Abstract
Batch cultivation of Candida rugosa for the production of lipase and esterase enzymes was carried out by employing two different inoculation strategies. The effects of triolein (2, 3 and 5 g/l) and oleic acid (3 g/l) as carbon sources of the enzyme production medium on the activity, productivity, and yield of enzymes were also compared for both strategies. Inoculation of the cells into the enzyme production medium either directly or after cultivation in a pre-culture medium rich in glucose affected the activity and yield of esterase more than those of lipase for both carbon sources. In both strategies, triolein and oleic acid yielded the same lipase activity (16.67 U/ml) whereas triolein provided higher esterase activity (0.0035 U/ml). Time courses of the extracellular and intracellular lipase and esterase enzymes indicated that lipase activity was growth-associated and the cells secreted esterase into the medium after a considerable level of extracellular lipase activity was reached. The role of protease in the enzyme activities was also discussed.
INTRODUCTION
Candida rugosa is among the most extensively studied microorganisms by biotechnologists with respect to its powerful lipase (E.C. 3.1.1.3) production capacity. Wide substrate specificity provides C. rugosa lipase (CRL) to be successfully used in a variety of hydrolysis and esterification reactions, and its high stereoselectivity and regioselectivity make possible the synthesis of several pharmaceuticals Citation[1]. C. rugosa synthesizes and secretes a mixture of lipase isoenzymes differing in biocatalytic properties. It has been established that at least seven genes are involved in the C. rugosa lipase-producing machinery and some isoenzymes exhibit esterase (E.C. 3.1.1.1) activity Citation[2]. Additionally, carboxyl-thioesterases were also described and characterized from commercial samples Citation[3], Citation[4]. Lipases can be distinguished from esterases by the phenomenon of interfacial activation, which was only observed for lipases. Lipases prefer water-insoluble substrates, typically triglycerides composed of long-chain fatty acids, whereas esterases preferentially hydrolyze simple esters Citation[5]. Since C. rugosa has a non-universal genetic code Citation[6], difficulties have arisen in obtaining recombinant CRL to produce the desired isoenzyme. Consequently, many research activities have been diverted into modulating lipolytic enzyme activities towards desired enzyme through changing culture and fermentation conditions or operation mode of bioreactors. As Dominguez et al. Citation[2] reviewed, carbon source (inducer), kind of operation (batch/fed-batch), and feed rate are important parameters in C. rugosa fermentation in terms of quantity and quality of the crude lipase. The literature has a consensus that lipidic substances such as fatty acids and their esters stimulate the production of CRL Citation[2], and addresses the importance of oleic acid as an inducer Citation[4], Citation7–9. Despite comprehensive studies on CRL production, the effects of inoculum features along with media formulation on the productions of lipase and esterase enzymes in the fermentation of C. rugosa are not discussed in the literature.
The aim of the present work is to represent a novel parameter, inoculation strategy, which affects the lipolytic and esterasic activities, productivities, and yields achieved in C. rugosa cultivation. In one-step inoculation strategy, the cells were inoculated directly into the enzyme production medium. In two-step inoculation strategy, the cells were transferred into the enzyme production medium after being inoculated into the pre-culture medium whose composition was different from the enzyme production medium. For both strategies, time variations in extracellular and intracellular lipase and esterase activities as well as biomass were followed in the presence of triolein and oleic acid—a triglyceride and its fatty acid—separately as carbon sources. The overall results indicated that, regardless of the carbon source used, inoculation strategy was a crucial parameter that affected the activity, productivity, and yield of C. rugosa enzymes.
EXPERIMENTAL
Chemicals
The chemicals were of reagent grade and purchased from commercial suppliers (Merck, Germany; Sigma-Aldrich, USA).
