Abstract
To evaluate the changes of gel scaffold and spongy scaffold in fibroblast culture in vitro, two kinds of collagen dermis were constructed in this research. Type I collagenase and Dispase were used to isolate dermis fibroblast from neonate prepuce. The gel dermis was constructed by mixing the fibroblast and collagen swelling solution, and the collagen spongy dermis was also constructed. After histological and immunohistochemical staining, these two dermis’ properties were compared. Compared with gel dermis, the spongy dermis possessed more superior tension strength, but it decreased significantly during the culture. The transition temperature in DSC reduced also. The rupture length of gel dermis increased, on the contrary. Gel dermis contained more moisture than spongy dermis. It had been observed that the collagen gel was more suitable for fibroblast in maintaining its morphology than spongy scaffold, though with less cell number. The reason might be due to the special structure of collagen gel. Water in gel is sub-bound water, which is different from free water, and it is suitable for fibroblasts to secrete extracellular matrix. It could be concluded that the collagen gel dermis is better than collagen spongy dermis in the biological property.
INTRODUCTION
For extensive skin defect, the key is closing the surface of the wound. But the self- plerosis is insufficient in the extensive cutaneous deficiency. Although autologous skin graft has been successfully used for wound closure in patients with skin defects, this kind of treatment inflicts additional injuries, and there are not sufficient autografts in extensive cutaneous deficiency. The surgeons have used cadaver skins and animal skins, but many implantation cases implied that they can only serve as temporary wound dressings; they can not cover the wound permanently and induce skin regeneration Citation1–4.
With the development of biomaterials and tissue engineering, especially the cell culture technology and the find of neutral proteinase Dispase, some skin substitutes were developed for grafting by culturing cells with biomaterials in vitro. In those skin substitutes, cells isolated from skin were seeded into matrices. The cell growth microenvironment has been a severe problem.
The most common kinds of carriers for fibroblast were the gelatin-cultured carrier and 3D scaffold carrier. In the gelatin culture method, cells were mixed with gelatin, such as collagen and chitosan. In the 3D scaffold culture method, the scaffold was constructed to form a 3D space structure; cells were seeded into the scaffold.
Although there was some research completed about those two artificial skins, the changes of matrices in the skins during in vitro culture was not clear, which would affect the use of artificial skin in the clinic. Because the properties of the matrix were changed continuously during in vitro culture, a suitable using juncture could get a more curative effect.
In this study, fetal dermis fibroblast was isolated by Dispase and type I collagenase according to the method of Hansburgh and Middelkoop. Those fibroblasts were cultured with collagen swelling solution and collagen 3D matrix. The physical-chemical properties were compared Citation[5], Citation[10].
MATERIALS AND METHODS
Preparation of the Two Dermises
Isolation and culture of dermis fibroblasts
The skin samples were obtained from neonate prepuce. It was cut into 1×1cm2 in asepsis, and washed in the D—Hanks solution containing penicillin (100u/ml), streptomycin (100ug/ml), and amphotericin B (2.5ug/ml). The skin spices were incubated in 10ml Dispase (0.25%) at 4°C for 1–2h. After removing the epidermis, the dermal layer was cut to pieces and then we added 10ml type 1 collagenase (0.25%). Digesting 0.5–1h at 37°C, the suspension was collected, and centrifuged 1500rpm for 5min. The cells were resuspended in culture medium (DMEM) with 10% fetal calf serum, 100IU/ml penicillin, and 100 IU/ml streptomycin. The fibroblast cultures were maintained at 37°C in air and 5% CO2Citation[2], Citation[3], Citation[6], Citation[7].
Preparation of collagen swelling solution
Collagen was extracted from bovine tendon and was treated by enzyme digestion described in Xhang et al. Citation[8], Citation[9]. 63.5ml 0.2M acetic acid was added in 36.5g 1.37% collagen swelling solution to get 0.3% (w/v) collagen swelling solution. The solution was homogenized in a high speed stirrer and was defoamed for 10 min in a freeze centrifuge (2000rpm, 5°C).
Preparation of collagen gel dermis
5ml concentrated DMEM (×10), 0.5ml L-glutamine (0.3M), 1.8ml sodium hydrogen carbonate (1M), and 6ml fetal calf serum were mixed. The mixture solution and 40ml 0.3% collagen swelling solution were poured into 100ml flask, we added 3ml fibroblasts suspension (5.0×106), and mixed at 4°C. 8 mL solution was poured into 60mm culture plate, and cultured at 37°C for 3–5 min. After the gel had been conformed, 1ml DMEM medium with 10% fetal calf serum was added into each well. The collagen gel dermis was cultured at 37°C 5% CO2, and the medium was changed every other day Citation[10].
