Abstract
The purpose is to prepare 2% w/v emulsions of dodecafluoropentane, perfluorodecalin, and perfluoroctylbromide and compare them for their ability to absorb oxygen.
The oxygen uptake capability and volume expansion of each emulsion and the blank vehicle were evaluated in water at 21°C and 37°C. The average particle size of the dodecafluoropentane emulsion is < 400 nm stored at room temperature for 6 months. In comparison to water treated with either the blank vehicle, the perfluorodecalin emulsion, or the perfluoroctylbromide emulsion, the dodecafluoropentane emulsion absorbs 3 times more oxygen at 21°C and 7 times more oxygen at 37°C. Furthermore, a significantly higher in vitro expansion (5 times) is observed with the dodecafluoropentane emulsion at 37°C. As such, DDFP has been hypothesized to be a better oxygen carrier and delivery agent in vivo. This may be applicable to a variety of hypoxic medical conditions where oxygen delivery might be therapeutically beneficial.
INTRODUCTION
There is a need to develop an intravenous injectable that can increase the exchange of respiratory gases in certain clinical and field situations (e.g. hemorrhagic shock) Citation[1], Citation[2]. It is well known that the body needs a constant supply of oxygen as well as an efficient carbon dioxide removal mechanism in order for the cells to maintain healthy metabolic processes. In addition, the extent of benefit from efficient oxygen and carbon dioxide exchange may be dependent upon the time of restoration of this exchange process after a hypoxic event (ischemia-reperfusion) Citation[3], Citation[4]. The potential applications of a product that can conveniently supplement these needs are widespread, including tumor radiation sensitization Citation[5], elimination of tissue nitrogen in decompression sickness Citation[6], treatment of carbon monoxide poisoning Citation[7], and treatment of various hypoxic conditions that cause cellular damage. Stroke is a serious and widespread condition that can result in neural damage. Such damage is known to be caused in part by a reduction in the opportunity for local brain cells to exchange oxygen and carbon dioxide. Current approved treatments for stroke, such as aspirin, are designed to limit the process of thrombus formation but are not designed to restore tissue viability. Agents such as tissue plasminogen activator (tPA) can currently only be utilized for a three-hour window of time following onset of stroke due to an increased incidence of intra-cranial hemorrhage when administered later Citation[8], Citation[9]. It would be interesting and quite beneficial if an agent could be used to extend the window of time for tissue viability so either thrombus dissolving or recanalization life-saving treatment paradigms can be used beyond the 3-hour limit. A 2% w/v sub-microemulsion of dodecafluoropentane (DDFP) (also known as perfluoropentane) was developed and has been shown to be safe in humans Citation[10]. This emulsion was proven by Lundgren et al. Citation[11] to carry out respiratory gas transport and maintain tissue viability as well as normal physiological processes in severely anemic rats. Furthermore, Koch et al. Citation[5] have shown that by facilitating the oxygenation of anaerobic tumor cells the formulation can render those cells aerobic, thus making them vulnerable to radiation. The in vitro experiments detailed herein suggest physical characteristics and a gas transport mechanism that indicate how this 2% w/v DDFP emulsion (DDFPe) may function to help maintain tissue oxygen perfusion. The data may also offer insight into how the formulation might be useful as a cellular protectant during stroke or heart attack. When administered in relatively small doses these submicron-sized particles (5–10×smaller than red blood cells) may be able to perfuse to the distal end of vascular occlusions, when blood cells are unable, and provide vital oxygen.