Microorganism, Culture Media, and Conditions
C. rugosa DSMZ 2031 purchased from Deutsche Sammlung von Mikrooganismen and Zellkulturen GmbH (DSM, Braunschweig, Germany) was used in the study. The stock cultures were maintained on universal yeast medium (UYM). The microorganisms incubated at 30°C for 22 h on UYM were inoculated into either pre-culture medium or enzyme production medium. The composition of the pre-culture medium was as follows (per liter): 5 g peptone (from soybean), 3 g yeast extract, 3 g malt extract, 10 g dextrose. The enzyme production medium was prepared by adding carbon sources of different concentrations into basal medium of the following composition (per liter): 15 g KH2PO4, 5.5 g K2HPO4, 1 g MgSO4.7H2O, 10 mg FeCl3.6H2O, 4 g urea, and 10 ml vitamin solution (pH = 6.2). The vitamin solution contained per liter 400 mg of thiamine, 2 mg of biotin, and 2 g of inositol. Enzyme production was carried out in shake flasks at 30°C and 150 rpm, and the changes in extracellular and intracellular activities of lipase and esterase enzymes as well as biomass with time were followed. Extracellular and intracellular protease activities were also measured at the end of cultivations.
Enzyme Assays
Alkalimetric final titration was used to determine the lipase enzyme activity by adapting the method by Cernia et al. Citation[10]. The mixture, containing 2.5 ml phosphate buffer solution (0.1 M pH 7.2), 0.5 ml olive oil, and 0.1 ml sample, was incubated at 37°C under magnetic stirring for 30 min. After terminating the reaction with 2.5 ml acetone:ethanol mixture 1:1 (v/v), the solution was titrated with 0.1 M NaOH in the presence of phenolphthalein as indicator. One unit lipase activity (U) was defined as the amount of enzyme that catalyzes the release of fatty acid per min under the conditions mentioned above.
The esterase enzyme activity was measured spectrophotometrically (Shimadzu 1601A, Tokyo, Japan) by using p-nitrophenyl acetate as substrate Citation[11]. One unit esterase activity (U) was defined as the amount of enzyme produces 1 µmol p-nitrophenol per min at pH 7.5 and 25°C.
For intracellular enzyme activity assay, cells were disrupted by adapting the procedure reported by Dalmau et al. Citation[12]. After harvesting by centrifugation at 12000×g for 10 min at 4oC, cells were washed in Tris-HCl buffer (10 mM pH = 8.0) and resuspended to a 3 ml final volume with the same buffer. The cell suspension was disrupted with glass beads (Biospec Mini-Beadbeater, U.S.A.) for 8 periods of 30 s. The disrupted cells were centrifuged at 12000×g at 4°C for 10 min and the supernatant was used as the cell extract for the determination of intracellular activity. The cell viability was inspected with a microscope (Olympus CX21FS1, USA) after staining the cells with methylene blue.
Protease activity was assayed spectrophotometrically using casein as substrate Citation[13]. One unit of enzyme activity is defined as 4 nmol tyrosine released/min per ml.
All experiments and biochemical assays were carried out in duplicate. The range of duplicate values was within 5%.
Biomass
Biomass was determined by dry weight as follows Citation[12]: samples were filtered (0.45 µm, Millipore, USA) and washed with a mixture of dioxane-propionic acid (1:1) and distilled water in sequence. The filters were then dried at 85 °C to constant weight.
RESULTS
Enzyme Production by One-Step Inoculation in the Presence of Triolein
C. rugosa cells from stock cultures were transferred into the enzyme production medium without using any pre-culture medium. Triolein at the initial concentrations of 2, 3 and 5 g/l was used as carbon source of the production medium. The variations in biomass, extracellular enzyme activities, and intracellular enzyme activities with time are given in , respectively.
Figure 1. Variation in biomass with time in the cultivation of C. rugosa (one-step inoculation; carbon source: triolein; nitrogen source: 4 g/l urea; T = 30°C; N = 150 rpm).
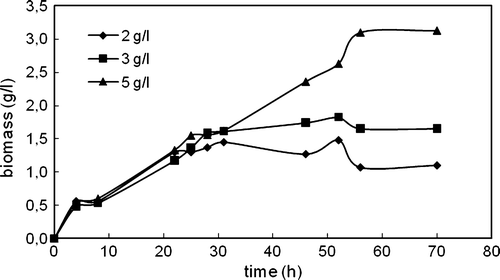
Figure 2. Variations in extracellular lipase and esterase activities with time in the cultivation of C. rugosa (one-step inoculation; carbon source: triolein; nitrogen source: 4 g/l urea; T = 30°C; N = 150 rpm).
