Abstract
Lethal dose experiments in animals have demonstrated that second-generation perfluorocarbon oxygen carriers are remarkably non-toxic. However, this non-toxicity has not previously been demonstrated in a liver failure scenario. A surgical liver damage and regeneration model in rats was selected using a well-controlled cross tabulated study design. A large number of physiological, biochemical, and hematological parameters were measured. No indications were found that intravenously injected perfluorooctyl bromide emulsion was toxic at the concentrations employed, in either healthy or severe liver injury scenarios. Neither was there any significant impact on the rate of liver regeneration following the injuries. Bearing in mind prior human clinical studies, it is therefore safe to assume that perfluorocarbon emulsions are also non-toxic in bioartificial liver treatments.
INTRODUCTION
Perfluorocarbon (PFC) polymers have the properties of exceptional chemical and biological inertness. PFCs also have very high dissolving capacities for oxygen (O2) and carbon dioxide (CO2), making them attractive candidates as artificial O2 carriers in erythrocyte replacement applications including, for example, plasma perfused bioartificial livers Citation1–3. The intravascular (IV) administration of a PFC requires the development of a heat sterilizable, sub-micron droplet-size emulsion that is stable in non-frozen conditions for at least two years Citation[4], Citation[5].
In mammals the in vivo distribution and elimination of PFC is characterized by its half lives in the circulation and in the reticulo-endothelial system (RES). In the first 24 hours, the PFC is cleared from the circulation by the mononuclear phagocyte system accumulating in the liver, spleen, and bone marrow. In the second phase, lasting days to weeks, it is cleared from the RES via lipid compartments in the blood into the lungs. Thus, PFC is not metabolized; it is excreted from the respiratory system into the air. During this phase, flu-like symptoms with myalgia and light fever have been reported in clinical studies Citation[5]. Factors affecting the clearance half lives of PFC are emulsion droplet size, molecular weight, surfactant-type, complement activation, animal species and, in humans, racial differences Citation6–8.
In second generation PFC emulsions the toxicity problems associated with earlier attempts Citation[9] have been overcome. Oxygent (Alliance Pharmaceuticals, San Diego, USA), a PFC composed of (predominantly) perfluorooctyl bromide (PFOB) (C8F17Br) emulsified in egg yolk phospholipid (lecithin) as a surfactant, has successfully progressed through both stage II and III clinical trials. In the above trials Oxygent was not found to significantly initiate either immunological or coagulative reactions in healthy volunteers. Furthermore, no subsequent perturbation of normal blood hemostatic or viscosity behavior could be found (in fact, viscosity improved), there was no reduction in clot formation or strength, and no increase in red cell hemolysis Citation[10], Citation[11].
Lethal dose experiments in animals have shown that the value for PFOB is 41g/kg, which is remarkably non-toxic Citation12–16. However, the non-toxicity of PFCs has not previously been demonstrated in a liver failure scenario. The clinical progress of acute liver failure (ALF) involves the development of a hyperdynamic circulation, a disseminated intravascular coagulopathy (DIC), renal, and eventually, multi-organ failure Citation[17]. Since the IV administration of a toxin may produce a similar profile to the above, experiments such as these must discriminate between the two potential clinical syndromes. In this study a highly reproducible model of reversible liver failure in the form of a ¾ partial liver resection in rats was selected. This model emulates a seriously compromised liver, with failure in the beginning followed by progressive regeneration. Several previous studies have established that 100% of such animals will recover Citation18–23. Thus, the purpose of these experiments was, by using an animal model, to investigate the effects of IV administered PFOB on the recovery of a liver failure patient. They also served to preclude institutional manufacturing differences as the formulation of the UP-CSIR PFOB is similar to that of Oxygent.
METHODS
Animals
The experiments were conducted on 56 healthy female Sprague-Dawley rats of approximately 200g each. These were housed under temperature-controlled conditions in Macrolon type 3 cages with a 12 hour light-dark cycle, with sterilized wood shavings for bedding, access to standard rat food pellets and water with 10% glucose at the University of Pretoria biomedical research center.