Hemoglobin is the sole natural transporter of oxygen in the body. By volume comparison, fluorocarbons are known to dissolve gases more efficiently than other fluids or packed red blood cells Citation[12]. Thus, there are other fluorocarbon emulsions under investigation for gas transport. These other products in development contain relatively large, non-volatile perfluorocarbons (PFCs) such as perfluorodecalin (PFD) and perfluorooctylbromide (PFOB) Citation[13], both having boiling points of 142°C–144°C. They are formulated in high concentration (20% to 60% w/v) in order to provide adequate oxygen Citation14–16. The solubility of oxygen is reported to be 42% and 50% v/v in PFD and PFOB Citation[17], Citation[18], respectively. These 2 PFCs also are known to experience a large volume of distribution due to their extreme hydrophobicity. While they are not attracted to lipids, they are strongly repelled by the hydrogen bonding of water, rendering them strongly hydrophobic but only relatively lipophobic. Therefore, they predominantly distribute to the tissues, are notably taken up by the RES macrophages and, due to the high degree of accumulation, they exhibit relatively long secondary phase half-lives Citation[19], Citation[20]. This makes their utility as oxygen therapeutics somewhat limited. Alternatively, because the DDFP molecule is smaller, dissolves a higher concentration of oxygen (80% v/v), has a boiling point of 29°C, and thus may volatilize to some degree at biological temperature (further enhancing oxygen load), a much smaller volume of it is needed to supply sufficient oxygen in vivoCitation[6], Citation[21], Citation[22]. In addition, studies show that DDFP has a 2-minute half-life in the blood and is 99% cleared through the lungs in 2 hours after intravenous administration Citation[23]. Although this short half-life may appear to be a disadvantage, animal studies suggest that a low dose of DDFPe, around 0.7 cc per kg, administered one time as an IV infusion, may be sufficient for resuscitation of severely anemic animals Citation[6], Citation[11]. Another consideration is that an oxygen therapeutic does not need to replace blood; it might still have a useful role to stabilize patients with critical anemia or ischemia until additional definitive therapy (e.g. blood transfusion) can be administered. As previously stated, it has been suggested that DDFP has an advantage in solubilizing gases over many other PFCs due to its short linear chain length Citation[1], Citation[17] and low boiling point Citation[6], Citation[21]. This more efficient oxygen absorption may be attributed to the fact that DDFP has the highest ratio of primary CF3 groups relative to secondary CF2 groups compared to longer chain perfluorocarbons (PFD and PFOB). The CF3 groups, being strongly electronegative, are largely responsible for the attraction of gases Citation[24]. Thus, on a volume basis, liquid DDFP can dissolve more respiratory gases than other linear liquid PFCs. Possibly more important for oxygen-carrying capacity is the fact that DDFP expands from the liquid to the gaseous state Citation[7], Citation[25], Citation[26] at biological temperatures and as a result transfers gases based on local pressure gradients. It is believed that the gaseous state of DDFP can absorb, deliver, and exchange much more oxygen not only in comparison to its own liquid state but also in comparison to the liquid states of other larger PFCs Citation[21], Citation[27]. The current goal is to compare the ability of 2% w/v DDFPe with equivalent emulsions of PFD and PFOB for their abilities to pick up oxygen, thus simulating a scenario where oxygen would be available to be carried and delivered to a hypoxic environment. Furthermore, as the 2% DDFPe is the main focus of this manuscript, the physical stability of only the DDFPe is addressed.
METHODS
Preparation of the Product
Three separate batches of 2% w/v PFC (DDFP, PFD and PFOB) emulsions were prepared as previously described by Lundgren et al. Citation[6]. In addition, a blank formulation was prepared in the same manner as the 3 emulsions but without any PFC. Specifically, for each 1 liter batch, 3 grams of PEG-Telomer B and 20 grams of PFC (except for the blank) were homogenized along with a 33% sucrose solution using a custom-built semi-closed stainless steel containment system attached to an Avestin Emulsiflex-C5 homogenizer. Each homogenate was processed for 6 passes through the chamber at 14,000 psi and then terminally sterile filtered immediately prior to filling into 5 mL vials. The vials were stoppered and crimped and then stored at room temperature.
Product Analysis
Particle Sizing
Three vials were selected at random from each formulated batch. Vials were vortexed for 5 seconds and 10 uL aliquots of the liquid formulation were removed by syringe, injected into 3 mL cuvettes containing 2 mL of a phosphate buffered saline diluent (of known viscosity), and chilled in an ice bath. The cuvettes were covered and gently inverted 3 times. The temperature of the sample was then measured and each sample was analyzed using a Malvern Zetasizer HS100 at the temperature and viscosity settings determined. The 9 selected vials were each sampled once, unless an error message was given by the Malvern, in which case sampling was repeated until a passing test was achieved. Because it is the main focus of this study, only the DDFPe particle size was monitored over a 6-month time period.
pH Analysis
A Symphony SB21 pH meter and a Symphony 850 pH probe were used to determine the pH of the final formulation. Analysis of the hydrogen ion concentration was performed in triplicate. The meter was calibrated with pH standards at 4.0 and 7.0 and then, in order to confirm intra-batch uniformity, 1 vial was selected from the beginning, middle, and end of each batch for measurement.
