Abstract
To investigate the changes of calcitonin gene-related peptide (CGRP) in rat's blood plasma, spinal anterior motorneuron, and dorsal root ganglion (DRG) after fractures combined with central or peripheral nerve injuries and its influence on fracture healing, 72 healthy adult SD rats (male or female) were divided into 4 groups (18 rats in each group): group A, simple(left) tibial fracture; group B, left tibial fracture combined with left sciatic nerve injury; group C, left tibial fracture combined with T9-11 spinal cord transection injury; group D, left tibial fracture combined with right cerebral cortex injury. Group A was the control group. The concentration of serum CGRP was measured immediately, 1w, 2w, and 4w after injury using radio immunoassay. X-ray photograph was taken at 1w, 2w, and 4w after injury to assess fracture healing. The concentration of serum CGRP in spinal anterior motorneuron and dorsal root ganglion was measured 1w, 2w, and 4w after injury. Bony callus at 2w after injury using H.E.staining was observed. 1w and 2w after injury, the fracture line was still clear on the X-ray of all groups, but 4w after injury the fracture line disappeared with complete healing except the peripheral nerve injury group. By H.E. staining, we found lesser bony callus contents in the peripheral nerve injury group than the simple fracture group at 2w after injury; irregular bone trabecula and healing defect were found in the former group. While the spinal injury group and cerebral cortex injury group represented more bony callus than the simple fracture group, increased bone trabecula and regularity, medullary cavity occluded and finally solid bony connections were found. CGRP concentration in blood plasma and spinal anterior motorneuron represented no apparent differences among all groups during each observation period. For the dorsal root ganglion group, 1w after fracture, there was no apparent difference of CGRP concentration in the peripheral nerve injury group and cerebral cortex group compared with the control group (P > 0.05), but the spinal injury group showed more CGRP than the control group (P < 0.01). 2w after injury, the peripheral nerve injury group and cerebral cortex group also showed no difference compared with the control group, but the cerebral cortex group had more CGRP contents than the peripheral nerve injury group (P < 0.05), and the spinal injury group showed more CGRP than the control group (P < 0.01). 4w after injury, the peripheral nerve group, spinal injury group, and cerebral cortex injury group all showed higher concentration of CGRP than the control group. Among the 3 groups, the spinal injury group is the highest (P < 0.01). When fracture combined with peripheral nerve injury, the healing process can be slowed down. In contrast, fracture combined with spinal injury and cerebral cortex injury will accelerate the healing process. The CGRP in dorsal root ganglion in spinal injury group and cerebral cortex injury group increased, which may have positive effects on fracture healing.
In our clinical work, we found that when fracture combined with peripheral nerve injury, the healing process can be slowed down. In contrast, fracture combined with spinal injury and cerebral cortex injury will accelerate the healing process. Our research was aimed to investigate the changes of calcitonin gene-related peptide (CGRP) in rat's blood serum, spinal anterior motorneuron, and dorsal root ganglion (DRG) after fractures combined with central or peripheral nerve injuries, and its influence on fracture healing.
MATERIALS AND METHODS
Animal Models
Seventy-two healthy adult SD rats (male or female, weight 220-250g) were divided into 4 groups according to the operation methods, with 18 rats each group. All the operations were allowed by the animal operation experiment committee of Peking University.
Group A, simple(left) tibial fracture;
Group B, left tibial fracture combined with left sciatic nerve injury;
Group C, left tibial fracture combined with T9-11 spinal cord transection injury;
Group D, left tibial fracture combined with right cerebral cortex injury.
Group A was designed as control group.All the rats were provided by the Science Animal Center of Peking University Health Science Center.
SD rats were anaesthesized with 5% ketamine hydrochloride (0.2−.03ml/100g) by intraperitoneal injection. We built the superior segment of the rat left tibial fracture model, after anatomy reestablishment, and fixed the bone with a Kirschner wire. Group B: left tibial fracture combined with left sciatic nerve injury models were constructed by cutting the left sciatic nerve at 10 mm above sciatic nerve crotch, turning over the distal end and suturing it to the subcutaneous. Group C: we made a median incision at the back of the rat, exposed the thoracic vertebra, cut off and resected about 2mm spinal cord at the 9∼11 lever simultaneously, built the left tibial fracture combined with T9-11 spinal cord transection injury model. Group D: the right cerebral cortex injury model was constructed by the Allen method; we built the left tibial fracture combined with right cerebral cortex injury model. The rats were raised in different cages according to the grouping; we supplied the rats with enough food and water, and gave a massage to the bladder of the spinal cord injury rats every 6h after operation.
Dection Items
X-ray photographs were taken at 1w, 2w, and 4w after injury to assess the fracture healing. Bony callus at 2w after injury using H.E staining was observed.
