Abstract
The Labscale tangential flow diafiltration system in conjunction with polyethersulfone Biomax filters (both purchased from Millipore Inc.) were used in the following studies: (1) A crucial step in the preparation of polyhemoglobin (polyHb) is the removal of tetrameric hemoglobin (Hb), which can cause adverse side-effects. The efficiency for this depends on the integrity of the 100kDa filters. Those with lower integrity were not effective whereas those with a higher integrity of 0.075ml/min were more effective. A filter with an integrity of 0.075ml/min can reduce the initial 11.2% tetrameric Hb concentration to 1.3% using a flow rate of 0.2ml/min. Higher flow rate of 2.2ml/min was not as effective. (2) Filtration with a 100kDa filter was quick and efficient in separating stroma-free hemoglobin solutions from cellular debris from hemolysate. (3) Both 5kDa and 100kDa filters are efficient in concentrating hemoglobin solutions.
INTRODUCTION
Red blood cell substitutes have many advantages over human donor blood: they are sterilized, free of infectious agents, universally bio-compatible, have a longer shelf life, and can be made available in large quantities Citation[3], Citation[6], Citation[9]. There are different approaches for the preparation of hemoglobin-based blood substitutes: one of these is polyhemoglobin. Bifunctional agents can crosslink hemoglobin into polyhemoglobin (polyHb) Citation[1], Citation[2]. This has been developed by many groups Citation[3–6], Citation[9], Citation[10]. PolyHb have been studied in Phase III clinical trials in the USA, and are approved for routine clinical use in South Africa Citation[6], Citation[9], Citation[10]. In the preparation of polyHb, it is first necessary to isolate hemoglobin and purify it from contaminants such as red blood cell membrane, remnants of platelets, or other components. After polymerization, the polyHb solution should be treated to remove tetrameric hemoglobin that would otherwise cause adverse effects Citation[11]. The resulting PolyHb solution should then be concentrated. All these steps are very laborious, involving different equipment including large and expensive molecular sieve chromatography, molecular sieve filtration, diafiltration, centrifugation, and others. For industrial production, these steps are time-consuming and expensive; for laboratory research they are hardly feasible. This present study aims to test whether a single laboratory scale tangential flow ultrafiltration (TFF) system can be used to (1) remove tetrameric hemoglobin from polyhemoglobin, (2) purify hemolysate, and (3) concentrate polyhemoglobin.
MATERIALS AND METHODS
A Labscale TFF System (consisting of a reservoir, a pump, and supports for a filter) and Pellicon XL 50 Biomax filters () purchased from Millipore Inc. were used. The Biomax membrane is made of polyethersulfone; Biomax membranes with nominal molecular weight limits (NMWL) of 5, 100 and 500 kDa, as specified by the manufacturer, were used. The catalase assay kit used (CAT100) was purchased from Sigma (St. Louis, USA). The determination of mass distribution was done using a column chromatography setup.
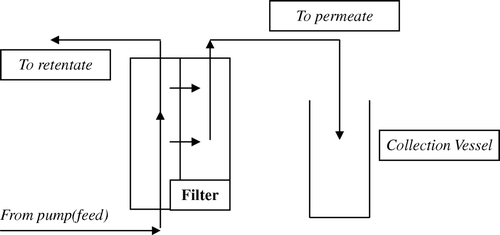
Figure 1. Schematic representation of a Tangential Flow Filtration filter. The direction of the flow through the filter is parallel (or tangential) to the membrane, which reduces the clogging of the filter and improves its efficiency. The pressure difference between the retentate out and feed in ports provides driving pressure for passage through the filter.
The experimental setup used for the three experiments is illustrated in .
Figure 2. Schema of experimental setup. A: Outflow from reservoir. B: Feed In port of filter. C: Permeate Out port of filter. D: Retentate Out port of filter. E: Retentate In port of reservoir. F: Diafiltration medium In port of reservoir. Upper left: additional elements for diafiltration setup (vessel with distilled water, tubing attached to airtight opening in reservoir).