Preparation of collagen matrix dermis
8ml collagen swelling solution was poured into 60mm culture dishes. It was dried in freez dryer to form the collagen lattice. The membrane was cross-linked by 0.3% formaldehyde (PH 8.4) for 2h. The cross-linked membrane was washed in double-distilled water repeatedly. At last the membrane was frozen at −40°C 2h, and lyophilized in the frozen dryer again Citation[11].
The collagen scaffold was sterilized by γ-irradiation. After culturing for 3–4 generation, 3 mL medium of cells suspension at a density of 1.0×107 cells/ml were implanted into the collagen scaffolds on the culture plate; the cells had been immersed in the DMEM solution for 30min before implantation. After 3 hours, 5ml DMEM culture solution with 10% fetal calf serum was added to each well. The culture plate was cultured at 37°C, 5% CO2, the culture solution was changed every other day Citation[5].
Physical Characterization of Collagen Dermis
Detection of diameter
Every four days, five specimens from each kind of artificial dermis were taken from the culture plate. Every sample's diameter was measured, and the average was calculated.
Hydrophilicity
Hydrophilicity analysis was performed to determine the water wettability of the collagen gel dermis and the collagen matrix dermis after being cultured for 12 days. Samples were weighed (Wh) at room temperature. Then all samples were dried by baking, and weighed again (Wd). The hydrophilicity (Wa) was calculated according to the following equation Citation[1] (statistical analysis by STATA 8.0).1
DSC
Every four days, the thermal characteristics of the collagen gel dermis and collagen matrix dermis were determined by differential scanning calorimeter (TADSC2910) under nitrogen environment from 30°C to 250°C with a rate of 10°C/min.
Mechanical property
Every four days, the specimens of collagen gel dermis and collagen matrix dermis were cut into strips (40mm×10mm), and their intensity of snap were measured by XL-50A pull detector at a pulling rate of 5mm/min.
Histochemical Analyze
SEM
After being cultured for 8 days, the morphology of fibroblast on the matrices was observed by HITACHI X-650 model SEM. All samples were washed with phosphate-buffered saline (PBS), and fixed with 2.5% glutaradehyde for 2 h at 4°C. After fixation, specimens were dehydrated through a series of graded alcohols and critical point dried, then gold-sputtered in vacuum and observed.
H&E staining
After being cultured for 4 and 8 days, the specimens from each dermis were fixed by 4% paraformaldehyde, dehydrated by graded alcohol, embedded in paraffin, and sliced into sections (the sections were 4um thick) hematoxylin and eosin (H&E), observed for the comparison of density and attachment of fibroblasts on both kinds of scaffolds.
Immunohistochemical analysis
Every four days, the immunohistochemical demonstration of fibronectin (FN) and type I collagen were performed by standard techniques. Vectastain ABC Kit and monoclonal rat antibody against human FN and monoclonal rat antibody against human type I collagen were used.
RESULTS
Physical Characterization of the Collagen Dermis
Detection of diameter
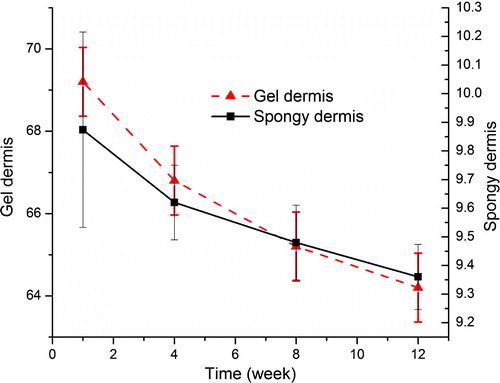
The diameters of both dermises are shown in . The diameter of collagen gel dermis decreased obviously with the elongation of the culture time. But during the last four days, the shrinking speed of the dermis had decreased. The diameter of the collagen spongy dermis had not decreased obviously, other than the last four days.
Table 1. Diameter of the dermises
Hydrophilicity
The moisture content of the dermis is shown in . The moisture content of the collagen gel was as high as about 69%, and it was almost stable, which is near to the water content of normal human tissue. But the moisture content of the collagen spongy dermis was much lower than that of the gel.
