:330000U ().
Table 1. Catalase and SOD activity after cross-linking with hemoglobin in PolyHb, PolySFHb and PolyHb-SOD-CAT
Scavenging of Hydrogen Peroxide (H2O2) PolySFHb Solution
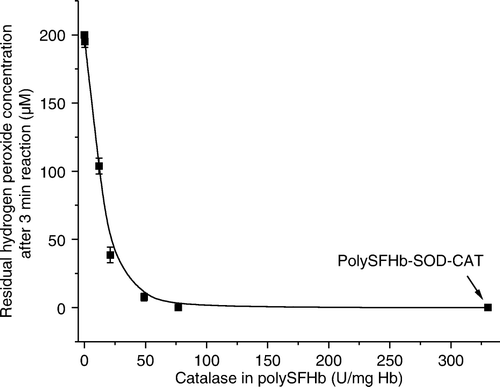
As and show, catalase in PolySFHb (23.0 U/mg Hb) could decompose low concentration of added hydrogen peroxide. The H2O2 concentrations in mixture decreased with increasing concentrations of polySFHb.
Catalase Activity Retained Following Polymerization
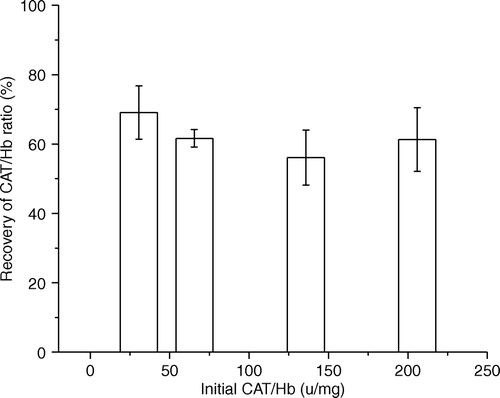
Increasing amounts of catalase were added before polymerization. After cross-linking with glutaradehyde (molar ratio 17:1 glut:Hb), 60% of the original catalase activity was recovered ().
Figure 1. Peroxidase-catalyzed measurement of hydrogen peroxide 3 min after the addition of 200uM of hydrogen peroxide in polySFHb solution (Catalase: 23.0 U/mg Hb).
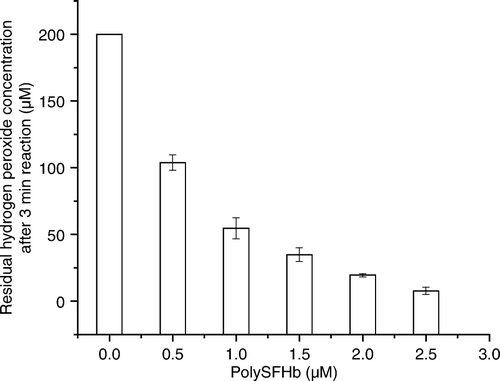
After enrichment with extra catalase, H2O2 levels were markedly reduced in the PolySFHb-CAT solutions. When the amount of catalase was increased to 77.0 U/mg Hb, it could efficiently eliminate 200 mM of hydrogen peroxide at 22 C in 3 min ().
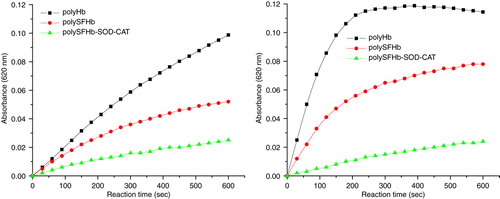
Cross-linked SOD in PolyHb Solution Reduces the Superoxide Anion (O2−) Concentration Generated by Xanthine Oxidase (XOD)
SOD catalyzes the dismutation of the superoxide radical (O2−) into hydrogen peroxide and elemental oxygen and as such provides an important defense against the toxicity of the superoxide radical. In the reaction, superoxide ions (O2−), generated by xanthine oxidase (XOD) conversion of xanthine to uric acid and hydrogen peroxide, convert NBT to NBT-diformazan, which absorbs light at 550 nm. SOD reduces the superoxide ion concentration and thereby lowers the rate of NBT-diformazan formation Citation[3].