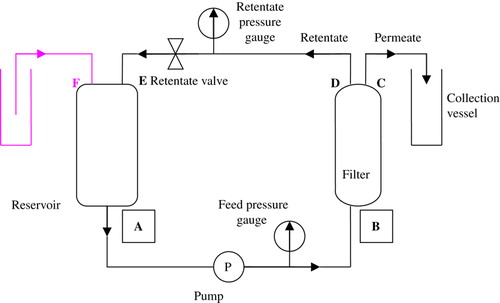
The sample is placed in the reservoir and then circulated by the pump to the filter. The fraction of the sample that passes through the filter is defined as the permeate, while that which has not passed through the filter is the retentate, and is then circulated back to the reservoir. An air-tight cap is fitted on the top of the reservoir; it has one opening for a tube coming from the retentate port of the filter, and another for a tube immersed in distilled water (in the diafiltration setup) or left open to room air (concentration setup). In the diafiltration setup, distilled water is sucked into the reservoir as sample is filtrated out into the permeate vessel; a constant volume is therefore kept in the reservoir. If the concentration of the sample is too high, the filter becomes clogged and the filtration fraction decreases. If a longer filtration is sought, the sample in the reservoir should be periodically diluted; the diafiltration setup is a way to keep the sample diluted without additional intervention from the experimenter, making overnight experiments possible. A diavolume is defined as the “total buffer volume introduced to the operation during diafiltration [divided] by the [initial] retentate volume” Citation[7], Citation[8]. After the end of filtration, the remaining hemoglobin solution in the reservoir, the tubing, the pump, and the filter is flushed out (by gravity or by the introduction of air in the retentate out port of the filter) into a collection vessel. Filters are flushed with 500ml of distilled water before and after experiment. Their integrity is measured right before use and after the final flushing; with the retentate valve closed, air is pumped into the filter until the pressure reaches 2.1 bar. If the flow of air from the permeate port if greater than 3ml/min, the filter is said to be defective Citation[7]; new filters generally have an integrity of 1ml/min, while filters used several times only average 0.35-0.5ml/min. Hemoglobin concentrations of the samples were calculated using the Drabkin method with a UV/visible light spectrophotometer (purchased from Ultrospec 2100 pro, GE Healthcare); absorbance values were taken at 540 nm. Mass distribution of the samples was assessed by molecular sieve column chromatography.
REMOVAL OF TETRAMERIC HEMOGLOBIN FROM POLYHEMOGLOBIN SOLUTION
Methods
Stock polyhemoglobin (polyHb) samples prepared in our laboratory were used. The time elapsed between the synthesis of the polyHb samples and their diafiltration ranged from 5 weeks to 2.5 years; the samples had been stored in a –80C refrigerator. The mass distribution of the particles in a polyHb solution was assessed by column chromatography, at the time of synthesis and shortly before diafiltration. Samples that had not been treated previously for the removal of tetrameric Hb were used. Those with over 10% of molecules smaller than 100 kDa were selected and filtered with a Biomax 100 filter (NWML of 100kDa). The diafiltration setup was used; tetrameric Hb were filtered out, while the polyHb was recuperated in the retentate. The concentration setup was used at the end of the experiment to reduce the volume of the retentate. Mass distribution of the final retentate samples was determined by column chromatography.
Results
As shown in and and , the efficiency for this depends on the integrity of the 100kDa filters. Those with a high integrity of 0.075ml/min were more effective. Thus, filter with an integrity of 0.075ml/min can reduce the initial 11.2% tetrameric Hb concentration to 1.3% using a flow rate of 0.2ml/min. Higher flow rate of 2.2ml/min was not as effective even when using filter with an integrity of 0.075ml/min. Those filters with lower integrity were not effective in removing tetrameric Hb even if filtration was performed for more than 8 hours or more than 10 diavolumes.
Figure 3. Removal of tetrameric Hb from polyHb with 100kDa filter. Chromatographic results. Left column: Initial Hb mass distribution. Right column: mass distribution post-diafiltration. The best result is obtained with a filter integrity of 0.075 using a flow rate of 0.020ml/min.
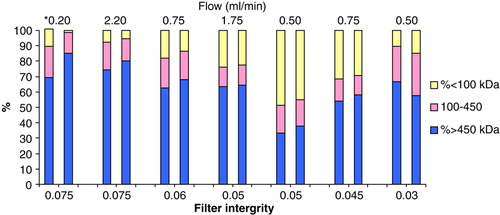
Figure 4. Relationship between filter integrity and percent change in tetrameric Hb in the solution (change calculated as [(Final-Initial)/Initial]%Hb < 100).
![Figure 4. Relationship between filter integrity and percent change in tetrameric Hb in the solution (change calculated as [(Final-Initial)/Initial]%Hb < 100).](/cms/asset/27deb292-68e9-4dd7-becf-48ec66fefb98/ianb19_a_374415_f0004_b.jpg)
Table 1. Removal of tetrameric Hb from polyHb (100kDa filter)
Discussion
100kDa filters with integrity values of 0.075ml/min were used successfully in reducing the percentage of tetrameric Hb in polyHb samples a final level of 1.3%. The integrity of a filter reflects its condition; a new filter typically has an integrity of 0.075-0.1 ml/min, with a decrease in integrity as its pores lose their patency. The filters used in this experiment were used an average of 7 times, with up to 8 months between the first and last use. The length of filtration, feed and retentate pressures and integrity value of the filter, as opposed to the diavolumes used, are most predictive of the reduction in percentage of single hemoglobin. The difference in percent reduction in tetrameric Hb concentration using two filters with the same integrity of 0.075 ml/min (filtrations 1 and 2 in ) may be explained by the lower flow used, as a high flow in this system can reduce the efficiency of removal of small particles.
