Abstract
The aberrant vascular architecture in solid tumors is the key limiting factor known to ameliorate hypoxia and increase circulating anticancer drugs, thus resulting in resistance to radiotherapy and chemotherapy in tumor treatment. Previous experiments have reported hemoglobin-based oxygen carriers (HBOCs) that are effective to improve tumor oxygenation, thereby serving as potential agents target-oriented to the hypoxic tumor. Herein, we draw the hypothesis that HBOCs combined with an anticancer drug may increase oxygen bioavailability and anticancer drug retention in solid tumors and in turn contribute to enhanced sensitivity of radiotherapy and chemotherapy. This novel drug will bring a new breakthrough in the field of the development of anticancer drugs and reveal the alternative clinical use of HBOCs in tumor treatment.
INTRODUCTION
Solid tumors are characterized by deregulated angiogenesis, which may result in highly disorganized, twisted, excessive branching, stenotic, and uneven diameter vessels, then lead to hypoxia Citation1–3. In addition to surgical excision, radiotherapy and chemotherapy are commonly accepted as the most effective treatments for solid tumors, and have been introduced into the clinics to treat tumors of different origins and stages. As we know, the efficacies of radiotherapy and chemotherapy are modulated by local oxygen (O2), partial pressure (PO2), and anticancer drug accessibility to tumor. Hypoxia in solid tumors not only reduces radiosensitivity, but also leads to resistance to most anticancer drugs, and appears to accelerate malignant progression and increase metastasis Citation[4]. Moreover, the aberrant vascular architecture in solid tumors may greatly limit anticancer drug delivery. Taken together, sufficient O2 supply as well as adequate anticancer drug delivery in solid tumors are critically important to enhancement of the sensibility of radiotherapy and chemotherapy.
Hemoglobin-based O2 carriers (HBOCs) were initially assessed as blood substitutes Citation[5], Citation[6]. Previous studies have reported HBOCs are also salutary to increase tumor oxygenation and radiation sensitivity Citation[7], Citation[8]. Hence, it is reasonable to consider that HBOCs are potentially target-oriented to tumor to delivery O2, which can be explained by their promising O2 transporting capacity, lower viscosity, and smaller mean diameter as compared to human red blood cells Citation9–11. In addition, the PO2 gradient between hypoxic tumor and healthy tissue can further promote HBOCs into the tumors.
THE HYPOTHESIS AND ITS PROOFS
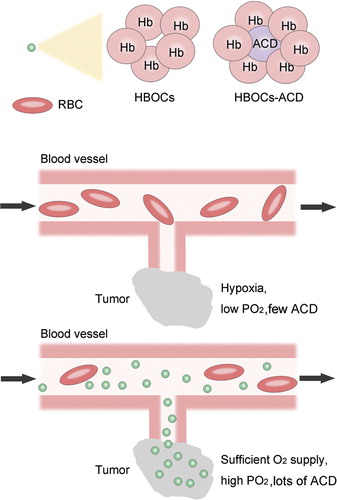
Based on the notion that HBOCs are theoretically target-oriented to hypoxic tumor and can improve tumor tissue PO2, we hypothesize that HBOCs combined with the anticancer drug may jointly enhance the sensitivity of radiotherapy and chemotherapy to solid tumors. HBOCs here serve as vehicles for the delivery of O2 and anticancer drug to tumors, thereby increasing O2 bioavailability and anticancer drug retention in tumors at the time of radiotherapy and chemotherapy. The combination between HBOCs and anticancer drug is feasible because there are many reactive groups on the hemoglobin and the research of HBOCs combined with antioxidants, superoxide dismutase (SOD), and catalase (CAT) has been reported Citation[5]. illustrates the theoretic feasibility and advantages of this hypothesis. However, it is noteworthy that, when designing such a novel drug, we should make sure the O2 transporting capacity of HBOCs is not damaged by the combined anticancer drug, including avoiding direct O2 scavenging and steric effect to reduce O2 affinity.
EVALUATION OF THE HYPOTHESIS
The theoretic feasibility and advantages have been described and evaluated above. Generally speaking, the experimental evaluation of this hypothesis can be conducted as follows. First, a solid tumor, such as liver cancer, lung cancer, or breast cancer, and corresponding anticancer drug are selected. Second, the combination of HBOCs and this drug is performed by use of a chemical or biological method. Finally, intravenous injection of the obtained compound and radiation therapy are administred to the animal model of solid tumor, the measured parameters including the PO2 and anticancer drug concentration in tumor tissue, tumor growth, tumor metastasis, mortality rate, and so on.
IMPLICATIONS
If the hypothesis that HBOCs combined with an anticancer drug enhance the sensitivity of radiotherapy and chemotherapy to tumors is demonstrated to be true, it may be the new hope and of greater value for patients suffering from solid tumors. This novel drug would not only improve the efficacy of standard care, but also allow radiation dose reduction to limit the toxicity to healthy tissues. Furthermore, if this hypothesis is practical, it will also bring a new breakthrough in the field of development of HBOCs-based anticancer drug and reveal the alternative clinical use of HBOCs in tumor treatment.
CONCLUSION
We believe that HBOCs combined with an anticancer drug is a promising carrier for delivery of O2 and the anticancer drug to solid tumors, therefore increasing the sensitivity of radiotherapy and chemotherapy in tumor treatment. The resulting improvement in therapeutic outcomes will be useful and practical in clinical situations.
Acknowledgements
This work was supported by the National Nature Science Foundation of China, Beijing, China (Grant No. 30801083 and Grant No. 30772084). Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hanahan D., Weinberg R.A. Cell. 2000; 100: 57–70
- Bergers G., Benjamin L.E. Nat Rev Cancer. 2003; 3: 401–410
- Sonveaux P. Radiotherapy and Oncology 2008; 86: 300–313
- Brown J.M. Cancer Res. 1999; 59: 5863–5870
- D'Agnillo F., Chang T.M. Nat Biotechnol. 1998; 16: 667–671
- Burmeister M.A., Rempf C., Standl T.G., Rehberg S., Bartsch–Zwemke S., Krause T., Tuszynski S., Gottschalk A., Schulte am Esch J. Br J Anaesth. 2005; 95: 737–745
- Robinson M.F., Dupuis N.P., Kusumoto T., Liu F., Menon K., Teicher B.A. Artificial Cells, Blood Substitutes, and Biotechnology 1995; 23: 431–438
- Nozue M., Lee I., Manning J.M., Manning L.R., Jain R.K. J Surg Oncol. 1996; 62: 109–114
- McNeil C.J., Smith L.D., Jenkins L.D., York M.G., Josephs M.J. J Trauma. 2001; 50: 1063–1075
- Standl T., Freitag M., Burmeister M.A., Horn E.P., Wilhelm S., Am Esch J.S. J Vasc Surg. 2003; 37: 859–865
- Li T., Yu R., Zhang H.H., Wang H., Liang W.G., Yang X.M., Yang C.M. Artif Cells Blood Substit Immobil Biotechnol. 2006; 34: 175–188