Abstract
Platelet gels (PG), activated by bovine thrombin (BT), have increasingly been used in orthopedic surgery. However, BT may induce immunological reactions and carry potential viral and prion risks. To avoid these side effects, thrombin derived from human plasma (human thrombin, HT) is becoming the preferred platelet activator to prepare PG. However, limited experience and data on the clinical benefits of HT-generated PG (HTPG) in orthopedic surgery is reported. Consequently, we designed and performed a series of studies in dogs to compare the impacts of promotion of bone growth by an artificial bone substitute (Osteoset®) in combination with HTPG or without it in the spinal repair experiments. X-ray observations and histological studies were performed at predetermined periods post-operation. The preliminary results revealed the preparation of HTPG was easy and required less than 30 minutes. HTPG was capable of embedding the artificial bone substitute Osteoset® to prepare a sticky and easily manipulated composite for the application into spinal defect. We found HTPG exhibited enhancement of grafting capacity in consolidation of bone mass. After 12 weeks, tissue reconstruction reached approximately 80% of the injury defects when treated by HTPG/Osteoset® combination, but only 30∼40% in the absence of HTPG. The physiological activity of artificial bone substitute combined with PG activated by HT may therefore open beneficial prospects for more successful and safer bone formation in spine procedures in the near future.
INTRODUCTION
Platelet gel (PG) is a new, promising biomaterial obtained by combining a platelet-rich blood fraction, such as a platelet concentrate or platelet-rich plasma (PRP), with calcified thrombin. Mixing calcified thrombin with such platelet-rich blood fraction leads to the formation of a thick adhesive gel Citation[1], Citation[2] and to the release of a variety of proteins and other substances, such as growth factors, known to be crucial to cell differentiation and tissue healing processes Citation[3]. More specifically, thrombin induces platelet activation leading to a α granules-release of a mixture of platelet-derived growth factor (PDGF), transforming growth factor (TGF–β), sub-family of bone morphogenetic protein (BMP), and epidermal growth factors (EGF), which sequester and concentrate in PG Citation[4], Citation[5]. These growth factors are thought to possess osteoinductive properties and could activate local mesenchymal and epithelial cells to migrate, divide, and increase extracellular matrix synthesis. Among these growth factors, PDGF has been shown to activate cellular proliferation and migration of cells and promote angiogenesis. TGF-β has a potent effect on cells associated with bone, and therefore increases and stimulates the deposition of extracellular matrix (ECM) onto bone. Moreover, PDGF and TGF-β were reported to possess mitogenic effects on osteoblasts and mesenchymal cells by stimulation of DNA synthesis and cell replication and to help bone formation in vitro and in vivo Citation[6–9]. Due to the presence of physiologically active growth factors, PG could facilitate ECM formation, thereafter promoting cell proliferation and migration and vascular invasion. Growth factors have also proved to possess chemotactic and mitogenic effects on undifferentiated stem cells in situ, favoring their multiplication, the secretion of growth factors, and the promotion of self-cell repair mechanisms Citation[10]. This may enhance cell differentiation into osteoblasts.
The surgical applications of PG are so far particularly prominent in oral and maxillofacial surgery. PG was also shown to support bone grafts by accelerating fracture healing and to promote wound healing. Until now, the thrombin used to activate PG is generally from bovine origin. As known, bovine thrombin (BT) carries the demonstrated risks of inducing immunological reactions, most specifically cross-reacting anti-Factor V antibodies that may lead to severe bleeding if BT is reused in the same patient Citation[11–13] and, at least theoretically, zoonotic infections, including variant Creutzfeldt-Jakob disease, the human form of bovine spongiform encephalopathy Citation[14], Citation[15]. Therefore, human thrombin (HT) would be a preferred platelet activating agent to avoid the potential risks of bovine components Citation[16]. Very recently, a stand-alone system to prepare HT from single human plasma donations has become available for surgical uses. In this device, HT is obtained by a physiological activation process of whole human plasma in the presence of calcium chloride Citation[17]. Such HT has already been shown to generate a quantity of platelet growth factors similar to that obtained when using BT upon mixing with human platelet rich plasma Citation[18]. However, still limited data is reported on the efficacy of a PG generated with HT in essentially orthopedic surgery fields. In this study, a dog model was used to compare the effectiveness and capacity of combining artificial bone substitute (Osteoset®) with (experimental group A) or without HTPG (control group B) to facilitate new bone formation in spine area.