The concentration of serum CGRP was measured immediately 1w, 2w, and 4w after injury using radio immunoassay.
2 ml blood were drawn from the angular vein immediately 1w, 2w, and 4w after injury. We put it into a centrifuge tube within 30 l EDTA (100g/L)and 40 l aprotinin, 3000r/min centrifuge for 10 min, drew the clear supernatant liquid 1ml, put it into a sample tube and preserved it in −70°C profound hypothermia environment.
We executed 6 rats in each group immediately and at 1w, 2w, and 4w after injury, cut for 1.5cm T12∼S1 ventro-spinal cord and 3 pairs of spinal cord dorsal root ganglion, ground for a short while and put it into a sample tube within 1ml HAC (1%), heated it to boil for 15 min, bene tritum to get homogenate, then 3000r/min centrifuge for 15 min. to draw the clear supernatant liquid. We put it into a sample tube and preserved it in −70°C profound hypothermia environment, then detected the CGRP concentration using radioimmunity method (CGRP kit was bought from Ferrer Biotechnology Companies in Beijing).
Statistical Analysis
The data were analyzed by SPSS 11.0 and compared by t-test method. A probability where p < 0.05 was considered significant for all statistical comparison. All values were presented as the mean±SD.
RESULTS
Observation According to the X-ray
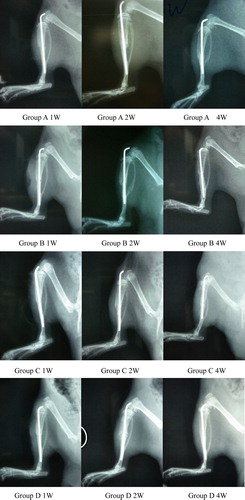
The overview morphous of the fracture is shown in : 1w and 2w after injury, the fracture line was still clear on the X-ray of all groups, but 4w after injury, the fracture line disappeared with complete healing except peripheral nerve injury group.
H.E Staining of the Bony Callus
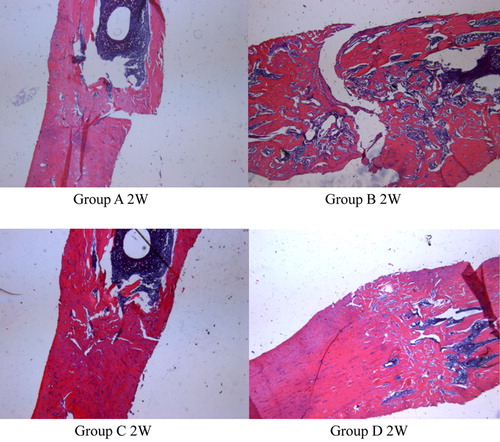
As shows, by H.E. staining, we found lesser bony callus contents in the peripheral nerve injury group than the simple fracture group at 2w after injury; irregular bone trabecula and healing defect were found in the former group. While the spinal injury group and cerebral cortex injury group represented more bony callus than the simple fracture group, increased bone trabecula and regularity, medullary cavity occluded and finally, solid bony connections were found.
The Variation of CGRP Concentration in Blood Serum
As can be seen in , the CGRP concentration in blood serum represented no apparent differences among all groups during each observation period.
Table 1. The variation of CGRP concentration in blood plasma (pg/ml)
The Variation of CGRP Concentration in Spinal Anterior Motor Neuron
As shown in , CGRP concentration in spinal anterior motorneuron represented no apparent differences among all groups during each observation period.
Table 2. The variation of CGRP concentration in spinal anterior motorneuron (pg/ml)
The Variation of CGRP Concentration in Dorsal Root Ganglion
For the dorsal root ganglion group, 1w after fracture, there was no apparent difference of CGRP concentration in the peripheral nerve injury group and cerebral cortex group compared with the control group (P > 0.05), but the spinal injury group showed more CGRP than the control group (P < 0.01). 2w after injury, the peripheral nerve injury group and cerebral cortex group also showed no difference compared with the control group, but the cerebral cortex group had more CGRP contents than the peripheral nerve injure group (P < 0.05), and the spinal injury group showed more CGRP than the control group (P < 0.01). 4w after injury, the peripheral nerve group, spinal injury group, and cerebral cortex injury group all showed higher concentration of CGRP than the control group. Among the 3 groups, the spinal injury group is the highest (P < 0.01).
