Abstract
A new procedure was developed to obtain high-quality polymerized human hemoglobin by modifying purified hemoglobin with PLP and polymerized with GDA. Comparing polymerized hemoglobin products obtained from different methods, the product from the new procedure has similar physical, chemical, and biological properties in the molecular distribution, methemoglobin concentration, oxygen carrier capacity, P50 and spectral analysis. Furthermore, the new procedure of modification after polymerization can save PLP greatly, and significantly reduce the cost. So the procedure of modification after polymerization is a better way in research and production.
Stablizing hemoglobin structure, prolonging the circulation half-life, and lowering the O2 affinity is very important in the research of hemoglobin-based oxygen carriers Citation[1], Citation[2]. American Northfield Corporation modified human hemoglobin with Pyridoxalated-5-phosphate (PLP) and polymerized hemoglobin with Glutaraldehyde (GA). The product which is on its phase III clinical trials is perspective. Chengmin Yang et al. reported the modification and polymerization of human placental hemoglobin and made great progress Citation[3]. This paper investigated a number of polymerization methods and further optimized the process of the placental hemoglobin modification.
MATERIALS
Blood Source
The normal human placental blood was supplied freshly by TianJin Union Stem Cell Gene Engineering Corp.
Chemicals and Reagents
5-Pyridoxalated was purchased from Sigma; all other chemicals used in this study were at analytical reagent grade.
Instrument
CARY50 ultraviolet-visible spectrophotometer (VARIAN); SuperdexTM 75Gel column (Amersham Biosciences); HPLC (VARIAN); Stat PHOX blood gas analyzer (NOVA).
METHODS
Preparation of Placental Hemoglobin
The placental hemoglobin was isolated and purified by established methods in our laboratory and the methods were described in reference Citation[4].
Study of the Process on Polymerization and Modification (All Steps were Carried out in 4°C Water Bath)
The procedure of PLP modification before GDA polymerization
The stroma-free hemoglobin solution obtained from step 2.1 is completely deoxygenated by flushing with nitrogen and vacuumizing. The solution is pyridoxylated using pyridoxa-5’-phosphate, which was dissolved in tris-hydrochloride buffer on about 4:1 molar ratio to hemoglobin. After 3-4h a reducing agent sodium borohydride, which was dissolved in sodium hydroxide, is added to the solution, and the PLP-HbF is obtained after incubation for 4h. Excess reagents were removed by dialysis against 0.9% sodium chloride. Subsequently, the PLP-HbF is polymerized using 1% glutaraldehyde. After 1-2h a reducing agent sodium borohydride, which was dissolved in sodium hydroxide, is added to the solution; the Poly-PLP-HbF is obtained after incubation for 4h. Excess reagents may be removed by dialysis against 0.9% sodium chloride.
The procedure of PLP modification after GDA polymerization
The stroma-free hemoglobin solution is completely deoxygenated by flushing with nitrogen and vacuumizing. The solution is polymerized using 1% glutaraldehyde. After 2h incubation a reducing agent sodium borohydride, which was dissolved in sodium hydroxide, is added to the solution; the Poly-HbF is obtained after incubation for 4h. Excess reagents may be removed by dialysis against 0.9% sodium chloride. Subsequently, the Poly-HbF is pyridoxylated using pyridoxa-5’-phosphate, which was dissolved in tris-hydrochloride buffer on about 4:1 molar ratio to hemoglobin. After 3-4h a reducing agent sodium borohydride, which was dissolved in sodium hydroxide, is added to the solution; the Poly-PLP-HbF is obtained after incubation for 4h. Excess reagents may be removed by dialysis against 0.9% sodium chloride.
Detection Methods
The concentration of Hb was determined using a standard method by converting Hb into cyanmethemoglobin and detecting the absorption at 540nm as described in reference Citation[5].The methemoglobin concentration was determined using standard spectral analysis methods described in the reference Citation[5].
The polymerization rate was detected by SDS-PAGE using 12.5% gel, and HPLC assay using SuperdecTM 75 Column (10mm×300mm) with 1/15M PBS (pH7.3) at flow rate of 0.5ml/min. The HPLC assay was conducted at 25°C, and monitered at wavelength 280nm.