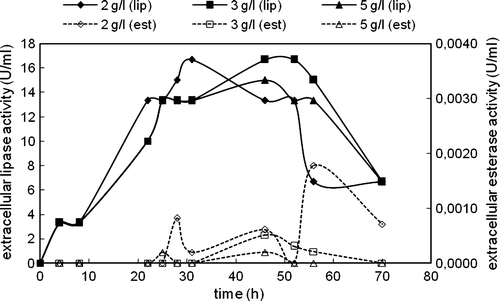
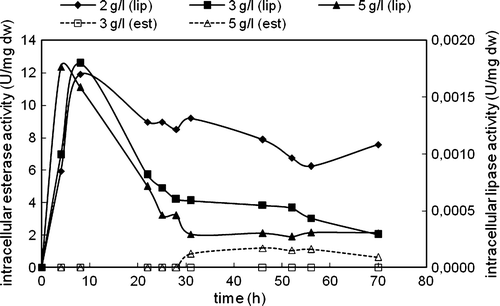
Figure 3. Variations in intracellular lipase and esterase activities with time in the cultivation of C. rugosa (one-step inoculation; carbon source: triolein; nitrogen source: 4 g/l urea; T = 30°C; N = 150 rpm).
The cells grew almost with the same low rate in the presence of 2, 3, and 5 g/l triolein for 30 h, and then the production of biomass continued only in the presence of 5 g/l triolein (). Stationary phases of the cells were reached after about 30 h of cultivation for 2 and 3 g/l triolein concentrations; however, after about 55 h for 5 g/l triolein concentration. The highest biomass obtained in the presence of 5 g/l triolein was 3.13 g/l.
The cells secreted lipase enzyme starting from the beginning of the cultivation almost with the same rate for three triolein concentrations (). The same maximum extracellular lipase activity (16.67 U/ml) was obtained in the presence of 2 and 3 g/l triolein; however, 5 g/l triolein yielded lower extracellular lipase. The cells secreted esterase enzyme after lipase activity started decreasing; therefore, esterase activity could only be detected in the media after about 25 h of cultivation time. Two g/l triolein favored the highest extracellular esterase activity (0.0018 U/ml).
On the other hand, intracellular lipase activity was determined in the beginning of the cultivation for all triolein concentrations; however, after a very short time it started to decrease and after 30 h of cultivation intracellular lipase activities almost held constant (). The highest intracellular lipase activity was almost the same at all triolein concentrations studied. After 30 h, intracellular esterase activities were also determined in the media. The highest intracellular esterase enzyme was determined in the presence of 3 g/l triolein, and no intracellular esterase enzyme was observed in the presence of 2 g/l triolein.
Protease activities were also measured at the end of cultivations to see to what extent the proteolytic activity affected lipolytic enzyme activities (). The highest extracellular proteolytic activity was obtained in the presence of 2 g/l triolein. This result showed that the reason for earlier decrease in the extracellular lipase activity observed at 2 g/l triolein than other concentrations might be the relatively high protease activity measured at this concentration.
Table 1. Protease activities for one-step and two-step inoculation strategies at the end of cultivations of C. rugosa in the presence of triolein and oleic acid (T = 30°C, N = 150 rpm, nitrogen source: 4 g/l urea)
Enzyme Production by Two-Step Inoculation in the Presence of Triolein
C. rugosa cells from stock cultures were transferred into the pre-culture medium for 22 h incubation before being transferred into the enzyme production medium. Triolein at the initial concentrations of 2, 3, and 5 g/l was used as carbon source of the enzyme production medium. The variations in biomass, extracellular enzyme activities, and intracellular enzyme activities are given in , respectively. Since a considerable level of lipase activity was determined in the pre-culture medium, the time expired in this medium was also taken into account while representing the total cultivation time.