Table 3. The variation of CGRP concentration in dorsal root ganglion (pg/ml)
DISCUSSION
Calcitonin gene-related peptide (CGRP) is one of several biologically active neuropeptides founded by Rosenfeld Citation[1] through DNA recombination and molecular biology techniques in 1983. CGRP is coded by calcitionin gene; the calcitionin gene is composed of 2800 bp, which contains 5 piece intervening sequence and 6 piece exons. They undertake recombination in different tissues to present different productions. For example, the calcitonin gene transcribes to calcitonin in the thyroid gland, but in the nervous tissue it transcribes to CGRP. The relative molecular mass of CGRP is 3786.91, and it contains 37 amino-acid residues Citation[1]. At the present realization, CGRP is the most fortis vasodilator material, and its regulation effect to bone metabolism is clearer as the investigation developed Citation[2].
Hukkanen Citation[3] et al. verified that there was rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture, suggesting that neural involvement is present in the bone growth and remodeling process. In addition, Onuoha Citation[4]designed a study to examine the circulatory levels of wound modulatory peptides–calcitonin gene-related peptide (CGRP) in patients with muscle injuries with bone fractures and within 24 h of the injury. The peripheral plasma levels of the sensory nerve peptide were measured on hospital admission (OA) and 24 h post-injury (PI) using ELISA technique. The result of this study shows that mean (s.d) ng/liter of CGRP was higher in patients OA than the controls; sensory nerve peptides are increased in bone-fracture-related injuries up to 24h after injury. It hints that CGRP may participate in the tissue repairment, and an intact nociceptor system of primary afferent sensory nerves is important for the initiation of successful tissue repair as dysfunction of this system could be a contributing factor for a delayed wound healing.
We had observed the fracture healing status and the variation of CGRP concentration in different positions, seen from results of this experiment. When fracture combined with peripheral nerve injury, the healing process can be slowed down. In contrast, fracture combined with spinal injury and cerebral cortex injury will accelerate the healing process. By H.E. staining, we found lesser bony callus contents in the peripheral nerve injury group than the central nerve injury group; the different type of injury of the nerve can result in different speed of fracture healing. It demonstrated that nerve factors can affect the healing process of the fracture. The theory that nerve factors can affect the healing process of the fracture had already been put forward by Aro et al. Citation[5] and was confirmed by some researchers in the last decade, but its mechanism still remains unclear. So we presume that CGRP is one of the nerve factors that affect the healing process of fracture. J.M. Garcia-Castellano Citation[6] demonstrated that CGRP has a great effect on angioectasia to capillary, so it can accelerate the proliferation of osteocyte.
In our research, we observed that the CGRP concentration in blood plasma represented no apparent differences among all groups during each observation period, so we presume that the way of CGRP regulated healing process of fracture is not by humoral regulation. Zaidi et al. Citation[7] thought that the CGRP in osseous tissue plays its role by means of paracrine secretion, and the CGRP in normal serum has no effect on bone metabolism. The CGRP concentration in spinal anterior motorneuron represented no apparent differences among all groups during each observation period. It demonstrated that spinal anterior motorneuron did not participate in the regulation to the healing process of fracture. In other words, the regulation of CGRP to healing process of fracture is not carried by means of the spinal anterior motor neuron.
As we all know, the distribution of CGRP is mainly concentrated in dorsal root ganglion. Through the analysis of the variation of CGRP concentration in dorsal root ganglion, we can see that CGRP concentration is the highest, and decreased gradually as time passed. There were two possible reasons for the CGRP concentration decrease: (1)The reduction of synthesis; (2) The augmentation of consumption. CGRP is a neurotransmitter, which is synthesized and secreted by neurone, but there is no obvious variation of CGRP concentration in spinal anterior motor neuron in the simple fracture group. So we can presume that the synthesis of CGRP did not decrease after fracture, but the consumption of CGRP is increasing for its participation in the regulation of the fracture healing process. Compared with the control group, the CGRP concentration in dorsal root ganglion degraded during the two weeks after injury in the peripheral nerve injury group, and the extent of degrade is obvious. The result agrees with what K.C. Kajander Citation[8] had observed: the CGRP concentration in dorsal root ganglion degraded obviously when the peripheral nerve was injured. However, in our research, compared with the control group, the CGRP concentration of peripheral nerve injury group decreased obviously at 4 weeks after injury. We presumed that because o the CGRP cannot be transported to the target organ through axoplasmic transportation and accumulated in dorsal root ganglion, so the CGRP concentration in dorsal root ganglion increased relatively. The CGRP concentration in dorsal root ganglion is high, but it cannot be transported to the target organ and play its regulation role, so the speed of healing is slower than the normal group, and contents of local bony callus is low. Compared with the control group, the CGRP concentration in spinal cord dorsal root ganglion of spinal cord injury group is lower, and increased gradually at 2 weeks and 4 weeks after injury. The extent is higher than the control group. The speed of fracture healing in the spinal cord injury group is faster than the control group, and contents of local bony callus are high. It demonstrated that the reason spinal cord injury can accelerate the healing speed is the participation of massive CGRP synthesized and secreted by neuron.