Oxygen carry capacity assay and P50 determination were performed according to refrence Citation[7]. Testing samples were prepared by dialysis against saline solution (pH 7.2 – 7.4), and then adjust Hb concentration to 6.5%∼7%.
Spectral assay was conducted at wavelength 220∼1000 nm with Hb concentration at 5mg/100ml.
RESULTS
The principal Hb in adults is HbA and in placental is HbF. Normal Hb consists of four chains (α2β2, α2γ2). The α chain and β (γ) chain combine tightly into a dimmer, and two dimmers combine loosely into a tetramer. The affinity of Hb for O2 is regulated by 2,3-diphosphoglyerate (2,3-DPG). However, lots of 2,3-DPG was lost during the purification procedure. Due to the depleting of 2,3-DPG, isolated Hb therefore cannot readily release O2 until the tissue O2 tension is much lower (low P50). PLP is an analogue of 2,3-DPG, which could bind to the 2,3-DPG pocket within Hb. PLP-modified Hb would have a lower oxygen affinity and a higher P50. The reported paper is mainly on the procedure of modification with PLP before polymerization with GDA. The paper studied the physical, chemical, and biological properties of Hb products from different procedures between modification before polymerization and modification after polymerization.
The Molecular Distribution of the Products from Different Procedures
Molecular distribution of Hb products from 2 different procedures is shown in . Molecular distribution is an excellent indication of the conformation of Hb product. Molecular distribution of modification before polymerization is: 52. 6% (above 64KD), 47.4%(equal or below 64KD); the molecular distribution of modification after polymerization is: 54.1% (above 64KD), 45.9% (equal or below 64KD). So the molecular distribution can be controlled by regulating the reaction condition.
Table 1. Molecular distribution of different procedures
MetHb Concentration and Oxygen Carrying Capacity
Product from the new procedure has no significant difference on MetHb concentration and oxygen affinity compared to the product from the tradition procedure as indicated by data from five separate experiments (). Products from PLP modification before GDA polymerization contain an average of 4.74% MetHb and the oxygen affinity capacity is 1.02mL/g, while product from modification after polymerization has 4.95% of MetHb and oxygen affinity capacity is 1.07mL/g, respectively. There is no marked difference between the two processes and oxygen affinity capacity maintained in the adult blood level.
Table 2. Comparation of MetHb concentration and oxygen capacity volume
P50 of Products from Different Processes
summarizes the product P50, an important functional property, from different processes. The Hb products from two different processes of PLP modification and GDA polymerization have similar P50 values (29.6 mmHg and 27.5 mmHg, respectively) and P50 values are higher than that of purified Hb. P50 increased from 11.3 mmHg (purified) to 29.6 mmHg in process of modification before polymerization, while P50 increased from 11.3 mmHg (purified) to 27.5 mmHg in process of modification after polymerization. There is no marked difference in P50 between the two processes.
Table 3. Comparison of P50 from of different modification processes
Biological Activities
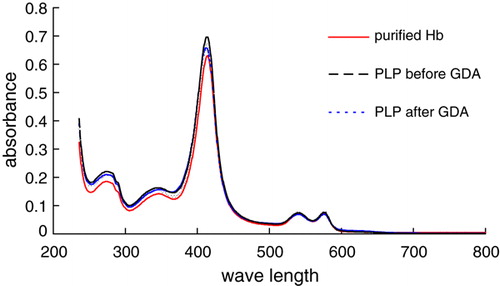
The change of biological activities of a protein is highly related to changes of its structural conformation. Using spectral analysis, we demonstrated that polymerized Hb from different procedures have almost identical absorption spectrums with purified Hb on a maximum absorption 415nm (), which indicates no significant structural changes. So we could infer that a series of modification and polymerization procedures have no adverse effects on the structure of Hb that was the premise of biological activity. Additionally, the purified and modified Hb showed absorption peaks at 540nm and 577nm, respectively, which were the symbols of carrying O2. The absorption peaks suggest the O2 carrying capacity might be reserved in the modified Hb, and the procedure has no effects on the oxygen carrying capacity.