Figure 4. Variation in biomass with time in the cultivation of C. rugosa (two-step inoculation; carbon source: triolein; T = 30°C; N = 150 rpm).
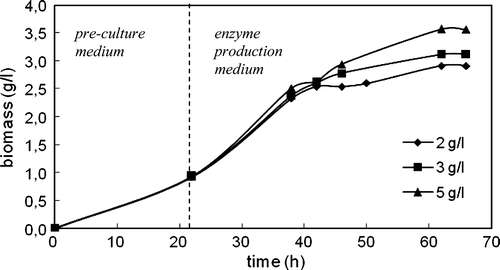
Figure 5. Variations in extracellular lipase and esterase activities with time in the cultivation of C. rugosa (two-step inoculation; carbon source: triolein; T = 30°C; N = 150 rpm).
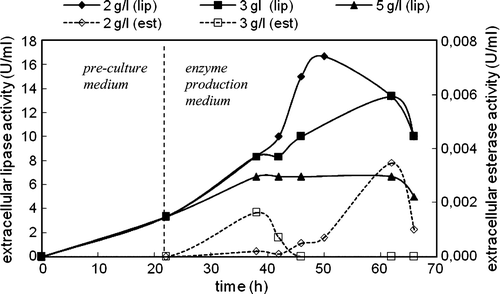
Figure 6. Variations in intracellular lipase and esterase activities with time in the cultivation of C. rugosa (two-step inoculation; carbon source: triolein; T = 30°C; N = 150 rpm).
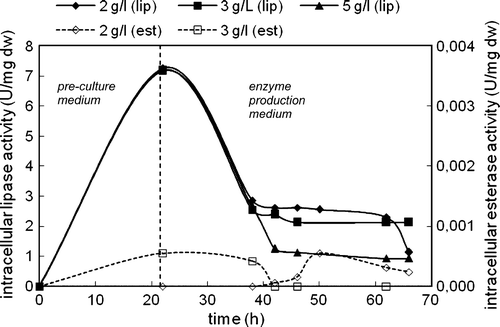
The concentration of cells increased with time in both media and after about 40 h of total cultivation the cells entered into stationary phase for all triolein concentrations. The highest biomass obtained at 5 g/l initial triolein concentration was 3.57 g/l ().
Lipase secretion started in the pre-culture medium (). In the enzyme production medium, the extracellular lipase activity increased and after reaching a maximum level a decrease was seen for all triolein concentrations. The highest extracellular lipase activity was achieved in the presence of 2 g/l triolein as 16.67 U/ml. On the other hand, extracellular esterase activity was observed only in the enzyme production medium (). The highest extracellular esterase activity was achieved in the presence of 2 g/l triolein as 0.0035 U/ml. Considerable decreases were observed in extracellular esterase activities at high cultivation times. Extracellular lipase and esterase activities decreased with increasing triolein concentration, and no esterase activity was seen at 5 g/l triolein.
By contrast with extracellular activity, intracellular lipase activity decreased as soon as the cells transferred into the enzyme production medium (). After about 40 h of total cultivation where the cells entered into stationary phase and extracellular lipase activities reached their maxima, intracellular lipase activities remained at low levels. However, at the cultivation time where the cells slowed down to produce intracellular lipase enzyme, intracellular esterase activity started to increase (). At high cultivation times, intracellular esterase activities decreased. The highest intracellular lipase and esterase activities were obtained in the presence of 2 g/l triolein. No intracellular esterase activity was detected in the presence of 5 g/l triolein.
Extracellular protease activity was detected in all of the media except for that including 2 g/l triolein. Higher proteolytic activity was obtained at higher triolein concentration ().
Comparison of Inoculation Strategies in the Presence of Triolein
The results indicated that extracellular lipase activity was growth-associated, and the cells produced and secreted lipase before esterase enzyme was produced. The cells secreted esterase into the cultivation medium after a considerable level of extracellular lipase activity was reached. Considerable levels of protease activities were also detected in the last period of cultivation for both inoculation strategies.