Statistical Analyses
All descriptive statistics, such as mean and standard deviation calculations for particle size, pH, oxygen uptake, and volume expansion, were performed using the Microsoft Excel 2003 Data Analysis Kit.
In Vitro Performance Testing
Oxygen Transport
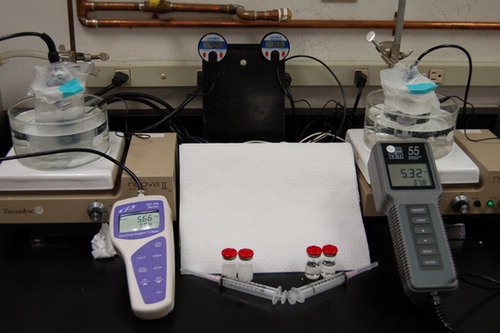
The in vitro set–up to measure oxygen uptake by the formulations, shown in , was adapted from Lundgren et al. Citation[11]. Three hundred mL beakers with stir bars were filled with 250 mL of deionized water and placed in temperature-controlled water baths. The water baths were set on top of stirring hotplates. The probes of portable oxygen meters were submerged into the beakers and dissolved oxygen readings were allowed to stabilize. Once stabilization was established, the water surfaces were first covered with Styro-foam® disks and then sealed in Parafilm® to eliminate any headspace and prevent further gas exchange with the atmosphere. A needle and syringe were used to inject 5 mL of formulation through the parafilm and into the 250 mL volume of water (1:50, v:v). The injection hole was resealed each time with adhesive tape. Dissolved oxygen readings were recorded at 30-second intervals on a computer using custom-designed communications port data logger software Citation[28] for 1 hour after each injection. This procedure was carried out in triplicate for the DDFPe, PFDe, PFOBe, and the blank formulation at both temperatures of 20°C and 37°C.
Volume Expansion
Because DDFP is expected to volatilize at 37°C, the volume expansion of each formulation upon injection was tested using a manometer apparatus shown in .In parallel experiments, 5 mL volumes of the DDFPe, PFDe, PFOBe, the blank formulation, and deionized water were injected into 250 mL of stirred DI water while the temperature of the stoppered 250 mL Erlenmeyer flask containing the 250 mL of DI water was maintained at 37° C. A 25 mL burette was inserted through the stopper and its tip submerged into the water such that any volume increase would be forced up into the burette and then could be measured.
Results
Particle Sizing
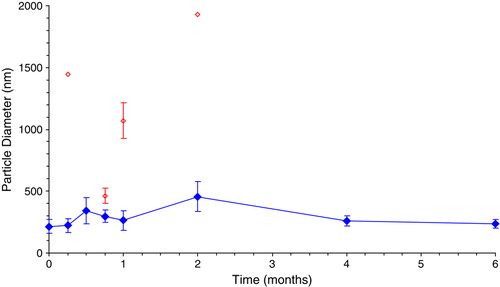
The initial average particle diameters were determined to be 215±56 nm, 103±8 nm and 155±6 nm for DDFPe, PFDe and PFOBe, respectively. shows that the DDFPe particle size remains stable at a diameter below 400 nm for 6 months at room temperature (23°C±2°C). Note that the open diamonds represent less than 2% of the particles in the sample and these bimodal distributions were only observed for the first 2 months.
pH Analysis
The pH values of DDFPe, PFDe and PFOBe were found to be 5.5, 6.1 and 5.7, respectively.
In Vitro Performance Testing
Oxygen Transport
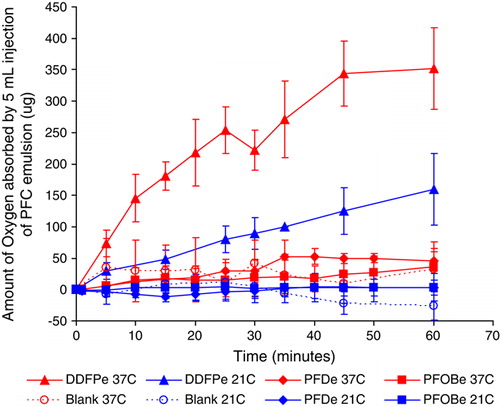
shows the oxygen uptake data for all of the samples and controls tested. The PFDe and PFOBe formulations were determined to absorb no more oxygen than the blank formulation at both test temperatures of 21°C and 37°C. By contrast, DDFPe absorbed significantly more oxygen than the PFDe, the PFOBe, and the blank formulation at both temperatures. Specifically, at 60 minutes, DDFPe absorbed approximately 3 times more oxygen at 21°C (p = 0.03) and 7 times more oxygen at 37°C (p = 0.001). This can be attributed to two important things. One is the fact that the DDFPe contains approximately twice the molar amount of perfluorocarbon vs. PFC wt amount and the other is the higher ratio of trifluoromethyl groups present per unit volume compared to the other formulations.