DISCUSSION
Hemoglobin based oxygen carrier has to obey the physical and chemical index as well as retain the biological activity of carrying and releasing oxygen. First of all, depleting 2,3-DPG during purified procedure leads to an increased oxygen affinity, decreased P50, and then lost the biological functions. In addition, after separation from red blood cells, hemoglobin tetramer divides into the dimmer easily, and the dimers could not be used as blood substitutes because dimmers discrete from kidneys, which damage kidney function. Appropriate modification and polymerization processes must be introduced to the isolated Hb to keep Hb in polymer or oligomer formation and to maintain its basic biological function, such as O2 transporting. GDA has been a widely used polymerization agent and the polymerization mechanism has been well established. The aldehyde of GDA mainly reacted with lysine -NH2 residues as well as the αterminal -NH2 and the sulfhydryl group of cysteine in the polymerization process. The reaction is random and the product is Schiff based. And in the modified process, PLP (which is the analog of 2,3-DPG) reacts with the site of βstrain. Therefore GDA and PLP react with different sites of Hb, respectively, without interference, and thus it must be concluded that they do not compete with each other for the same amino acid –NH2 groups within the Hb chains. So the physical-chemical and biological properties haven't a marked difference between products from two processes.
In summary, data from this report demonstrated that the molecular distribution, MetHb concentration, oxygen carrying capacity, and spectral analysis are not markedly different between the products from the two procedures. Furthermore, the new procedure of modification after polymerization can save a great amount of PLP compared to that of traditional procedure and subsequently significantly reduced the total production cost of Hb-based blood substitutes. We believe that the procedure of modification after polymerization has great potential both in research and industrial-scale production.
References
- Chang, T.M.S., Montreal, P.Q. 1993. Blood Substitutes: Principles, Methods, Products and Clinical Trials, pp.3–12.
- Wang C.L., Xiu R.J. The artificial blood substitutes and application development. Foreign Medical Sciences and Blood Transfusion and Hematology 2005; 28(3)260–263
- Yang, C.M., Li, J.Z., Ji, Y. 2001. Science Basis of Transfusion. People's Science and Technology Publishing House, Beijing, pp. 547–569.
- Yang, C.M., Wang, H., Zeng, M., , et al. The preparation methods of stroma free and pyrogen free hemoglobin for red blood cell substitutes. Patent no. 01108643.
- Litao, 2006. Master's degree dissertation, The College of Huaxi Pharmaceutical, SiChuan University.
- He, Z.X., Zhang, S.Z. 1999. Electrophoresis, 2nd ed. Science Publishing House, Beijing, pp. 67–90.
- Zhu, Z.Y., Chen, Z.H. 1978. Clinic Medical Detection. Shanghai Scientific & Technical Publishers, Shanghai, pp.381–384.
- Li, Tao, Zhang, Honghui, Li, Hongying, , et al. 2005. A modified process for purification of human hemoglobin from placenta blood. The Xth International Symposium on Blood Substitutes, Brown University, Providence, p.51.
- Zeng, Y.T. 2002. Human Hemoglobin. Science Publishing House, Beijing, pp. 1–12.
- Liang S.G., Tong M.R., Pan J.L., Yu Y.T. Preparation and charactaristics of pyridoxylated polyhemoglohin. Ion Exchange and Adsorption 1994; 10(4)294–299
- Chad R. H., Paul W. B., Anil G. Purification and chemical modifications of hemoglobin in developing hemoglobin based oxygen carriers. Advanced Drug Delivery Reviews 2000; 40: 153–169
- Judy A. W., Craig R. J., Joe H. S. Shifts in affinity and enthalpy of oxygen binding to human hemoglobinA induced by pyridoxal and pyridoxal 5-phosphate. J. Nutr. Biochem. 1997; 8: 19–24
- Lu Zhang, Abraham Levy, Joseph M. R. Autoxidation of hemoglobin enhanced by dissociation into dimers. The Journal of Biological Chemistry 1991; 266(36)24698–24701