High concentration of triolein favored cell growth, and almost the same amount of biomass was achieved in both strategies. However, the increase in the initial triolein concentration decreased the enzyme production. The same level of extracellular lipase activity was obtained in both strategies, although the time to reach the highest activity was lower in one-step inoculation strategy. Therefore, lipase productivity and specific productivity were higher when one-step inoculation strategy was employed (). On the other hand, two-step inoculation strategy provided higher extracellular esterase activity and productivity. The comparison of the highest yields with respect to initial substrate concentration indicated that the yield of lipase was the same in both strategies or was higher in one-step inoculation strategy. However, higher yields of esterase and biomass were achieved when two-step inoculation strategy was employed.
Table 2. Comparison of maximum extracellular lipase and esterase productivities and yields of C. rugosa in the presence of triolein and oleic acid by using two different inoculation strategies (T = 30°C, N = 150 rpm, nitrogen source: 4 g/l urea, Y: yield, L: lipase activity (U/ml), E: esterase activity (U/ml), So: initial substrate concentration (mg/ml), X: biomass concentration (mg/ml))
Enzyme Production by One-Step Inoculation in the Presence of Oleic Acid
C. rugosa cells were directly transferred from stock cultures into the enzyme production medium containing 3 g/l oleic acid without using any pre-culture medium. The reason for using 3 g/l concentration of oleic acid was the achievement of higher lipase activity at this concentration than 0.5 and 1.5 g/l values in the preliminary experiments performed (data not shown). The variations in biomass, extracellular, and intracellular enzyme activities with cultivation time are given in .
Figure 7. Variations in biomass, extracellular and intracellular enzyme activities with time in the cultivation of C. rugosa (one-step inoculation; carbon source: 3 g/l oleic acid; T = 30°C; N = 150 rpm).
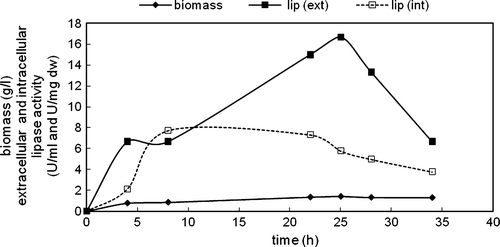
The growth rate of cells was low and the highest biomass was obtained as 1.43 g/l at 25 h of cultivation. The highest extracellular lipase activity was obtained also at 25 h of cultivation as 16.67 U/ml. Lipase activity further slowed down. Intracellular lipase activity increased up to about 10 h of cultivation and then a decrease was observed. Protease activity, which was followed with time in this experimental set-up, could be detected in the fermentation medium only after 25 h of cultivation (). These data indicated that protease enzyme might have an effect on the decrease of extracellular lipolytic activity observed at high cultivation times. No extracellular and intracellular esterase activities were detected in the presence of 3 g/l oleic acid.
Enzyme Production by Two-Step Inoculation in the Presence of Oleic Acid
C. rugosa cells from stock cultures were transferred into the pre-culture medium for 22 h incubation before being transferred into the enzyme production medium containing 3 g/l oleic acid. The variations in biomass, extracellular, and intracellular enzyme activities with time are given in .
Figure 8. Variations in biomass, extracellular and intracellular enzyme concentrations with time in the cultivation of C. rugosa (two-step inoculation; carbon source: 3 g/l oleic acid; T = 30°C; N = 150 rpm).
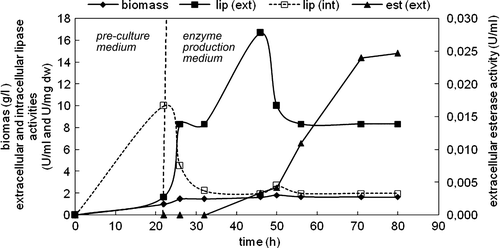
Considerable levels of biomass as well as extracellular and intracellular lipase activities were measured at the end of the pre-culture period, indicating that the pre-culture medium stimulated not only biomass production but also the lipolytic activity. However, extracellular esterase activity could only be detected in the enzyme production medium after extracellular lipase activity started to decrease. The highest biomass was obtained as 1.83 g/l, and the cells yielded the highest extracellular lipase and esterase activities as 16.67 U/ml and 0.0247 U/ml, respectively. The highest intracellular lipase activity was obtained at the end of the pre-culture period. No intracellular esterase activity was detected both in pre-culture and enzyme production media. However, extracellular and intracellular protease activities were detected at the end of cultivation ().