Experimental Design ()
The experimental groups were composed of 12 sub-groups in a cross-tabulated design aimed at discriminating between the effects of the surgery and the test substance (PFC). Half the animals received surgery (LI: liver injury) and the other half did not. In turn, half of each of the above received IV injections of either the test substance (PFC) in high (3 ml, 5 g/kg) or low doses (1 ml, 2 g/kg), or saline controls. Acute and sub-acute toxicity effects were investigated by terminating experiments at either short (2-day) or longer (4-day) durations.
Table 1. The experimental sub-groups
Perfluorocarbon Composition and Dosing
Perfluorooctylbromide-lecithin emulsions were prepared according to the method of Moolman et al., as previously described by our group Citation1–3. The emulsions had a Sauter mean droplet size of 0.2 µm for optimal oxygen mass transfer characteristics, and were prepared at 40% v/v with deionized water. For every 100g of 40% PFC the contents were as follows: water, 42.70 g, NaCl, 0.32 g, NaHCO3, 0.10 g, Perfluoro-octyl bromide (Interchim SA 0, 53.24 g, egg-yolk lecithin, 3.30 g (Lipoid E80S, Lipoid AG) and Vitamin E as antioxidant 0.10 g. Following steam autoclaving at 121oC for 20 minutes, the emulsions were allowed to cool to room temperature. Sterile, deionized, autoclaved water was used to make up the PFOB-lecithin emulsion to 20% v/v concentration. pH was adjusted to 7.35 prior to drawing up the IV injections.
PFC doses were provided as either “high” or “low.” The high dose (5 g/kg) was 3 ml of 20% PFC in a 200 g rat, which was calculated to simulate exposure to the IV entry of one liter of 20% PFC into an adult human. Hypervolumia was prevented in the animals by prior blood sampling of an equivalent volume. The low dose (2 g/kg) was chosen as 1 ml, i.e. 1/3 of the high dose. Saline dose controls (3 ml) were used as controls for the test substance. Surgical controls, i.e. surgeries without any additional treatments, were also performed. All doses were introduced through the tail vein.
Liver Injury (LI) Model
As per the protocol first described by Higgins and Andersen in 1931 Citation18–23, 3/4 liver resections were carried out (on day 0) on one half (N = 24) of the animals. Briefly, while the animals were under isoflurane anesthesia, a midline incision was made, followed by complete liberation of all liver ligaments to allow the ligation of the pedicles of the median and left lateral lobes, i.e. they were scissor clamped, tied off with suturing line, and resected. Thereafter, the midline was sutured shut and the animals were allowed to recover. Each procedure took approximately 10 minutes. Prior to the above, 8 rats were used for perfecting the surgical procedure and their organ weights and blood indices were included as healthy controls (baselines) relative to the experimental groups.
Anesthesia Protocol
Caprofen (Rimadyl®, 5 mg/kg BW SC) was injected pre-operatively, followed by isoflurane (Safe Line pharmaceuticals) inhalation using a Boyle's isofor inhalation machine with 100% O2. Buprenorhine (Temgesic®, 0.1 ml/100g BW IM) was given at the time of incision. To manage pain post-operatively, carprofen was given once daily with buprenorphine adjusted to 30% of normal liver weight every 12 hrs. On termination, all animals were euthanased through inhalation of a lethal overdose of isoflurane.
Recovery, Pain and Toxicity Scoring
The National Society for the Protection and Care of Animals (NSPCA) pain and toxicity scoring sheets were completed once daily to assess possible toxicity, pain, and humane end-points for the experiments.
Analyses
On days 0, 2, and 4, 1 ml blood samples were taken from the tail vein of all animals for blood biochemistry and haematology ( and ). Upon termination of the 2 and 4 day groups, body, liver, left kidney, lungs, and spleen weights were measured. The organs were first examined for macroscopic pathology, followed by preservation in formalin. For histology, 5 µm sections were cut from paraffin wax tissue blocks of the above organs using an automated tissue processor. Slides stained with haematoxylin and eosin (H and E) were then examined for cellular changes.