DSC
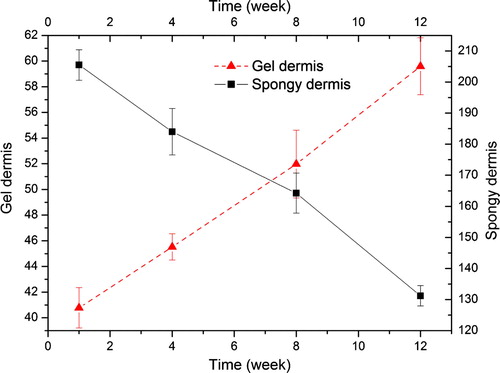
The thermodynamics of the biomaterials were investigated to show the interaction among the components of the biomaterials. The denaturation of the collagen protein is caused by the damaging of the protein super-helix structure. shows the denatured temperature of matrices in DSC. The transition in DSC is due to the denaturation of the collagen protein. The transition temperature reduces from 112.3°C to 98.4°C during the process of culturation. This implies that the cell culture in spongy dermis decreases the stabilization of the matrices. But in the gel dermis the denature temperature increased from 82.4°C to 92.1°C, although the whole denature temperature was lower than that of the spongy dermis.
Table 2. The denature temperature of the dermises (°C)
Histochemical Analysis
SEM
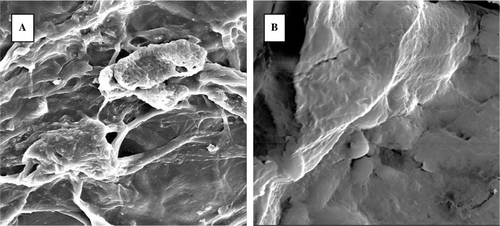
shows the morphology of fibroblasts cultured on both scaffolds. After being implanted for 8 days, the cells disperse among the spongy dermis scaffold, and sometimes aggregate in clusters. Some extracellular matrix (ECM) secreted by fibroblasts is observed around the cells. For gel dermis groups, the cells were generously proliferate, spread among the fiber of collagen. No cells could be observed on the surface of the gel dermis. The results of SEM suggest that the matrix could provide a favorable space for cell adhering and proliferating. Obvious differences in the density and the form of cells growth were observed between scaffolds.
H&E staining
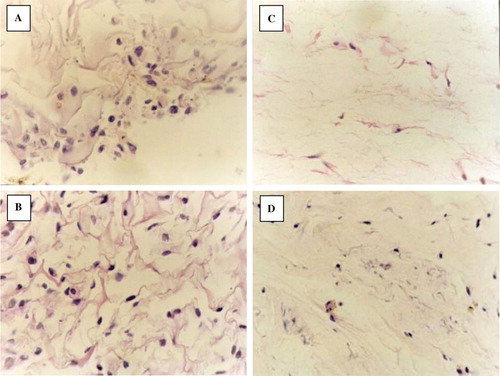
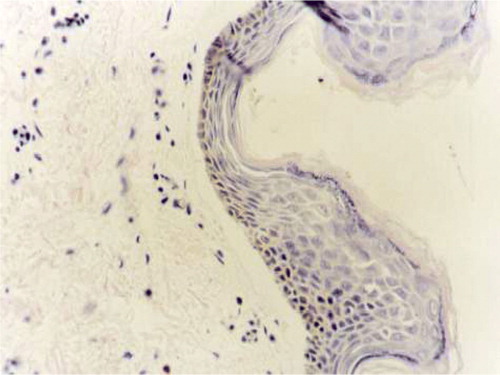
After being implanted for 4 days, the number of cells in the dermis is very few. The cells on the collagen spongy scaffold were lying between the fibers of scaffold. The number of cells in the collagen gel is fewer than the cells on the collagen. The cells in both dermises were round and oval. The range of cells in both dermises was in random array. The ECM secreted by the fibroblast in the dermises was very minimal. From 8 days, the number of cells in both dermises had risen distinctly. The cells in the collagen gel were changed into long spindles. Some slight collagen fibro could also be seen in the collagen gel. The fibroblasts were parallel with the collagen fibro. Though the number of fibroblasts in the collagen spongy dermis is much greater than the collagen gel dermis, they were in random array all the same. The shapes of those cells were round and oval. During the processing, we could see that part of the collagen structure began to degrade ().