Comparison of Inoculation Strategies in the Presence of Oleic Acid
The results showed that the time course of C. rugosa enzymes in the presence of oleic acid was similar to that obtained in the presence of triolein. One-step and two-step inoculation strategies were compared in in terms of extracellular maximum productivity, specific productivity, and yield of C. rugosa enzymes. Although the same activity and yield of lipase were obtained in both strategies, higher productivity and specific productivity of lipase were achieved by one-step inoculation. Conversely, higher biomass and yield of biomass were obtained by two-step inoculation strategy. However, esterase enzyme was only produced when the two-step inoculation strategy was employed. Considerable levels of protease activities were also detected for both inoculation strategies.
DISCUSSIONS AND CONCLUSION
The present work provides outputs to discuss not only the inoculation strategy in the cultivation of C. rugosa, but also the time courses of the extracellular and intracellular lipolytic and esterasic enzyme activities in the presence of two different carbon sources. Although the role of inoculum features such as quality, volume, etc., in the successful execution of a fermentation process are considered to be important, none of the studies on the C. rugosa fermentation refers to the changes in the enzyme activity and productivity, i.e. in the cultivation progress, with inoculum properties.
In most fermentations for CRL production, the inocula were grown in shake flasks that contained the same media as that in the final fermentation Citation[4], Citation[7], Citation14–16. In the rest of the studies, the cells that were previously cultured in agar plates, were suspended in 0.9% NaCl solution and then added into the flasks containing the fermentation medium Citation[12], Citation[17]. Only Sokolovska et al. Citation[18] reported that C. cylindracea inoculum was prepared by inoculation of the cells, first in glucose-rich medium and then in the medium containing olive oil. However, no reports appeared in the literature comparing the progress of C. rugosa fermentations carried out in sequential pre-culture and enzyme production media of different compositions with that carried out using only enzyme production medium. In the present work, we found that the utilization of different compositions for pre-culture and cultivation media affected the activity and productivity of C. rugosa enzymes. Regardless of the carbon source of the enzyme production medium, two-step inoculation strategy was found to have an advantage for esterase enzyme activity, productivity, and yield in C. rugosa cultivation. However, no considerable difference in the production of lipase was observed when two different inoculation strategies were compared. This result is mainly attributed to the effect of glucose content of the pre-culture medium, which probably stimulated esterase production rather than lipase enzyme. To see the glucose effect on the esterase activity, we accomplished two supplementary experiments employing one-step inoculation strategy. First, we started the cultivation with glucose (2 g/l) only and the second with glucose (2 g/l) and oleic acid (0.5 g/l) together as carbon sources of the enzyme production medium. We found that C. rugosa cells yielded higher extracellular esterase activity (0.0097 U/ml) in the presence of glucose than in the presence of a mixture of glucose and oleic acid (0.0027 U/ml). The evaluation of these data with the extracellular lipase activities obtained in the presence of glucose (10 U/ml) and of a mixture of glucose and oleic acid (11.67 U/ml) led us to conclude that the presence of glucose in the early periods of the cultivation had a greater effect on esterase activity than lipase. There is no literature report on the influence of inoculation strategy in C. rugosa cultivation to compare with the results of the present work. Furthermore, although the inhibitory or repressing effect of glucose on the lipase activity was suggested by several authors Citation[12], Citation[15], Citation[19], the effect of glucose on the esterase activity was not discussed. Dalmau et al. Citation[12] used glucose in batch cultivation of C. rugosa; however, they did not determine the esterase activity in the medium.