MATERIALS AND METHODS
Materials
Human fresh frozen plasma (FFP) and platelet-rich plasma (PRP) were obtained from whole blood donations collected in the presence of CPD anticoagulant/stabilizing solution at the Taiwan Blood Services Foundation (TBSF, Taipei, Taiwan). The Thrombin Generation Device (TGD® -001) and 10% CaCl2 solution used to produce HT were purchased from Merries International Inc (Taipei, Taiwan) and Taiwan Biotech Co., LTD. (Taoyuan, Taiwan), respectively. Artificial bone substitute (Osteoset®) was purchased from Wright Medical Technology, Inc., Arlington, U.S.A.
Preparation of Human Thrombin
10 ml of FFP and 0.3 ml of 10% CaCl2 solution were injected into the chamber of TGD®. The device was shaken gently for 30 s and then put aside to let the plasma activation reaction proceed at room temperature. After 20 minutes, the device was shaken vigorously to isolate the formed fibrin clot, and to aseptically draw the thrombin-rich solution using a 10 ml-syringe.
Preparation of the Platelet Gel-artificial Bone Substitute Implants
Two kinds of bone grafts were prepared and evaluated as bone growth (near spine area) implants in this study: 5 g of pure, commercially available artificial bone substitute (Osteoset®, medical grade, 3×5 mm, calcium sulfate pellets), or a mixture of artificial bone substitute Osteoset® and HTPG. HTPG was prepared as follows:
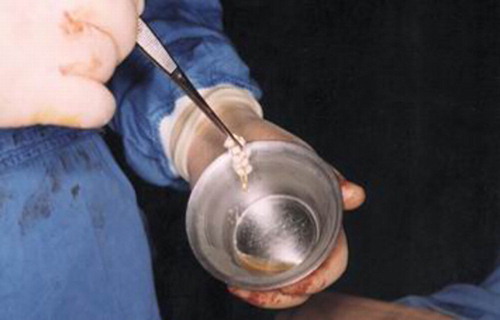
Platelet-rich plasma (5 ml) was added and mixed uniformly with the artificial bone substitute Osteoset® (5 g) in a stainless steel bowl and the resulted pellets were left in contact with the platelet concentrate for 1 to 2 min. Subsequently, the same volume of HT (5 ml) was added and the mixture was gently stirred for a few seconds. A jelly product comprising HTPG and artificial bone substitute (HTPG) was formed within 10 to 20 sec. As can be seen (), a viscous and sticky HTPG was obtained, which adhered well to the artificial bone substitute, forming a fibrin-platelet-artificial bone substitute jelly structure that could easily be implanted (experimental group A).
Animal and Surgical Operation Procedures
Eight dogs (male, average weight = 20 Kg) were used for the animal experiments. The dogs were anesthetized by mixing Ketamin (0.11 mg/kg) and 2% Rompun (Cheng Shen Agri-Med Co., Taipei, Taiwan) at a 1: 1 volume ratio. The hair over the back of the incision was shaved completely and was sterilized by iodine. The skin incision ran straight in the midline of the back. The subperiosteal dissection was extended from spinous processes and laterally to the ends of the facet joints. The same procedure was subsequently applied to the contralateral side. The adjacent laminas of L1/2 and L4/5 were decorticated. The joints capsules of the segments were removed, together with all the remnants of the tendon insertions between the spinous processes and the interspinal ligaments. Following the above procedures, a graft of mixture of HTPG/Osteoset® (Group A) was put on the facet joints of L1/2 and Osteoset® alone (Group B, that could be viewed as the control group) was put on L4/5 of the prepared laminas of each dog, ensuring the same amount of the respective grafts were put on each dog. Wound closure was affected by suture of paraspinal musculature and skin without drainage. The lumbar spines were evaluated radiologically 2, 4, 8, and 12 weeks after surgery. The dogs were killed to evaluate histologically at the end of 12th week.
Radiographic Assessment of New Bone Formation
Radiographs in the lateral planes were obtained to evaluate the fusion masses 2, 4, 8, and 12 weeks after the operation. A radiographically fused segment was defined as a continuous callus over laminas and facet joints with no radiolucent clefts. The presence of any cleft resulting in the specimen would be deemed a nonunion. The new bone formation of spine area was assessed visually and expressed as a percentage of new bone observable on the X-ray observations (semi-quantitative evaluation).