Table 2. Measured variables and units
Table 3. Weight changes, biochechemistry and hematology
Statistics
Microsoft Excel (ver. 2003) was used as a spreadsheet while Statistix (ver. 8, Tallahasee, FL, USA) was used for data analysis. The mean and standard deviations were calculated for all variables. Non-parametric Wilcoxon rank sum tests, appropriate for small groups, were used to determine the statistical significance of differences between groups. Since no difference could be detected between the high-dose and low-dose PFC groups in the raw data, these were included as one group in subsequent statistical comparisons. The following sub-groups were compared for each of the measured variables to discriminate between the effects of the surgery and the PFC:
The surgical versus non-surgical groups at 2 and 4 days (PFC + saline).
The 2 versus 4 day surgical groups (PFC + saline).
The PFC versus saline non-surgical groups.
The PFC versus baseline (no interventions) non-surgical groups.
The saline versus baseline non-surgical groups.
The 2 and 4 day surgical groups versus the baselines.
The 2 and 4 day non-surgical groups versus the baselines.
Only significant (p ≤ 0.05) or marginal (p > 0.05 ≤ 0.1) differences between groups are mentioned below.
RESULTS
provides an explanation of the measured variables and their units. provides the mean±standard deviation of the relevant organ and body weights, blood biochemistry, and hematological indicators.
General Observations
All animals in the control and experimental sub-groups survived for the duration of the trial. In the first two days all surgical animals demonstrated signs of trauma in the form of hunched postures, pilo-erection (ruffeled coats), red circles around their eyes, and gnawing at the wood-shaving bedding material (pain). These signs decreased from 2 to 4 days. In the non-surgical sub-groups (baselines, PFC or saline) this behavior was not present. One animal was lost from the trial due to a disembowlment following its gnawing off its abdominal sutures. This loss did not impact the results as the animal was of the surgical control group.
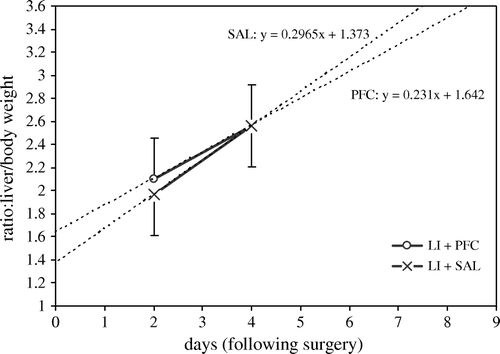
Body weight loss was found in all groups save the non-surgical saline injected sub-group. This was greatest in the surgical groups, but did not significantly differ in the PFC or saline, 2 or 4 day sub-groups. No increases in the spleen to body weight ratios were found in the PFC versus saline sub-groups. Although kidney and lung weights were measured, no differences between the sub-groups and the baseline animals were detectable, and this data was consequently excluded from . A significant increase in the liver to body weight ratios was found in all surgical versus non-surgical groups at 2 days (p ≤ 0.001) and at 4 days (p = 0.003). In the surgical PFC or salines at 2 days and 4 days there was no difference in the liver to body weight ratios. The rate of regeneration of the livers of the surgical PFC or saline sub-groups was also not different. Thus, the PFC did not impact liver re-generation following severe injury ().
Figure 1. Liver regeneration projections, assuming linear re-growth. The rate of regeneration after liver injury and IV dosing of PFC or saline is not significantly different. The y-intercept indicates the amount of liver initially resected. A liver/BW value of 1.5 equates to approximately a 60% liver weight resection. The projected time to complete liver weight regeneration, i.e. to a liver/BW ratio of 3.5, is 7-8 days. This duration is in agreement with prior experience with this surgical model Citation18–23.
Biochemistry
Blood albumin in the surgical versus non-surgical groups (PFC + saline) was significantly decreased at 2 days (p = 0.005) and 4 days (p = 0.003). The non-surgical PFC sub-groups had significantly lower levels than both the salines and the baselines (p = 0.001 and p < 0.001, respectively). Thus, both the surgeries and the PFC decreased albumin production by the liver.