It has been shown that there are different genes in C. rugosa, whose expression can be modulated by culture conditions Citation[20]. In the present work, the synthesis and secretion of C. rugosa enzymes were also found to be regulated by the nature and structure of the substrates in the culture media. The effect of inoculation strategy on the enzyme production by C. rugosa was investigated by using triolein and oleic acid individually to be able to find whether a triglyceride or its fatty acid predominated over the other in producing lipase or esterase. The same concentrations of oleic acid and triolein yielded similar extracellular activity and yield of C. rugosa lipase; however, oleic acid provided higher lipase productivity and specific productivity than triolein. On the other hand, when one-step inoculation strategy was employed, no extracellular esterase activity was detected in the presence of oleic acid. This result indicated that the presence of triolein ensured esterase production in C. rugosa more than oleic acid. Conversely, when the two-step inoculation strategy was employed, more esterase activity, yield, and productivity were obtained in the presence of oleic acid than triolein due to the glucose content of the pre-culture medium.
The low solubility of long-chain fatty acids and their esters in water usually creates difficulties in mass transfer from bulk to the cell surface in microbial productions. In the present study, the reason for the decrease seen in enzyme activities at high triolein concentrations can be attributed to the lower solubility of higher concentrations of triolein in the culture medium. However, the increase in biomass with increasing triolein concentration led us additionally to consider this situation as a substrate inhibition on the enzyme production, which needs further investigations.
The employment of triolein and oleic acid as carbon sources in batch cultivation of C. rugosa was also compared by Dalmau et al. Citation[12]. The authors reported that almost the same biomass and extracellular lipase activities were obtained with both substrates whereas higher extracellular esterase activity was detected with oleic acid. Higher cell-bound lipase activity than intracellular one was achieved with triolein by Dalmau et al. Citation[12]. On the contrary, Wei et al. Citation[17] reported that triolein significantly benefited lipase production, giving an extracellular lipase activity 65.3% higher than that with oleic acid. The results of the present work are consistent with those reported by Dalmau et al. Citation[12] in terms of biomass and extracellular lipase activity; however, we observed almost the same intracellular lipase activity with triolein and oleic acid.
When the time courses of the C. rugosa enzymes are compared with those available in the literature, a similarity is found between the report of Gordillo et al. Citation[14] and this paper. Gordillo et al. Citation[14] also reported that, in the presence of oleic acid, extracellular lipase production was growth-associated, and the maximum of intracellular lipase activity was observed in the middle-exponential phase. The authors also showed that intracellular lipase activity remained constant at the end of the batch. In the present work, we additionally demonstrated that C. rugosa cells produced lipase and esterase enzymes in a sequential pattern.
Proteolytic activities were measured at the end of cultivations to see if protease was responsible for the downfall seen in lipase and esterase enzyme activities. Although protease activity was detected in the presence of triolein and oleic acid regardless of the inoculation strategy employed, no correlation was found between the proteolytic activity and the substrate type or concentration. Puthli et al. Citation[16] also observed that protease enzyme appeared in the fermentation broth at the end of the exponential growth phase of the biomass and then its concentration gradually increased.
In conclusion, it has been demonstrated that the inoculation strategy is a crucial parameter that canalizes C. rugosa cells to produce either lipase or esterase isoform. Glucose is suggested to be responsible from the changes in esterase enzyme synthesis and secretion with the inoculation strategy employed. The overall results also indicate that the proper formulation of the pre-culture and cultivation media allows a high production level of desired enzyme(s) in C. rugosa cultivation.