Immunohistochemical analysis
At 4 days, the stains of the FN and type I collagen of the two scaffolds were all light. At 8 days, the stains were increased. By comparing the two scaffolds, the collagen spongy scaffold's stains were deeper than the collagen gel scaffold's ().
Table 3. The stains of the FN and type I collagen of the two scaffolds *
DISCUSSION
Dermis fibroblast is a kind of mesenchymal cell, and it can secrete ECM, growth factor, and so on, which play an important role in the formation and development of tissue. With the development of cell culture technology, several methods of isolating and culturing dermis fibroblasts have been set up, which include the tissue culturing method, collagenase digestion method, the combination digestion method with collagenase and Dispase. In this study, the fibroblast was isolated from neonate prepuce, for this kind of fibroblast has low antigenicity and high multiplication potential. After two generations, we obtained fibroblasts without any other kinds of cells, for the fibroblasts adhere more quickly than other cells () Citation[5], Citation[12].
In the process of preparing the collagen solution, malonic acid is usually used. But collagen can be more easily distributed in acetic acid, acetic acid volatilizes more quickly than malonic acid, and can be rinsed out in a collagen-based scaffold, so we used acetic acid in preparing the collagen distribution solution.
We measured the diameter of collagen gel in the culturing process. The diameter decreased with the elongation of culturing time. From the first day to the fourth day, shrinking speed of the collagen gel dermis’ diameter decreased distinctively. After that, it slowed down. In spongy dermis, shrinking speed was slower during the first four days than the gel dermis. But the rate became quicker from the eighth day.
For all materials, if their structure has an asymmetry characteristic, there is surface tension that causes its surface shrinking. In the whole culturing process, fibroblasts secrete some kind of extracellular matrix, such as type I collagen, so the component ratio of collagen gel is gradually altered, and fibroblasts redistributed along the collagen fibro; at the same time, the strength of collagen gel was intensified with the yield of extracellular matrix in gel. In Lopez Valle's study, he cultured collagen gel on a fiberglass loop, which decreased the shrink speed of collagen gel, but his loop can interfere in the culturing process of gel. Therefore it is still a problem in preventing the shrinking of gel in preparation Citation[13].
After being transplanted, the artificial dermis will contact with different kinds of body fluid. The anti-coagulation must be considered in the preparation of artificial dermis. For membrane biomaterials, the interaction with fluid occurs at its surface. The anti-coagulation ability is defined by its wetness. The measurement result of moisture content is shown in . The moisture content is an important factor, which influences its blood biocompatibility and also influences the adherence of blood platelet on it. With its high water content, collagen gel has low phase energy, so it is hard for blood platelet to adhere to it. The moisture content of the collagen gel is as high as 69.2% at the first day. It is almost stable (64.2% at the twelfth day), which is near to the water content of normal human tissue. The moisture content in spongy dermis was 9.874% at the first day, and 9.36% at the twelfth day Citation[14].
It is important that the dermis substitute has some extent of strength, which is suitable for manipulation of implantation. From our measurement result, the strength of our collagen gel was gradually increased, but its absolute strength quantity is still much lower than the value of normal human dermis (245 Kpa), and is also lower than that of the collagen spongy scaffold. To improve the strength of the collagen gel, some methods, such as purification and cross-linking, could be used. But the effects need more validation.
From the H&E staining result, we can find, during the first four days, fibroblasts in spongy dermises were round and distributed on the surface of the scaffold. In gel dermis the fibroblasts were spindle-like and distributed uniformly in the scaffold. But the cell number was much fewer than in the spongy dermis. At the second four days, the number of fibroblasts increased. The shape of fibroblast in collagen gel dermis changed into shuttle, and there was also interweaved collagen fibril in the collagen gel. The fibroblasts distributed along the fibril. The fibroblasts in membrane spongy were still round. The shape of fibroblasts in the collagen gel is simulated to that in normal dermis tissue (). In collagen spongy, the number of fibroblasts increased, collagen scaffold degraded partially, there was a lot of extracellular matrix yielded in the scaffold, and the component ratio of scaffold was altered, but the fibroblasts were distributed in disorder along the collagen fibril, and the collagen fibril showed interweaving. In the whole, the structure of the collagen spongy was far from the normal structure of dermis tissue.