There was no apparent variation of CGRP concentration of the cerebral cortex injury group, respecting the serum CGRP concentration of the simple fracture group degraded gradually after injury, so we can conclude that after the cerebral cortex injuring the synthesis and secretion of CGRP increased compared with the simple fracture group, but the extent of CGRP increasing is lower than the spinal cord injury group.
When hematencephalon, hemorrhagic shock, endotoxic shock, and septic shock happened,the secretion of CGRP increased obviously Citation[9]; meanwhile, the mRNA lever of CGRP in spinal cord dorsal root ganglion increased simultaneously. Why did the content of CGRP increase? The mechanism is still unclear.
Seen from the results of this experiment, when fracture combined with peripheral nerve injury, the healing process can be slowed down. In contrast, fracture combined with spinal injury and cerebral cortex injury will accelerate the healing process. The CGRP in dorsal root ganglion in the spinal injury group and cerebral cortex injury group increased, which may have positive effects on fracture healing. CGRP is one of the most powerful endogenous hemangiectasia peptidehasten. It was transported to the fracture region by means of axoplasmic transport and played its hemangiectasia roles, which could increase the blood supply of the fracture region and hasten the healing of fracture.
Aoki et al. Citation[11] demonstrated that CGRP is abundantly distributed in bone via sensory nerves, especially in the epiphyseal trabecular bones. CGRP is shown to be expressed endogenously by the osteoblasts. Transgenic mice with osteoblasts overexpressing CGRP are characterized by increased bone formation rate and enhanced bone volume, suggesting that CGRP indeed acts on bone metabolism not only via the nervous route but also via autocrine loop. Calcitonin gene-related peptide has an osteogenic stimulating effect, either by stimulating stem cell mitosis or osteoprogenitor cell differentiation (or both) Citation[12] in vitro. CGRP also inhibited bone resorption potentially Citation[13].
Our study provided powerful evidence that CGRP may play a local role in bone metabolism to promote the healing of fracture, but the mechanism of its action need further research.
Acknowledgements
This research project was funded by Chinese National Natural Science Fund for Outstanding Youth (30625036), Chinese 973 Project Planning (2005CB522604),Chinese National Natural Science Youth Fund (30801169),Beijing City Science & Technology New Star Classification A-2008-10 and Chinese ministry of education for Doctor Position New Teacher (20070001780).
References
- Rosenfeld M.G., Mermod J.J., Amara S.G., et al. Production of novel neuropeptide encoded by the calcitonin gene via issue-specific RNA processing. Nature 1983; 304: 129–135
- Bjurholm A., Kreicbergs A., Brodin E., et al. Substance P- and CGRP-immunoreactive nerves in bone. Peptides 1988; 9: 165–171
- Hukkanen M., Konttinen Y.T., Santavirta S., et al. Rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodeling. Neuroscience 1993; 54: 969–979
- Onuoha G.N. Circulating sensory peptide levels within 24 h of human bone fracture. Peptides 2001; 22: 1107–1110
- Aro H., Eerola E., Aho A. J. Development of nonunions in the rat fibula after removal of periosteral neural mechanoreceptors. Clin Orthop 1985; 199: 292–299
- Garcia-Castellano J.M., Diaz-Herrera P., Morcuende J.A. Is bone a target-tissue for the nervous system? New advances on the understanding of their interactions. Iowa Orthop J. 2000; 20: 49–58
- Zaidi M., Chambers T.J., Bevis P.J.R., et al. Effects of peptides from the calcitonin genes in bone and bone cells. Q J Exp Physiol 1988; 173: 471–485
- Kajander K.C., Xu J. Quantitative evaluation of calcitonin gene-related peptide and substance P levels in rat spinal cord following peripheral nerve injury. Neurosci Lett 1995; 186(2-3)184–8
- Wang X., Han C.D., Fiscus R.R., et al. Hypertension and endotoxin induced alterations in calcitonin gene-related peptide: Modulation by dexamethasone. Circ Shock 1991; 34: 217–223
- Poyner D, Marshall I, Brain SD. The CGRP Family1st eds. Texas, Landes BioscienceUSA 1999; 173–252
- Aoki M., Tamai K., Saotome K. Substance P and calcitonin gene-related peptide-immunofluorescent nerves in the repair of experimental bone defects. Int Orthop 1994; 18: 317–324
- Shih C., Bernard G.W. Calcitonin gene-related peptide enhances bone colony development in vitro [J]. Clin Orthop 1997; 334: 335–344
- Valentijn K., Gutow A.P., Troiano N., Gundberg C., Gilligan J.P., Vignery A. Effects of calcitonin gene-related peptide on bone turn over in ovariectomized rats [J]. Bone 1997; 21(3)269–274