The liver enzymes ALT and AST, reflecting liver damage, were significantly increased in all surgical versus non-surgical groups, at 2 days (ALT p = 0.001, AST p = 0.001) and at 4 days (ALT p = 0.001, AST < 0.001). At 4 days, levels were significantly higher in the surgical groups (PFC + saline) versus the baselines (ALT p = 0.001, AST p < 0.001). Therefore, the surgeries rather than the PFC caused liver damage.
Bilirubin, reflecting hepatic bile removal, was significantly increased in the 2 versus 4 day surgical groups (p = 0.001) and also increased in the (PFC + saline) surgical versus non-surgical sub-groups at 2 (p = 0.001) but not at 4 days. The (PFC + saline) 4 day surgical group was significantly increased relative to the baselines (p = 0.003), but not the non-surgical group. Thus, surgery immediately decreased bilirubin clearance, followed by a return to normal by day 4. PFC had no effect.
Urea, reflecting hepatic (nitrogenous-waste) metabolism, was significantly lower in the non-surgical PFC and saline groups versus the baselines (PFC p = 0.009 and SAL p = 0.024, respectively). It was also significantly lower in the (PFC + saline) surgical and non-surgical 4 day sub-groups versus the baselines (+LI p = 0.005 and −LI p = 0.008, respectively). Interestingly, urea was slightly higher in the 2 versus 4 day surgical groups. It appears that neither the surgeries nor the PFC had any effect on urea production.
Ammonia, reflecting blood nitrogenous toxin levels, was increased in the 2 day versus 4 day surgical groups (but not significantly). The (PFC + saline) surgical groups at 2 days were significantly increased relative to the non-surgicals (p = 0.002), but only marginally increased at 4 days (p = 0.064). The non-surgical PFC groups (2 + 4 days) were marginally lower than the salines (p = 0.068) and significantly lower than the baselines (p = 0.006). Thus, similar to bilirubin, surgery decreased ammonia clearance with recovery to normal by day 4. Of interest, the presence of the PFC was associated with decreased ammonia levels.
Hematology
Red cell count (and hematocrit) were significantly decreased in both the (PFC + saline) 2 and 4 day surgical versus non-surgical groups (for RCC p = 0.005 and p = 0.004 respectively). The 4 day surgical group was significantly lower than the baselines (p < 0.001), but not so in the non-surgical group. The non-surgical saline group was marginally lower than the baselines (p = 0.055). RCC and Hkt were therefore decreased by the surgeries rather than the PFC.
White cell counts were increased in surgical versus non-surgical groups, significantly so at 2 days (p = 0.011) but not at 4 days. The baselines were significantly increased relative to the non-surgical PFC groups (p = 0.005) but not the salines. Ab-Neutr was significantly increased in the surgical versus non-surgical groups at both 2 days (p < 0.001) and 4 days (p < 0.001). In the 2 day group this was significantly larger than in the 4 day surgical group (p = 0.031) and at 4 days the surgical groups had significantly larger values than the baselines (p < 0.001). In Plt-C the surgical 2 day groups had significantly higher counts than 4 day groups (p = 0.004). The surgery therefore substantially increased the WCC (especially the neutrophils) while the PFC had no apparent effect.
Macroscopic Observations and Histology
White droplets were macroscopically noted in the spleens and to a lesser extent in the kidneys and livers in the PFC-injected 2-day animals, both surgical and non-surgical. In the 2-day surgical groups (PFC + saline) the liver remnants were blanched and tough relative to healthy livers. In the 4-day surgical groups the livers had grown back to approximately ¾ their original size and were more similar in color and texture to the (healthy) livers of the baselines than the 2-day group. In the 4-day PFC injected groups, no white droplets could be discerned in any of the organs.
H and E histology of the livers revealed vacuolar swelling with cytoplasmic droplets and an increase in mitosis and apoptosis that correlated with the surgeries. This was more severe in the 2 versus 4 day groups. Vacuolated Kupffer cells, associated with the PFC, were especially detected in the 2-day high-dose animals in the non-surgical groups. Low-dose animals did not demonstrate this. Micro-granulomas were noted in the Kupffer cells in the 4-day PFC-injected animals. In the spleens of the PFC injected animals vacuolated reticulo-endothelial cells in the blood sinuses of the red pulp were visible. Kidney sections demonstrated no specific findings. In the lungs, atelectasis, presumably associated with anesthetic euthanization, was found in the majority of the animals. Leucocyte aggregations and alveolar macrophage hypertrophy were observed in several of the PFC-injected surgical and non-surgical animals in the 2 day and 4 day groups.