In normal human dermis tissue, the extracellular matrix includes collagen protein, proteoglycan, and firbronection. Type I and III collagen protein distribute broadly in the dermis, which consists of 80% Type I collagen protein and 20% type III collagen protein. Fibronection (FN) is a kind of Nianlian, and it distributes mainly in the surface of cells or in theextracellular matrix. In a wound, FN promotes the moving of fibroblasts and epidermal cells towards the wound, which can accelerate the healing process. Type I collagen protein and FN are the main markers of dermis tissue. From the immunohistochemical staining result, it can be found that the quantity of extracellular matrix in collagen gel increased with the elongation of culturing time. During the second four days, the immunohistochemical staining strength of collagen gel is lower than that of the collagen spongy dermis; this might be due to the low seeding density of fibroblasts in collagen gel, which was 1.0×105↑/cm2, but in collagen spongy its seeding density was 1.0×106↑/cm2. In the second four days, comparing the structure of collagen gel with collagen spongy, no obvious differences were observed between them Citation[7], Citation[11], Citation[15].
It can be concluded that the collagen gel is more suitable for fibroblasts in maintaining morphology than spongy scaffold. The reason might be due to the special structure of collagen gel. Water in gel is sub-bound water, which is different from free water, and it is suitable for fibroblasts to secrete extracellular matrix.
The financial support of the National Natural Science Foundation (30700848 and 30772443) and Natural Science Foundation of Beijing (7042058 and 7082090) is gratefully acknowledged.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cairus Bruce A., Deserres Suzan, Peterson H.D. Skin replacement. Arch Surg. 1993; 128: 1246–1250
- Dermarchez M., Hartman D.J., Regnier M., et al. The role of fibroblasts in dermal vascularization and remodeling of reconstructed human skin after transplantation onto nude mouse. Transplantation 1992; 54(2)317–326
- Regnier M., Vaigot P., Darmon M., et al. Onset of epidermal differentiation in hyperproliferative basal keratinocytes. J Invest Dermatol. 1986; 87: 472–476
- Asselineal D., Dernard B.A., Barlly C., et al. Epidermal morphogenesis and incubation of 67KD keratin polypeptide by culture at the liquid-air interface. Exp Cell Res. 1985; 159: 536–539
- Belle E., Ivarsson B., Merrill C. Productive of a tissue like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 1979; 76(3)1274–1278
- Hansbrough J.F., Boyce S.T., Cooper M.L., et al. Burn wound closure with cultured autologous keratinocytes and fibroblasts attached to a collagen-glycosaminoglycan substitute. JAMA 1989; 262: 2125–2130
- Bell E., Bengt I., Charlotte M. Production of a tissue like structure by contraction of collagen lattice by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 1979; 76(3)1274–1278
- Qiqing Zhang, Kangde Yao, Lingrong Liu, et al. Evaluation of porous collagen membrane in guided tissue regeneration. Art. Cell, Blood Subs., and Immob. Biotech. 1990; 27(3)245–253
- Qiqing Zhang, Ling-rong Liu, Lei Ren, et al. Preparation and characterization of collagen-chitosan composites. Journal of Applied Polymer Sciences 1997; 64: 2127–2130
- Osborne C.S., Barbenel J.C., Smith D., et al. Investigation into the tensile properties of collagen/chondroitin-6-sulphate gels: the effect of crosslinking agents and diamines. Med Biol Eng Comput. 1998; 36(1)129–34
- Dermarchez M., Hartman D.J., Regnier M., et al. The role of fibroblasts in dermal vascularization and remodeling of reconstructed human skin after transplantation onto nude mouse. Transplantation 1992; 54(2)317–326
- Heck E.L., Bergstresser P.R., Baxter C.R. Composite skin graft: frozen dermal allografts support the engraftment and expansion of autologous epidermis. J Trauma 1985; 25(2)196–201
- Lopez Valle C.A., Germain L., Rouabhia M., et al. Grafting on nude mice of living skin equivalent produced using human collagens. Transplantation 1996; 62: 317–23
- Yang H., Zhang Q. Preparation and characterization of collagen-GAGs bioactive materials for tissue engineering. J Mate Sci Technol. 2001; 17(5)495–500
- Middelkoop E, de Vries HJ, Ruuls L., et al. Adherence, proliferation and collagen turnover by human fibroblasts seeded into different types of collagen sponge. Cell Tissue Res. 1995; 280(2)447–453