Histological Preparation and Evaluation
Histological evaluation was done after the healing period of 12 weeks. The dogs were sacrificed and the status of bone formation around the spine area was evaluated by histomorphological observations from the regenerated tissues, and the detection of newly formed bone mass. Briefly, the regenerated bone mass was removed and fixed in 10% neutral-buffered formalin, decalcified in HCl solution, dehydrated in an ascending graded series of ethanol solutions and toluene, and the full-faced section was embedded in paraffin. The celloidin-paraffin double-embedding procedure took 96 h by using an automatic specimen processor. The specimen was impregnated with paraffin wax and embedded. Transverse sections encompassing the original and regenerated bone component were prepared by using a microtome and stained with hematoxyline-eosin (HE) and then subjected to optical microscopic observation.
RESULTS AND DISCUSSION
Radiographic Assessment
shows X-ray radiographs after 2, 4, 8, and 12 weeks’ of implantation. Due to the viscosity and stickiness of the HTPG used to prepare HTPG/Osteoset® material, the implant was not drained out upon application, in contrast to the implanted Osteoset® material alone. As shown in (a), HTPG/Osteoset® material adheres more firmly on the pre-decorticated lamina and facet joint whereas the Osteoset® material used alone exhibited a loose contact with the same area. Notable callus formation phenomena began to be observed in the areas of combined HTPG/Osteoset® (Group A) after 2-week implantation and they achieved further consolidation in the following weeks. The Osteoset® was evidently resorbed gradually ((b) & 2(c)). On the contrary, in the control group, Osteoset® particles were visible throughout the whole observation period, although some of the graft particles were somewhat resorbed as well (Group B). Significantly, after 12 weeks, a more solid bone formation was clearly evident from the view of X-ray on the laminas and facet joints where combined HTPG/Osteoset® material was implanted (Group A). These X-ray observations demonstrated that the HTPG/Osteoset® material could successfully promote the new bone formation in the spinal area as compared to the Osteoset® material used alone. We have included a table based on the reading of radiographs during the healing periods. As shown in , tissue reconstruction reached approximately 80% of the injury defects when treated by HTPG/Osteoset® combination, but only 30∼40% in the absence of HTPG after 12 weeks.
Figure 2. X-ray radiographic observations at (a) 2 weeks, (b) 4 weeks, (c) 8 weeks, and (d) 12 weeks. Group A: HTPG/Osteoset® material. Group B: Osteoset® material alone.
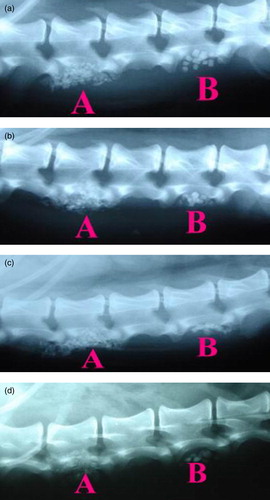
Table 1. The semi-quantitative evaluation of new bone formation
Histological Observations
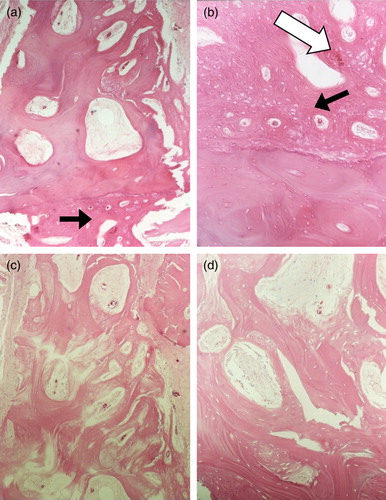
Varying degrees of new bone formation were observed in Group A and Group B. shows a transverse section of tissues on the fusion sites 12 weeks after surgery. As shown, new bone tissue was observed around the spinal area that implanted with Group A ((a) & (b)). On the contrary, there was no obvious new bone formation in the control group after 12 weeks of implantation ((c) & (d)). An angiogenesis effect (new vascular formation) was also observed in the HTPG/Osteoset® combination (Group A) ((b)), whereas new blood vessels were absent in the control group (i.e. Group B). These results demonstrated that the HTPG/Osteoset® materials would be beneficial to new bone formation in spine area.
Figure 3. Histological observations of new bone structures at 12 weeks implanted with HTPG/Osteoset material or Osteoset® alone. New bone structures (black arrow) and blood vessels (white arrow) could be observed for cases in combination of HTPG and Osteoset®. (a) implanted with HTPG/Osteoset® material (Group A)×40; (b) implanted with HTPG/Osteoset® material (Group A)×100; (c) implanted with Osteoset® material only (Group B)×40; and (d) implanted with Osteoset® material only (Group B)×100. Hematoxylin and eosin stain.