DISCUSSION
The surgical method employed in this study was selected to model the potentially reversible acute liver failure syndrome seen in human patients. However, animal models make extension to the human clinical scenario difficult due to, amongst other reasons, species differences in response to test substances, the degree of reversibility, the disease process duration, and the degree of involvement of other organ systems Citation[24], Citation[25]. The ¾-partial liver resection in rats is an attractive model in that it is well-described, technically feasible, highly reproducible, non-toxic yet severe, but reversible within a time period sufficient to enable study. Although species differences must obviously exist, extension to the human scenario is reasonable in view of prior findings of PFOB non-toxicity in clinical studies Citation[6], Citation[10], Citation[11].
The lack of PFOB toxicity was evident in the absence of differences between the control and experimental sub-groups for the parameters studied. Specifically, no changes in the hematological indices as markers of systemic toxicity were apparent in the PFC versus saline injected groups. Similarly, the biochemical indices including bilirubin clearance, urea production, and liver enzymes levels were also not impacted by the PFC. Bearing in mind the findings, it is therefore safe to assume that this study was successful in meeting its aims. That is, PFC non-toxicity may be extended to include the liver failure case. This was possible owing to the non-toxic surgical model not complicating the effects of the test substance, the cross-tabular design, the measurement of a large number of variables, and the given ability to investigate the impact of the PFOB on the rate of liver regeneration following the injury.
The finding that PFOB did not impact the rate of liver regeneration following damage is of particular interest in view of the severity of liver failure in patients undergoing bioartificial liver treatments. In compromised livers, metabolic hypoxia may be significant. We found that PFOB actually decreased blood ammonia levels, possibly owing to improvements in blood oxygenation facilitating liver toxin clearance. A potential benefit may therefore lie in ameliorating the deleterious effects of ammonia. Of additional physiological interest: The decrease in albumin production associated with the PFC may have been due to the presence of the phospholipid lecithin surfactant in the emulsion. This may have activated a negative feedback mechanism regulating blood albumin levels and thereby blood viscosity. As previously stated, prior clinical studies Citation[10], Citation[11] have demonstrated improvements in blood viscosity following PFOB injection. The second phase flu-like symptoms post-operatively found Citation[6] may also be correlated with the macrophage hypertrophic changes and leucocyte aggregations found in our 4-day lung histology specimens.
To conclude, this study did not provide any indication that IV--injected PFOB was toxic at the concentrations employed in either healthy or severe liver injury scenarios. PFOB also had no impact on the rate of liver regeneration following the surgically induced damage. Bearing in mind the results of prior human clinical studies it is reasonable to assume the safety of using a PFOB emulsion in bioartificial liver support system treatments.
References
- Moolman, F.S. 2004. Oxygen carriers for a novel bio-artificial liver support system. PHD thesis, University of Pretoria, http://upetd.up.ac.za #etd-09092004-162043.
