Abstract
We established a HPLC method for content determination of aniracetam in aniracetam inclusion complex. The chromato column was Agilent ODS (4.6mm×150mm,5µm), the mobile phase was methanol-0.01 mol/L Potassium dihydrogen phosphate buffer solution (25:75, pH 3.0), with the flow rate of 1.0 ml/min, column temperature of 30° and the detection wave at 280 nm, the sample size was 20µL. A good linear relationship was obtained between the peak areas and the concentrations of aniracetam in the range from 5~80µg/ml (r=0.9998), the mean recovery was 100.1% (n=15), RSD=0.19%. This method is convenient, rapid, accurate, and brings about good recovery; it can be used for content determination of aniracetam in aniracetam inclusion complex.
INTRODUCTION
Aniracetam is an intellegence-promotion drug, a deravative of Piraceta, could act on the brain cells selectively, protect the brain functions from lack of oxygen, and improve the abilities of learning and memory. Aniracetam nasal gel was prepared with 2,6-dimethyl-β- cyclodextrin (DM-β-CD) through inclusion method, therefore more aniracetam could reach the brain tissue through nasal mocosa and olfactory nerve of regio olfactoria. UV spectrophotometry couldn't be used to determine the concentration of aniracetam in aniracetam inclusion complex because of the interference between aniracetam's UV absorption and DM-β-CD's. We established the method of content determination of aniracetam in aniracetam inclusion complex by HPLC Citation1–3.
MATERIALS
Aniracetam, provided by Lunan Pharmaceutical Co. Ltd., Lot 0610091. DM-β-CD, Shanxi Liquan Chemical Co. Ltd, Lot 060821.
LC-10Avp HPLC, SPD-Avp Detector, CTO-10Avp Column Oven, Class-vp workstation, UV-265FW spectrophotometer, Shimadzu Co. Ltd., Japan. Autoscience AS2060 ultrasonic oscillator.
METHODS AND RESULTS
Chromatograph Conditions
Stainless steel C18 5µm chromatography column, 150mm×4.6mm (Angilent); mobile phase: methanol-0.01mol/L KH2PO4 (25:75, pH3.0); flow speed: 1.0ml/min; detection wavelength: 280nm; column temperature: 30°C; injection volume: 20µl.
Preparation of Standard Solution
We weighed 10mg aniracetam precisely, dissolved it in a 250ml measuring flask with dilute hydrochloric acid, and prepared a standard solution whose concentration is 40µg/ml.
Preparation of Test Solution
We weighed 50mg comminuted aniracetam inclusion complex precisely, dissolved it in 250ml measuring flask with dilute hydrochloric acid, then filtered it with 0.45µm membrane; the subsequent filtrate was the test solution Citation4–6.
Chromatography Behavior of Aniracetam and Standard Curve
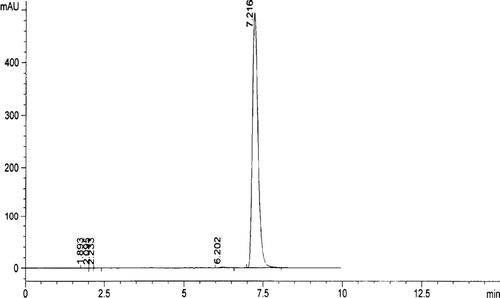
We injected 20µl standard solution into the HPLC under the above chromatograph conditions. The retention time of aniracetam was 7.04 min, the number of theoretical plates was 8018 according to aniracetam peak. is the HPLC behavior of aniracetam.
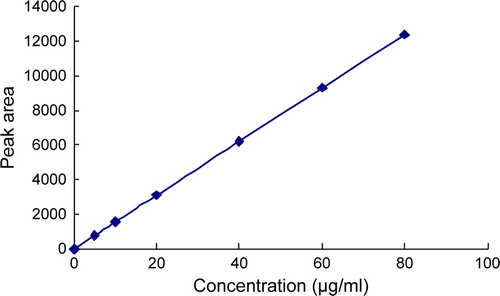
We measured 0.5, 1.0, 2.0, 4.0, 6.0, 8.0ml standard solution and put it into 10ml measuring flasks diluted with dilute hydrochloric acid. We injected the diluted solution into HPLC, then determined their absorption spectrums. We could get the linear relation from peak area (Y) and concentration (X): Y = 1.730×10−3X + 2.946×10−2 (r = 0.9998). The linear range was 5~80µg/ml. was the standard curve of aniracetam.
Recovery Test of the Determination Method
We weighed 2.0, 2.5, 3.0mg aniracetam precisely, put it and the corresponding DM-β-CD according to the formula into a 50ml measuring flask, dissolved it with dilute hydrochloric acid, then filtereditwith0.45µm membrane. The subsequent filtrate was injected into HPLC; the mean recovery of the method was 100.1% (n = 15), RSD was 0.19%. shows the results of the recovery test.
Table 1. Results of recovery tests
Repetition Test of the Determination Method
We injected 40.0µg/ml aniracetam standard solution repeatedly; the RSD of the repetition test was 0.38%.
Stability Test of the Determination Method
We injected the 40.0µg/mlaniracetam standard solution at the time of 0, 6, 12, and 24 hours after preparation. The aniracetam peak areas were 6210.6, 6251.3, 6200.8, and 6259.4, respectively; RSD was 0.35%, which suggested that the aniracetam solution was steady in 24 hours.
Assay of Test Solution
We injected 20µl test solution into HPLC, according to the external reference method to calculate the content of aniracetam. shows the results of content determination of samples.
Table 2. Results of content determination of samples (n = 3)
DISCUSSION
Selection of detection wavelength: Aniracetam had the maximum and steady absorbency in hydrochloric acid solution, λmax was 280nm, the concentration of hydrochloric acid had no effect on the absorbance of aniracetam, and showed good peak form and sensitivity.
A good linear relationship was obtained between the peak areas and the concentrations of aniracetam in the range from 5~80µg/ml (r = 0.9998); the mean recovery was 100.1% (n = 15), RSD = 0.19%, the RSD of the repetition test was 0.38%, and RSD of stability test was 0.35%.
Acknowledgements
This work was supported by the 2003 Award Foundation of Shandong Province for Excellent Young Scientists.
References
- Hao X., Liang C., Jian-Bin C. Preparation and spectroscopic studies of an inclusion complex of adenine with beta-cyclodextrin in solution and in the solid state. Analyst. 2002; 127(6)834–7
- Zhang J., Liang J., Tian Y., Zhang Z., Chen Y. Sensitive and selective liquid chromatography-tandem mass spectrometry method for the quantification of aniracetam in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 858(1–2)129–34
- Zhiquan Z., Yong S., Qiqing Z. Assay of CCNU in tumor by RP-HPLC. Artificial Cells, Blood Substitutes and Immobilization Biotechnology 2005; 33: 371–375
- Xu, Lisa,, Sun, Yong, Xu, Ping, Ma, Baohua.2007. Assay of levodopa in brain by CE. Artificial Cells, Blood Substitutes, and Immobilization Biotechnology, 35: 415–420.
- Ogiso T., Uchiyama K., Suzuki H., Yoshimoro M., Tanino T., Iwakai M., Uno S. Pharmacokinetics of aniracetam and its metabolites in rat brain. Biol Pharm Bull. 2000; 23(4)482–6
- Ogiso T., Iwaki M., Tanino T., Ikeda K., Paku T., Horibe Y., Suzuki H. Pharmacokinetics of aniracetam and its metabolites in rats. J Pharm Sci. 1998; 87(5)594–8