. shows that polyHb did not have any effects on the increasing levels of superoxide anions (O2−) produced by xanthine/xanthine oxidase system. The rate of NBT-diformazan formation in polyHb mixture is almost the same as that in buffer alone. PolySFHb contained sufficient SOD activity to effectively lower the rate of superoxide radical production.
Figure 4. Conversion NBT to NBT-diformazan, by xanthine/xanthine oxidase–generated superoxides radical(O2−), in the presence of PolyHb or PolySFHb or PolySFHb-SOD-CAT (1 µM heme).
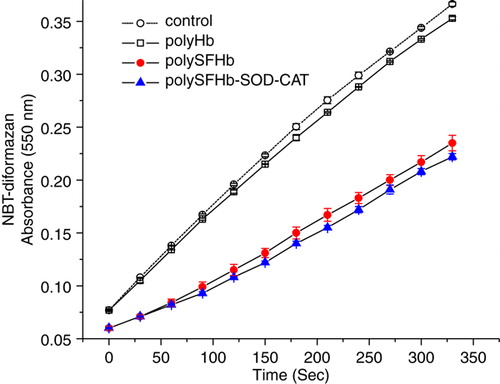
SOD converts superoxide anion into hydrogen peroxide. Thus, a minimal ratio of catalase is needed to remove the increased production of H2O2 from SOD. Therefore, in polySFHb-SOD-CAT a higher amount of catalase than SOD was added to maintain a correct SOD:CAT ratio ().
Polyhemoglobin Oxidation by Hydrogen Peroxide (H2O2)
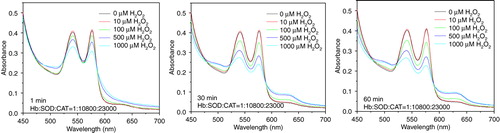
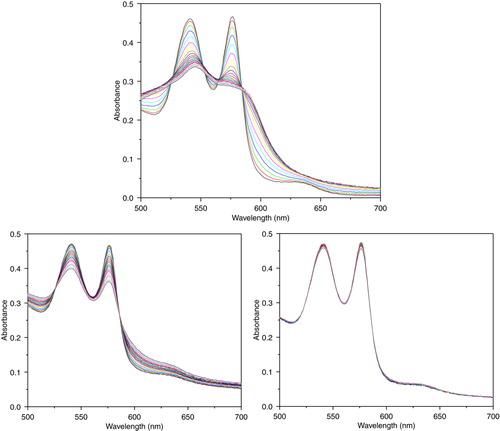
Absorbance spectra were used to follow the effects of hydrogen peroxide on the Hb components of PolyHb, PolySFHb, and PolySFHb-SOD-CAT. Addition of H2O2 into polyHb solution caused a decrease in the absorbance at 540 nm and 577 nm accompanied by the occurrence of two new peaks at 545 and 580 nm (), which indicated that ferrohemoglobin (reduced normal hemoglobin, which contains iron in the +2 (or reduced) oxidation state Fe2 +) was oxidized to ferrihemoglobin (Fe4 +). An additional peak observed at 630 nm was consistent with the formation of methemoglobin, whose iron in the heme group is in the Fe3 + state, which is also unable to carry oxygen. As results in show, without antioxidant enzymes protection, hemoglobin was oxidized and degraded easily and quickly.
Figure 5. Absorbance spectra of hemoglobin (10µM) in PolyHb following H2O2 addition of 10, 100, 500, and 1000 µM.
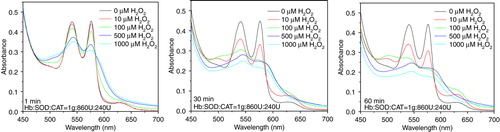
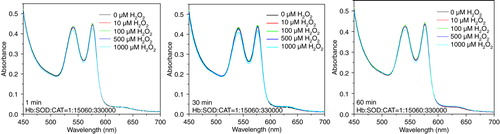
PolySFHb showed its resistant capability to low concentration of H2O2, but is still vulnerable to high concentration of hydrogen peroxide (). PolySFHb-SOD-CAT, with enriched antioxidant enzymes, efficiently protected Hb from oxidative attack (). Even though it was under oxidative stress of 1000 µM H2O2 for 60 min, Hb absorbance spectra did not change much.