In conclusion, Pellicon XL 50 Biomax 100 filter (NWML = 100 kDa) with integrity values of 0.075ml/min are useful in reducing the proportion of tetrameric hemoglobin in a polyHb solution. The efficiency of filtration is best predicted by the integrity of the filter, the flow and feed/retentate pressures used. Low integrity values are a sign of deterioration of the filter, due to increased use or time stored. Indeed, filters kept in storage for over 2 months or used for more than 10 filtrations did not successfully reduce the percentage of tetrameric hemoglobin in our experiments.
PURIFICATION OF RED BLOOD CELL HEMOLYSATE
Methods
Red blood cell hemolysate were prepared as described Citation[4]. Biomax 100 (NMWL = 100kDa) and Biomax 500 (NMWL = 500kDa) filters were used in order to remove contaminants from the hemoglobin and enzymes. The diafiltration setup was used in order to permit longer filtration times without concentrating the sample above the threshold of the filter. The final permeate sample, which is the purified stroma-free Hb, was recuperated and analyzed; the final retentate sample was discarded. The concentration of hemoglobin in the final retentate and final permeate and the corresponding volumes were used to calculate the mass of hemoglobin, and the efficiency of the filtration. The catalase concentration of a set of initial retentate, and final retentate and permeate samples was measured by the Catalase Assay (CAT100, purchased from Sigma, USA). The catalase yield in the permeate (the purified Hb) was calculated.
Results
The filtration of stroma-free Hb with a filter of NMWL = 500kDa was quick, and provided a superior hemoglobin yield ( and ). The final permeate Hb mass was >80% of the initial Hb mass, and >90% of the total final Hb mass. The average flow rate used was >1.5ml/min for less than 1 diavolume, and the filtration time between 30 and 90 minutes. A diavolume is defined as the “total buffer volume introduced to the operation during diafiltration [divided] by the [initial] retentate volume” Citation[7]. The hemoglobin yield was calculated as (total final Hb mass)/(initial retentate Hb mass); it was used as a measure of Hb retained by the filter.
Table 2. Diafiltration SFHb with 500kDa filter
Table 3. Diafiltration SFHb 100kDa filter
For the stroma-free Hb samples filtered with a filter of NMWL = 100kDa, a longer duration was necessary to achieve similar hemoglobin yields in the final permeate (). The samples were diafiltered for 2 or more diavolumes (DV). The final hemoglobin yield in the permeate was >73% of the initial mass, and >80% of the total final mass; only one diafiltration experiment (4.5 DV) achieved a permeate Hb yield over 85%.
In a final set of experiments () with a NMWL = 100kDa, in which the catalase concentration of the final permeate was measured as a measure of the amount of larger molecules that remain in the purified solution; for diafiltration with DV > 1 and average flow rate 0.5-1 ml/min, the permeate contained an average of 5% Units catalase/mg hemoglobin compated to the initial retentate sample. If we want to recover both Hb and enzymes like catalase and superoxide dismutase from the hemolysate to perpare PolyHb-catalase-superoxide dismutes, a filter with NMWL = 500kDa or higher can be used.
Table 4. Diafiltration with 100kDa filter, catalase assay
Discussion
A filter is said to retain with certainty molecules with a molecular weight three to five times its NWML Citation[7]; the 100kDa filter used in this experiment therefore theoretically retains molecules larger than 300-500 kDa, contaminants such as fragments of red blood cell membrane or platelets. In our experiment, the concentration of catalase, an enzyme present in red blood cells with MW = 247 kDa, was measured in the initial and final samples, in order to estimate the amount of molecules with molecular weight larger than 250 kDa that pass through the filter. With diafiltration with DV > 1, the permeate contained an average of 5% units catalase/mg hemoglobin compared to the initial retentate sample. The filter with NMWL = 100kDa has therefore been shown to retain ≥95% of the molecules with molecular weight greater than or equal that of catalase, 247kDa. The optimal recovery of hemoglobin in the permeate was obtained at between 4 and 5 diavolumes, with higher feed and retentate pressures, and a smaller difference between the retentate and feed pressures. If, however, the difference is too small, the flow will be reduced to zero. The optimal difference depends on the viscosity, concentration (the more concentrated, the lower the maximum retentate pressure), and mass distribution of the sample, and so varies during the filtration.