Acknowledgements
The authors gratefully acknowledge the financial support provided by the Scientific and Technical Research Council of Turkey (TUBITAK-MAG 105M052)
References
- Benjamin S., Pandey A. Candida rugosa lipases: molecular biology and versatility in biotechnology. Yeast 1998; 14: 1069–1087
- Dominguez de Maria P., Sanchez-Montero J.M., Sinisterra J.V., Alcantara A.R. Understanding Candida rugosa lipases: An overview. Biotechnology Advances 2006; 24: 180–196
- Diczfalusy M.A., Alexson S.H.E. Isolation and characterization of novel long-chain acyl-CoA thiosterase/carboxylesterase isoenzymes from Candida rugosa. Archives of Biochemistry and Biophysics 1996; 334: 104–112
- Sanchez A., Ferrer P., Serrano A., Pernas M., Valero F., Rua M.L., Casa C. Characterization of the lipase and esterase multiple forms in an enzyme preparation from a Candida rugosa pilot-plant scale fed-batch fermentation. Enzyme and Microbial Technology 1999; 25: 214–223
- Bornscheuer U.T. Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiology Reviews 2006; 26: 73–181
- Kawaguchi Y., Honda J., Taniguchi-Morimura K., Iwasaki S. The codon CUG is read as serine in an asporogenic yeast Candida cylindracea. Nature 1998; 341: 164–166
- Obradors N., Montesinos J.L., Lafuente F. J., Sola C. Effects of different fatty acids in lipase production by Candida rugosa. Biotechnology Letters 1993; 15(4)357–360
- Alcantara A.R., Dominguez de Maria P., Fernandez M., Hernaiz M.J., Sanchez-Montero J.M., Sinisterra J.V. Resolution of racemic acids, esters and amines by Candida rugosa lipase in slightly hydrated organic media. Food Technology and Biotechnology 2004; 42(4)343–354
- Lopez N., Pernas M.A., Pastrana L.M., Sanchez A., Valero F., Rua M.L. Reactivity of pure Candida rugosa lipase isoenzymes (Lip1, Lip2, and Lip3) in aqueous and organic media. Influence of the isoenzymatic profile on the lipase performance in organic media. Biotechnology Progress 2004; 20: 65–73
- Cernia E., Deflini M., Cocco E.D., Palloci C., Soro S. Investigation of lipase-catalysed hydrolysis of naproxen methyl ester: use NMR spectrescopy methods to study substrate-enzyme interaction. Bioorganic Chemistry 2002; 30: 276–284
- Chamorro S., Sanchez-Montero J.M., Alcantara A.R., Sinisterra J.V. Treatment of Candida rugosa lipase with short-chain polar organic solvents enhances its hydrolytic and synthetic activities. Biotechnology Letters 1998; 20: 499–505
- Dalmau E., Montesinosi M., Lotti M., Casas C. Effect of different carbon sources on lipase production by Candida rugosa. Enzyme and Microbial Technology 2000; 26: 657–663
- Takaç S., Elmas S., Çalik P., Özdamar H.T. Separation of the protease enzymes of Bacillus licheniformis from fermentation medium by crossflow ultrafiltration. Journal of Chemical Technology and Biotechnology 2000; 75(6)491–499
- Gordillo M.A., Montesinos J.L., Casa C., Valero F., Lafuente J., Sola C. Improving lipase production from Candida rugosa by a biochemical engineering approach. Chemistry and Physic of Lipids 1998; 93: 131–142
- Montesinos J.L., Dalmau E., Casa C. Lipase production in continuous culture of Candida rugosa. Journal of Chemical Technology and Biotechnology 2003; 78: 753–761
- Puthli M.S., Rathod V.K., Pandit A.B. Optimization of lipase production in a triple impeller bioreactor. Biochemical Engineering Journal 2006; 27: 287–294
- Wei D., Zhangi L., Songi Q. Studies on a novel carbon source and cosolvent for lipase production by Candida rugosa. Journal of Industrial Microbiology Biotechnology 2004; 31: 133–136
- Sokolovska I., Albasi C., Riba J.P., Bales V. Production of extracellular lipase by Candida cylindracea CBS 6330. Bioprocess Engineering 1998; 19: 179–186
- Lotti M., Monticelli S., Montesinos J.L., Brocca S., Valero F., Lafuente J. Physiological control on the expression and secretion of Candida rugosa lipase. Chemistry and Physic of Lipids 1998; 93: 143–148
- Lotti M., Alberghina L. Engineering of/with Lipases, F.X. Malcata. Kluwer Academic Publishers, DordrechtThe Netherlands 1996; 115–124