Volume Expansion
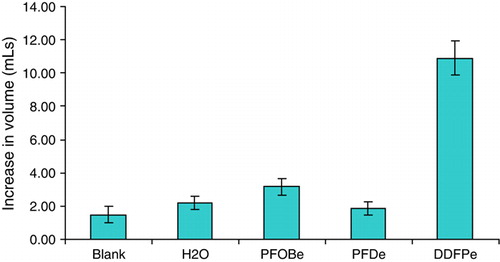
shows the differences in volume expansion of all the samples and controls when introduced into a 37°C semi-sealed flask. Although there are expansions observed with PFDe and PFOBe, neither is significantly larger than the expansion of an equal injection of water (p = 0.35 and p = 0.06 for PFDe and PFOBe, respectively). There does appear to be a modest difference between the volume increase of PFOBe and the blank formulation (p = 0.01) but not between water and the blank formulation (p = 0.12). The reason for the apparent difference between the blank and PFOBe is unknown; however, it is possible that PFOB expands more than PFD at raised temperature. The most notable result is seen in the expansion of the DDFPe. It is significantly greater (p < 0.00001) than all of the other test injections by at least 5 times.
DISCUSSION
It has been explained that liquid PFCs dissolve oxygen proficiently due to the relative positions of the fluorine atoms Citation[24]. Because the fluorine atoms are larger than hydrogen, the tetrahedral arrangement becomes slightly twisted around the carbon chain and renders the molecule relatively rigid. In addition, PFCs experience negligible Van der Waals attractions, thus making it easier for cavities to form in the liquid, allowing the entrance of gases that also lack attractive forces Citation[1]. Furthermore, the strongly electronegative fluorine atoms, especially the CF3 groups, create pockets where the electron density of gas molecules can be stabilized. These three combined aspects are responsible for the solubility of oxygen in liquid DDFP, PFD, and PFOB.
The fact that DDFP boils at 29°C may at first seem disadvantageous for oxygen delivery, as gas solutes are inherently less soluble at higher temperatures. This is likely to be true once a certain threshold of expansion is reached, but if one considers that PFCs neither reduce nor increase in attraction to each other when heated there will be an initial phase of expansion where solvent cavity space in the liquid simply increases. Nevertheless, it is not simply the inherent solubility of gas in DDFP that allows it to absorb so much more oxygen than other PFCs at biological temperature. In theory, this increased oxygen carrying capacity is primarily attributable to the relative lower boiling point of DDFP Citation[7]. The theory states that further expansion of the DDFP will eventually create bubbles, which are respiratory gas sinks into which dissolved oxygen, in the surrounding medium, can rapidly diffuse. Thus, once these oxygen rich microenvironments are present, they should be able to circulate and release their oxygen payload out into relatively hypoxic regions at a more rapid rate than would be true for larger PFCs that experience less expansion and consequently less oxygen absorption. We stress the theoretical aspect of how DDFP functions to carry oxygen because while a true expansion from nano-droplet to micro-bubble is known to occur in vitro and some degree of expansion must also occur in vivo, there is no solid proof that actual bubbles are formed in a living vascular system.
It should be clearly noted that the oxygen uptake concentrations measured in these in vitro experiments cannot be scaled quantitatively to an in vivo situation. The additional pressures exerted in the semi-closed circulatory system would be expected to result in a relative hindrance of the expansion of DDFP. It is even possible that, due to the blood pressure, DDFP does not form actual bubbles in vivo, but rather areas of a plasma-like interface between the edge of the DDFP droplet and the blood. In short, it is not concluded herein that the DDFP formulation forms true micro-bubbles in vivo. Rather it is suggested that the trend would be expected to remain the same in that DDFP should be able to hold more oxygen due its relatively higher ability to expand at biological temperature. Hence, it should deliver more oxygen than the same volume of PFD or PFOB. Another limitation of these experiments is that while they do show a superior ability of DDFP to absorb oxygen, they do not show the ability to release that oxygen under hypoxic conditions. Although evidence that the formulation does release oxygen in vivo has already been published Citation[6], Citation[11], it would be beneficial to provide in vitro support of the process. Future experiments are planned to investigate this.