In general, spinal fusions are major, often complex procedures in orthopedic surgery Citation[19–21]. Spine fusion usually requires a large volume of bone graft material. Harvesting autologous bone graft is associated with considerable secondary morbidity and therefore the use of allograft as graft extension is sometimes of great value Citation[22]. However, using allograft runs the risk of transmitting bacteria or viruses. For these reasons, bone graft substitutes are of great interest. The goals for bone-graft substitutes are to match fusion rates with autologous bone-grafting techniques while avoiding the morbidity of bone-graft harvest and extending the quantity of available graft material. Graft with bone substitute alone has an extreme high rate of non-union. An essential and successful fusion requirement in clinical practices is the presence of osteoinductive activity in the fusion site Citation[23]. Most commercial bone substitutes lack this property. Recent studies have reported the efficacy of bone morphogenetic protein (BMP) in facilitating spinal fusion and osteo-induction Citation[24–26]. BMP may act at the cellular level to cause mesenchymal stem cells to differentiate into bone precursor cells. Recombinant BMP-2 has received FDA approved for lumbar interbody application with titanium cages Citation[27]. However, BMP is very expensive and not commercially available in many countries. Besides, unresolved questions about dosage, carriers and patient variability still need to be explored. Recently, gene therapy and other bone healing applications are being examined as an alternative strategy for spine fusion Citation[28], but it still needs several years survey to become an affordable, alternative therapeutic tool.
In order to stimulate the entire cascade of healing events that are taking place in bone healing or spine fusion, use of the multiple growth factors released by activated human platelets remains a promising avenue of development Citation[3], Citation[29]. PG, obtained from platelet-rich plasma that activated with BT, has been increasingly used for the repair of lumbar spinal fusion. Combined with autograft or artificial graft, it has showed promising potential in posterior fusions or intradiscal fusions applications Citation[30], but contradictory results were also reported by others Citation[31], Citation[32]. Here we have evaluated the effects of a combination of HTPG and artificial bone substitute in enhancing bone healing around the spine area. X-ray radiographic observations and histological assessment were performed to examine the extent of new bone formation. The preliminary results obtained from X-ray and histological studies revealed that spine defects implanted with HTPG/Osteoset® combination material exhibited better bone formation as compared to those implanted with artificial bone substitute only. These results suggest that the calcium sulfate component in pure Osteoset® material was unable to achieve an adequate bone regeneration function due to insufficient osteo-induction capability after implantation Citation[33]. To our knowledge, the methodology utilized in the present study may be the first reported experimental work to evaluate the benefits of PG activated with HT and combined with artificial bone substitute on bone formation around the spine area. These results are new and open up interesting prospects for spine fusion procedures in future. Indeed, bone grafts are crucial in spinal operation to achieve stable fixation, especially for autologous bone grafts, which represent the most predictable material for its osteoinductive and osteoconductive properties. Nevertheless, the supplies of autologous bone grafts are limited and difficult to obtain in a sufficient amount for large segmental surgery.
Although the present study revealed beneficial results by using HTPG/Osteoset® material in bone formation around the spine area, we believe that some experimental designs or procedures could possibly be further improved. For example, the amounts and the release behavior of PDGF-AB and TGF-β1 growth factors obtained in HTPG and the effects of growth factors on the spine fusion still need to be carefully examined in real clinical practices. Nevertheless, mixing of PG with HT to prepare a growth-factor-rich HTPG was still capable of embedding the artificial bone substitute Osteoset® to prepare a novel composite for the application of spine fusion in this present study.
CONCLUSION
In summary, the preparation of HT, using TGD®, and of PG was easy and required less than 30 minutes. The experimental results obtained showed that a combined HTPG/artificial bone substitute Osteoset® material could be used successfully in regenerating new bone around the spine area and stimulating the implanted graft consolidation into a bone mass during spine healing procedures. Furthermore, artificial bone substitute combined with PG activated by HT avoids the immunological and infectious risks of BT and may open new interesting prospects for spine fusion procedures in future. Further experimental work will be conducted to evaluate whether the combination of platelet gel and fibrin glue, both activated by HT, with artificial bone substitute can further improve clinical outcomes in spinal fusion procedures.