Spectrophotometric Determination of Ferrihemoglobin (Fe4 +) and Methemoglobin (Fe3 +) Generation in PolyHb and PolySFHb and PolySFHb-SOD-CAT Exposed to a Continuous Flux of H2O2
Glucose /glucose oxidase system was used to generate a continuous flow of H2O2 in Hb mixture. Hydrogen peroxide generated by glucose /glucose oxidase led to a stepwise decrease in absorbance at 540 and 577 nm, showing that ferrohemoglobin (Fe2 +) was gradually oxidized to ferrihemoglobin (Fe4 +) and methemoglobin(Fe3 +). The transient appearance of two absorption peaks at 545 and 580 nm indicated a composite of Ferrylhemoglobin (Fe4 +), while increase in methemoglobin is shown as a stepwise increase at the absorbance of 630 nm. From the results of , PolySFHb-SOD-CAT with increase ratio on CAT/Hb (330000U/1g) and SOD/Hb (15060U/1g) could efficiently remove the hydrogen peroxide produced by glucose /glucose oxidase system and prevent ferrihemoglobin and methemoglobin formation.
Spectrophotometric Determination of Ferrihemoglobin (Fe4 +) and Methemoglobin (Fe3 +) Generation in PolyHb and PolySFHb and PolySFHb-SOD-CAT Exposed to a Continuous Flux of Superoxide
Xanthine /xanthine oxidase system was employed to generate superoxide (O2-), which oxidized reduced hemoglobin (Fe2 +) to methemoglobin (Fe3 +) (). Increase ratio on CAT/Hb (330 U/mg Hb) and SOD/Hb (15.1 U/mg Hb) protected hemoglobin from oxidation.
Figure 9. Absorbance spectra of reaction mixtures containing 10 uM of hemoglobin and 100 uM of xanthine, and 10 mU/ml xanthine oxidase for continuous generation of superoxide. Top: PolyHb. Lower left: PolySFHb. Lower right: PolySFHb-SOD-CAT.
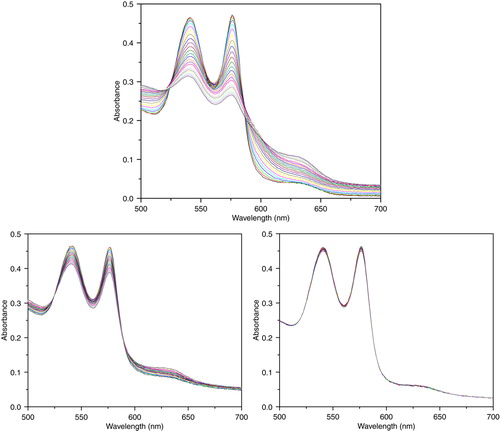
PolySFHb had relatively high SOD activity (10.8 U/mg Hb), but its catalase activity (23.0 U/mg Hb) was much lower than PolySFHb-SOD-CAT (330 U/mg Hb). Less catalase content could not prevent the Hb oxidation process, which means catalase play a more important role in antioxidant protection.
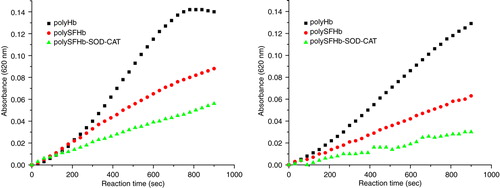
Ferrylhemoglobin Measurement
Ferrylhemoglobin (Fe4 +), is the oxidant product during the oxidation of oxyhemoglobin (Fe2 +) by H2O2, The oxidation of methemoglobin by hydrogen peroxide can also yield ferrylhemoglobin, a transient visible spectrum at 545 and 580 nm, generated by a heterolytic cleavage of the O-O bond of the coordinated peroxide. Ferrylmyoglobin is regarded as an oxidizing agent capable of promoting the peroxidation of fatty acids and the oxidation of p-carotene, ascorbic acid, and uric acid, as well as other molecules of less biological relevance that cause injury to cardiac cells Citation[11].
For PolyHb, without protection of antioxidant enzymes, ferrylmyoglobin concentrations increased quickly under oxidative stress of hydrogen peroxide. PolySFHb had some degree of antioxidant enzyme protection to prevent oxyhemoglobin from oxidation to ferrylmyoglobin, but only to some extent. Only PolySFHb-SOD-CAT, after antioxidant enzymes enrichment, could minimize such occurrence of ferrylmyoglobin production ( and ).