Thus, the Labscale TFF system in conjunction with a Pellicon XL 50 Biomax membrane with a NWML of 100kDa can be efficiently used to obtain a Hb solution purified from cellular debris larger than 300 kDa, from an original sample of stroma-free hemoglobin. Diafiltration for 4 to 5 diavolumes is recommended, with a feed pressure between 0.34 and 0.69 bar, and retentate pressures between 0.14 and 0.41 bar. The Biomax filters used are made of a polyethersulfone base, with hydrophilic additions to reduce protein adsorption Citation[7].
The effect of polarity of particles on their rate of filtration has, however, not been studied. For the preparation of PolyHb-catalase-superoxide dismutase we want to recover both Hb and enzymes like catalase and superoxide dismutase from the hemolysate. For this, a filter with a NMWL = 500kDa or higher can be used.
TO CONCENTRATION POLYHEMOGLOBIN SOLUTION
Methods
PolyHb solutions were filtrated with either a Biomax 5 (NMWL of 5kDa) or Biomax 100 filter (NMWL of 100kDa). The concentration setup was used; the filtration stopped when the reservoir was empty, indicating the total retentate volume was that of the tubing and filter, 20ml. The final retentate sample is the concentrated polyHb solution.
Results
As shown in , stroma-free polyHb samples of concentration <0.5g/dL and volume of 1 dL were concentrated over five times using a filter with NMWL = 100kDa in one hour, with a maximum flow rate of 0.9 ml/min. The permeate contained some hemoglobin, likely residual Hb. Using a filter with NMWL = 5 kDa, the same sample can be concentrated over ten times in one hour, with an average flow rate of 1.3 ml/min. In the latter experiment, the permeate was transparent and did not contain measurable amounts of hemoglobin when assessed by the Drabkin assay.
Table 5. To concentrate PolyHb solution
Discussion
When using a 100 kDa filter for a polyHb sample, it is expected that some tetrameric Hb (MW = 64 kDa) will be filtrated out, so the final hemoglobin mass will be slightly lower than the initial. This is not the case when using a 5kDa filter, where only small, non-protein molecules can be filtrated out. To increase the final concentration by reducing maximally the final retentate volume, it is possible to continue filtration once air has entered in the system (the volume of remaining retentate becoming lower than the volume of the circuit).
The results show that ultrafiltration using a Pellicon XL 50 Biomax filter (NWML of 5 or 100kDa) without volume replacement in the reservoir can efficiently concentrate polyHb solutions. The maximum concentration is dependent on the initial polyHb mass and the final retentate volume; concentrations as high as 4.8 g/dL have been reached. This study shows that a NMWL of 5 kDa is more efficient for the concentration of a polyHb solution, since it decreases the time of filtration and increases the concentration factor. A 100kDa filter, however, would be preferred if removal of tetrameric Hb from the polyHb mixture is sought.
Acknowledgements
Professor T.M.S. acknowledges the Operating Term Grant from the Canadian Institutes of Health Research and also the grant from the Quebec Ministry of Health, Hemovigilance Program administered by FRSQ.
References
- Chang T.M.S. Semipermeable microcapsules. Science 1964; 146(3643)524–525
- Chang T.M.S. Stabilization of enzyme by microencapsulation with a concentrated protein solution or by crosslinking with glutaraldehyde. Biochem Biophys. Res Com 1971; 44: 1531–1533
- Chang, T.M.S. 1997. Monograph on Blood Substitutes: Principles, Methods, Products and Clinical Trials. Karger (ed.), Basel, Switzerland.
- Chang T.M.S. Monograph on ARTIFICIAL CELLS: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, Bioencapsulation, Cell/Stem Cell Therapy. World Science Publisher, Singapore 2007
- Chang T.M.S. Nanobiotechnological modification of hemoglobin and enzymes from this laboratory. Biochimica et Biophysica Acta: Proteins & Proteomics 2008; 1784: 1435–1443
- Gould S. A., et al. The life-sustaining capacity of human polymerized Hb when red cells might be unavailable. J. Am. Coll. Surg. 2002; 195: 445–452
- Millipore. 2003. Protein Concentration and Diafiltration by Tangential Flow Filtration. From http://www.millipore.com/publications.nsf/docs/tb032.
- Millipore . 2009. Pellicon XL 50 Devices: Specifications. http://www.millipore.com/catalogue.nsf/docs/C1974.
- Moore E.E., et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: The USA multicenter trial. J Am Coll Surg. 2009; 208: 1–13
- Pearce L.B., Gawryl M.S., . HBOC- 201 (Hb Glutamer-250 (Bovine), Hemopure): clinical studies. Blood Substitutes, R. Winslow, et al. Academic Press, San Diego 2006; 437–450
- Yu B.L., Liu Z.C., Chang T.M.S. PolyHb with different percentage of tetrameric Hb and effects on vasoactivity and electrocardiogram. Artificial Cells. Blood Substitutes & Biotechnology 2006; 34: 159–175