Another concern with oxygen delivery is the potential accompanying increase in oxygen free radical production. It is well known that oxidative stress caused by the presence of free radicals causes cellular damage. Although some oxidative stress is physiologically unavoidable, unnecessary life-altering damage can result from an excess of such exposure. Since reactive oxygen species (ROS) are small molecules with unpaired electrons they may be scavengable by DDFP due to a combination of its electrophilic nature and tendency toward gaseous form at biological temperature. Furthermore, formulation changes to incorporate antioxidants in the DDFPe may provide chemical protection from ROS. Thus, additional studies are also planned to test the ability of the DDFPe to minimize cellular damage that can result from exposure to damaging oxygen free radicals.
CONCLUSION
A 2% w/v DDFP emulsion was prepared and tested against equivalent emulsion concentrations of PFD and PFOB for oxygen absorption ability. The final DDFPe has a pH of 5.5, appears milky white, and the initial particle size is 215±56 nm. The 2% DDFPe was found to carry 7 times more oxygen than 2% w/v emulsions of PFD or PFOB. These differences were determined to be significant at a p = 0.001 level of confidence. Thus, the ability of DDFP to expand at physiological temperature appears to provide it with a substantial advantage over PFD and PFOB to deliver a higher payload of oxygen. This is most likely the reason formulations with PFD and PFOB, intended for oxygen delivery, have commonly been prepared at 40% to 60% concentrations. It is clear that DDFP offers a strong advantage over PFD and PFOB in that a much smaller dose of PFC can be administered to achieve the desired result. This is not only less invasive for the patient but also for the environment, as it has been clearly documented that PFCs exit the body through the lungs Citation[23], Citation[29], Citation[30].
The data herein support the contentions of Burkard and Van Liew Citation[21] as well as Lundgren et al. Citation[6], Citation[11] in that DDFP should be able to provide enhanced oxygen delivery over other PFCs due to its expansion from a liquid to a gas at physiological temperature. These in vitro studies coupled with the in vivo results obtained by Lundgren et al. Citation[11] in rats appear to indicate that small doses of the DDFP emulsion may also be useful to manage preservation of tissue during acute hypoxic events. Furthermore, results may indicate an additional use for supporting neuroprotection during hypoxic episodes of cardiovascular accidents or stroke.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dias A.M.A., Freire M., Coutinho J.A.P., Marrucho I.M. Solubility of oxygen in liquid perfluorocarbons. Fluid Phase Equilibria. 2004; 222–223: 325–330
- Cheung A.T., To P.L., Chan D.M., Ramanujam S., Barbosa M.A., Chen P.C., Driessen B., Jahr J.S., Gunther R.A. Comparison of treatment modalities for hemorrhagic shock. Artif. Cells Blood Substit Immobil. Biotechnol. 2007; 35(2)173–190
- Schoenberg M.H., Beger H.G. Reperfusion injury after intestinal ischemia. Crit. Care Med. 1993; 21(9)1376–1386
- Grace P.A. Ischemia-reperfusion injury. Brit. J. Surg. 1994; 81: 637–647
- Koch C.J., Oprysko P.R., Shuman A.L., Jenkins W.T., Brandt G., Evans S.M. Radiosensitization of hypoxic tumor cells by dodecafluoropentane: a gas-phase perfluorochemical emulsion. Cancer Research. 2002; 62: 3626–3629
- Lundgren C., Bergoe G., Olszowka A., Tyssebotn I. Tissue nitrogen elimination in oxygen-breathing pigs is enhanced by fluorocarbon-derived intravascular micro-bubbles. UHM 2005; 32(4)215–226
- Van Liew H.D., Burkard M.E. Relationship of oxygen content to PO2 for stabilized bubbles in the circulation: theory. J. Appl. Physiol. 1996; 81(1)500–508