CONCLUSIONS
Under normal circumstances, hemoglobin is enclosed safely within the antioxidant environment in the red blood cells. The antioxidant enzymes superoxide dismutase and catalase help maintain the functional and structural integrity of hemoglobin within the space of the red blood cells under physiological conditions. However, under extreme conditions like sustained hemorrhagic shock, even red blood cells cannot protect against ischemia-reperfusion injuries from the large amount of oxidants produced under these conditions. PolyHb-SOD-CAT allows for a higher amount of these antioxidant enzymes to be included, as shown in this study, to more fully protect against oxidative stresses under extreme conditions like sustained hemorrhagic shock. The crosslinking of the antioxidant enzymes to PolyHb is important since free SOD and catalase are rapidly eliminated from the circulation with a half-time of <30 min. In the form of PolyHb–SOD–CAT these enzymes circulate with a half-time more comparable with PolyHb, which is about 24 h in human Citation[9]. Another advantage is that after cross-linking these antioxidant enzymes would locate at sufficient proximity to PolyHb, and could provide adequate protection.
The antioxidant enzymes in PolySFHb are extracted from red blood cells and therefore less expensive than other sources of enzyme. This is not enough to protect the hemoglobin from oxidation under more extreme conditions. On the other hand, after further enrichment of small amounts of SOD and catalase, PolySFHb-SOD-CAT could effectively defend the hemoglobin component against extreme conditions of superoxides anion and hydrogen peroxide.
Acknowledgements
The author acknowledges the ongoing research grants from the Canadian Institutes of Health Research and the Quebec Ministry of Health's Hemovigillance Program in the form of a FRSQ Research Group (d'equip) on Blood Substitutes in Transfusion Medicine.
References
- Alayash A.I., D'Agnillo F., Buehler P.W. First-generation blood substitutes: what have we learned? Biochemical and physiological perspectives. Expert Opin. Biol. Ther. 2007; 7(5)665–675
- Bannister J. V., Bannister W. H. Isolation and characterization of superoxide dismutase. Methods in Enzymology 1984; 105: 88–93
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels 1. Anal. Biochem. 1971; 44: 276–287
- Chang T.M.S. Stabilisation of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross-linking with glutaraldehyde. Biochem Biophys Res Common. 1971; 44(6)1531–1536
- Chang, T.M.S. (1997). Blood Substitutes: Principles, Methods, Products and Clinical Trials. Vol. 1, McGill University, Montreal ( full text and updates available for free online viewing at, , www.artcell.mcgill.ca).
- Chang T.M.S. Monograph on ARTIFICIAL CELLS: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, Bioencapsulation, Cell/Stem Cell Therapy. World Science Publisher, Singapore 2007
- Chang T.M.S. Nanobiotechnological modification of hemoglobin and enzymes from this laboratory. Biochimica et Biophysica Acta: Proteins & Proteomics 2008; 1784: 1435–144
- Chang, T.M.S. 2009. Nanobiotechnology for hemoglobin based blood substitutes. Critical Care Clinics, (in press).
- D'Agnillo F., Chang T.M.S. PolyHb-superoxide dismutase. catalase as a blood substitute with antioxidant properties. Nature Biotechnology 1998; 16(7)667–671
- Fossati P., Prencipe L., Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clinical Chemistry 1980; 26: 227–231
- Giulivi C., Davies K. J. A. A novel antioxidant role for hemoglobin: the comproportionation of ferrylhemoglobin with oxyhemoglobin. J. Biol. Chem 1990; 265: 19453–19460
- Gould S. A., et al. The life-sustaining capacity of human polymerized Hb when red cells might be unavailable. J. Am. Coll. Surg. 2002; 195: 445–452
- Moore E.E., et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009; 208: 1–13
- Pearce, L.B., Gawryl, M.S., Rentko, V.T., Moon-Massat, P.F. and Rausch, C.W. (2006). HBOC-201 (Hb Glutamer-250 (Bovine), hemopure): clinical studies. In. Winslow, R.. Blood Substitutes, Academic Press, San Diego, pp. 437–4509.