- Moolman F.S., Rolfes H., van der Merwe S.W., Focke W.W. Optimization of Perfluorocarbon emulsion properties for enhancing oxygen mass transfer in a bio-artificial liver support system. Biochem Eng J 2004; 19: 237–250
- Nieuwoudt M., Moolman F.S., Van Wyk A.J., Kreft E., Olivier B., Laurens J.B., Stegman F., Vosloo J., Bond R., van der Merwe S.W. Hepatocyte function in a radial-flow bioreactor using a perfluorocarbon oxygen xarrier. J Artif Org 2005; 29(11)915–918
- Riess J.G. Perfluorocarbon-based oxygen delivery. Artif Cell Blood Substit. Biotechnol 2006; 34: 567–80
- Krafft M.P., Riess J.G. Perfluorocarbons: Life sciences and medical uses. J Polym Sci Part A: Polym Chem 2007; 45: 1185–98
- Spahn D.R., Kocian R. The place of artificial oxygen carriers in reducing allogenic blood transfusions and augmenting tissue oxygenation. Canadian Jnl Anaesthesia 2003; 50(6)41–7
- Kim H.W., Greenburg A.G. Toward 21st century blood component replacement therapeutics: Artificial O2 carriers. Artif Cells, Bl subs. Biotechnol 2006; 34: 537–50
- Kuznetsova I.N. Perfluorocarbon emulsions: Stability in vitro and in vivo. Pharm Chem Jnl 2003; 37(8)415–20
- Ingram D.A., Forman M.B., Murray J.J. Activation of complement by Fluosol attributable to the pluronic detergent micelle structure. J Cardiovasc Pharmacol 1993; 22: 456–61
- Noveck R.J., Shanon E.J., Leese P.T. Randomized safety studies of IV perflubron emulsion. II. Effects on immune function in healthy volunteers. Anesth Analg 2000; 91: 812–22
- Noveck R.J., Shanon E.J., Shor J.S. Randomized safety studies of IV perflubron emulsion. I. Effects on coagulation function in healthy volunteers. Anesth Analg 2000; 91: 804–11
- Burgan A.R., Herrick W.C., Long D.M. Acute and subacute toxicity of 100% PFOB emulsion. Biomat Art Cells Art Org 1988; 16(1-3)681–2
- Sedova L.A., Kochetygov N.I., Berkos M.V. Side reaction caused by the PFC emulsions in IV infusion to experimental animals. Art Cells. Blood Subs Immob Biotech 1998; 26(2)149–57
- Sloviter H.A., Yamada H., Ogoshi S. Some effects of IV administered dispersed fluorochemicals in animals. Federation Proceedings 1970; 29(5)1755–7
- Mattrey R.F., Hilpert P.L., Long C.D. Hemodynamic effects of IV lecithin-based PFC emulsions in dogs. Crit Care Med 1989; 17(7)652–6
- Peck W., Mattrey R.F., Slutsky R.A. Perfluorooctyl bromide: acute hemodynamic effects in pigs of IV administration compared with standard ionic contrast media. Investigative Radiology 1984; 2: 129–32
- Nieuwoudt M., Kunneke R., Smuts M., Becker J., Stegmann G.F., Van der Walt C., Neser J., Van der Merwe S. Standardization criteria for an ischemic surgical model of acute hepatic failure in pigs. Biomaterials 2006; 27(20)3836–45
- Higgins G.M., Anderson R.M. Experimental pathology of the liver. Arch Pathol 1931; 12: 186–202
- Emond J., Capron-Laudereau M., Meriggi F., Bernau J., Reynes M., Houssin D. Extent of hepatectomy in the rat. Eur Surg Res 1989; 21: 251–59
- Kubota T., Takabe K., Yang M., Sekido H., Endo I., Ichikawa Y. Minimum sizes for remnant and transplanted livers in rats. J Hep Bil Pancr Surg 1997; 4: 398–404
- Topaglu S., Izci E., Ozel H., Topaglu E., Avsar F., Saygun O. Effects of TVE application during 70% hepatectomy on regeration capacity of rats. J Surg Res 2005; 124: 139–45
- Ijichi H., Taketomi A., Yoshizumi T., Uchiyama H., Yonemura Y., Soejima Y. Hyperbaric oxygen induces endothelial growth factor and reduces liver injury in regenerating rat liver after partial hepatectomy. J Hepatol 2006; 45: 28–34
- Urakami H., Abe Y., Grisham M.B. Role of reactive metabolites of oxygen and nitrogen in partial liver transplantation. Clin Exp Pharmacol Physiol 2007; 34: 912–9
- Vd Kerkhove M.P., Hoekstra R., van Gulik T.M., Chamuleau R. Large animal models of fulminant hepatic failure in artificial and Bioartificial liver support research. Biomaterials 2004; 25: 1613–25
- Seleverstov O., Bader A. Evaluation of liver support systems for preclinical testing by animal trials. Artificial Organs 2006; 30(10)815–21