Acknowledgements
This study was performed in accordance with the “Principles of Laboratory Animal Care” (NIH publication No. 86-23, revised 1985) and with the local laws prevailing for animal experiments.
References
- Burnouf, T., Radosevich, M., and Goubran, H. 2004. Local hemostatic blood products: fibrin sealant and platelet gel. Monograph, World Federation of Hemophilia 1–14.
- Burnouf T. Biomedical Engineering-Applications. Basis & Communications 2004; 16: 294–304
- Anitua E., Andia I., Ardanza B., Nurden P., Nurden A.T. Thromb Haemost 2004; 91: 4–15
- Marx R. E., Carlson E. R., Eichstaedt R. M., Schimmele S. R., Strauss J. E., Georgeff K. R. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 85: 638–646
- Whitman D. H., Berry R. L., Green D. M. J Oral Maxillofac Surg 1997; 55: 1294–1299
- Kasperk C. H., Wergedal J. E., Mohan S., Long D. L., Lau K. H., Baylink D. J. Growth Factors 1990; 3: 147–158
- Noda M., Camilliere J. J. Endocrinology 1989; 124: 2991–2994
- Pfeilschifter J., Oechsner M., Naumann A., Gronwald R. G., Minne H. W., Ziegler R. Endocrinology 1990; 127: 69–75
- Slater M., Patava J., Kingham K., Mason R. S. J Orthop Res 1995; 13: 655–663
- Caplan A. I. J Orthop Res 1991; 9: 641–650
- Banninger H., Hardegger T., Tobler A., Barth A., Schupbach P., Reinhart W., Lammle B., Furlan M. Br J Haematol 1993; 85: 528–532
- Israels S. J., Israels E. D. Am J Pediatr Hematol Oncol 1994; 16: 249–254
- Zehnder J. L., Leung L. L. Blood 1990; 76: 2011–2016
- Scott M. R., Will R., Ironside J., Nguyen H. O., Tremblay P., Armond S. J., Prusiner S. B. Proc Natl Acad Sci U S A 1999; 96: 15137–15142
- Will R. G. Acta Paediatr Suppl 1999; 88: 28–32
- Vankemmel O., Taille A., Burnouf T., Rigot J. M., Duchene F., Mazeman E. Urol Int 2000; 65: 196–199
- Tsai J. C., Kuo S. M., Chang S. J., Manousakas I., Chen T. M. Biomedical Engineering: Application. Basis and Communication 2007; 19(4)225–229
- Su C. Y., Chiang C. C., Lai W. F., Lin K. W., Burnouf T. Transfusion 2004; 44: 945–945
- Chosa E., Goto K., Totoribe K., Tajima N. J Spinal Disord Tech 2004; 17: 134–139
- Harris B. M., Hilibrand A. S., Savas P. E., Pellegrino A., Vaccaro A. R., Siegler S., Albert T. J. Spine 2004; 29: E65–70
- Zdeblick T. A. Spine 1993; 18: 983–991
- Eie N., Solgaard T., Kleppe H. Spine 1983; 8: 897–900
- Curylo L. J., Johnstone B., Petersilge C. A., Janicki J. A., Yoo J. U. Spine 1999; 24: 434–439
- Boden S. D., Grob D., Damien C. Spine 2004; 29: 504–514
- Boden S. D., Zdeblick T. A., Sandhu H. S., Heim S. E. Spine 2000; 25: 376–381
- Mummaneni P. V., Pan J., Haid R. W., Rodts G. E. J Neurosurg Spine 2004; 1: 19–23
- Xue Q., Li H., Zou X., Bunger M., Egund N., Lind M., Christensen F. B., Bunger C. Eur Spine J. 2005; 14: 222–226
- Cha C. W., Boden S. D. Spine 2003; 28: S74–84
- Lowery G. L., Kulkarni S., Pennisi A. E. Bone 1999; 25: 47S–50S
- Walsh W. R., Loefler A., Nicklin S., Arm D., Stanford R. E., Yu Y., Harris R., Gillies R. M. Eur Spine J 2004; 13: 359–366
- Carreon L. Y., Glassman S. D., Anekstein Y., Puno R. M. Spine 2005; 30: E243–247
- Castro F. P. J Spinal Disord Tech 2004; 17: 380–384
- Kelly C. M., Wilkins R. M., Gitelis S., Hartjen C., Watson J. T., Kim P. T. Clin Orthop Relat Res Jan 2001; 382: 42–50