- Fagan S.C., Morgenstern L.B., Petitta A., Ward R.E., Tilley B.C., Marler J.R., Levine S.R., Broderick J.P., Kwiatkowski T.G., Frankel M., Brott T.G., Walker M.D. The NINDS rt-PA Stroke Study Group. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke. Neurology. 1998; 50: 883–890
- Levi C.R. Tissue plasminogen activator (tPA) in acute ischaemic stroke: time for collegiate communication and consensus. The Medical Journal of Australia. 2004; 180(12)634–636
- Robbin M.L., Eisenfeld A.J. Perflenapent emulsion; a US contrast agent for diagnostic radiology-multicenter, double-blind comparison with a placebo. Radiology. 1998; 207: 717–722
- Lundgren C., Bergoe G., Olszowka A., Tyssebotn I. Intravascular fluorocarbon-stabilized microbubbles protect against fatal anemia in rats. Artificial Cells, Blood Substitutes, and Biotechnology. 2006; 34(5)473–468
- Johnson E.C., Erickson B.K., Podolsky A., Birks E.K., Keipert P.E., Faithfull N.S., Wagner P.D. Effects of a perfluorocarbon emulsion for enhanced O2 solubility on hemodynamics and O2 transport in dogs. J. Appl. Physiol. 1995; 79(5)1777–1786
- Lowe K.C. Fluorinated blood substitutes and oxygen carriers. Journal of Fluorine Chemistry. 2001; 109: 59–65
- Leese P.T., Noveck R.J., Shorr J.S., Woods C.M., Flaim K.E., Keipert P.E. Randomized safety studies of intravenous perflubron emulsion. I. Effects on coagulation function in healthy volunteers. Anesth. Analg. 2000; 91: 804–811
- Wahr J.A., Trouwborst A., Spence R.K., Henny C.P., Ceraianu A.C., Graziano G.P., Tremper K.K., Flaim K.E., Faithfull N.S., Clymer J.J. A pilot study of the effects of a perflubron emulsion, AF 0104, on mixed venous oxygen tension in anesthetized surgical patients. Anesth. Analg. 1996; 82: 103–107
- Kiral, R.M., Nicora, R., Evitts, D.P. 2002. Comparison of oxygen carrying capacity of a new perfluorocarbon (pfc) blood substitute in rats breathing room air or 100% oxygen. Presented at:. APS Intersociety Meeting, San Diego, CA.
- Dias A.M.A., Goncalves C.M.B., Legido J.L., Coutinho J.A.P., Marrucho I.M. Solubility of oxygen in substituted perfluorocarbons. Fluid Phase Equilibria. 2005; 238: 7–12
- Krafft M.P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Advanced Drug Delivery Reviews 2001; 47: 209–228
- Flaim S.F. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Art. Cells, Blood Subst., Immob. Biotech. 1994; 22: 1043–1054
- Tremper K.K. Perfluorochemical blood substitutes. Anesthesiology. 1999; 91: 1185–1187
- Burkard M.E., Van Liew H.D. Oxygen transport to tissues by persistent bubbles: theory and simulations. J. Appl. Physiol. 1994; 77: 2874–2878
- Takanori Y., Schmid-Schönbein G.W., Cotter B., DeMaria A.N. Flow dynamics of QW7437, a new dodecafluoropentane ultrasound contrast agent, in the microcirculation. J. Amer. Coll. Cardiology 1999; 34(2)578–586
- Correas J.M., Meuter A.R., Singlas E., Kessler D.R., Worah D., Quay S.C. Human pharmacokinetics of a perfluorocarbon ultrasound contrast agent evaluated with gas chromatography. Ultrasound in Med. Biol. 2001; 27(4)565–570
- Riess J.G. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Aritficial Cells, Blood Substitutes, and Biotechnology 2005; 33: 47–63
- Van Liew H.D., Burkard M.E. Bubbles in circulating blood: stabilization and simulations of cyclic changes of size and content. J. Appl. Physiol. 1995; 79(4)1379–1385
- Van Liew H.D., Raychaudhuri S. Stabilized bubbles in the body: pressure-radius relationships and the limits to stabilization. J. Appl. Physiol. 1997; 82: 2045–2053
- Van Liew H.D., Burkard M.E. Behavior of bubbles of slowly permeating gas used for ultrasonic imaging contrast. Investigative Radiology 1995; 30(5)315–321
- Kerschen, A. 2007. Universal COM Logger software (communications port data logger), Tucson, AZ.
- Lowe K.C. Perfluorochemicals in medicine. Chemistry and Industry 1991; 3(83)1–6
- Toft K.G., Hustvedt S.O., Hals P.A., Oulie I., Uran S., Landmark K, Normann P.T., Skotland T. Disposition of perfluorobutane in rats after intravenous injection of Sonazoid. Ultrasound in Med. Biol. 2006; 32(1)107–114