Abstract
Objective: To investigate whether it is feasible to use the chondrogenic microenvironment provided by cartilage cells to construct cartilage tissues in vitro with bone marrow stromal cells (BMSC). Materials and Methods: We isolated and cultured BMSC and cartilage cells from Sprague Dawley rats (SD rats). The supernatant of cartilage culture was used as inducing solution to cause differentiation of BMSC from the second generation of cells cultured in vitro. Cells were examined seven days later, using immunohistochemistry to determine the expression of collagen specific to type II cartilage. RT-PCR was used to detect the expression of type II collagen and aggrecan mRNA. BMSC and cartilage cells were isolated from SD rats and cultured in vitro. The BMSC and cartilage cells in culture were mixed evenly in an 8:2 ratio and inoculated into a polyglycolic acid/polylactic acid (PGA/PLA) scaffold to a final concentration of 5.0×107 cells/ml. PGA/PLA preparations with pure cartilage cells or pure BMSC served as the positive and negative controls, respectively. The control group of low-concentration cartilage cells consisted of PGA/PLA preparations containing cartilage cells at 20% of the abovementioned concentration (1.0×107 cells/ml). Samples were collected eight weeks later, at which time general observations, wet weight, and glycosaminoglycan (GAG) levels were determined, and histological and immunohistochemical examinations were performed. Results: Immunohistochemistry showed the induction of BMSC type II collagen, and RT-PCR indicated the expression of type II collagen and aggrecan mRNA. In the mixed-cell group and the positive control group, pure mature cartilage cells were produced after eight weeks of culture in vitro, and the size and shape of the scaffold were maintained throughout the culture period. The two groups gave rise to newly generated cartilage cells essentially identical in appearance and histological properties. The immunohistochemical results showed that the cartilage cells of both groups expressed abundant cartilage-specific type II collagen. The average wet weight and GAG content were more than 70% of the values in the positive control group. Only an extremely small amount of immature cartilage tissue formed in local regions in the BMSC-only sample, and the scaffold was obviously shrunken and deformed. Although the wet weight of newly generated cartilage tissue in the low-concentration cartilage cell sample reached 30% of the value of the positive control group, the scaffold was obviously shrunken and deformed. Only regional and discontinuous cartilage tissues were formed, and the amount of newly generated cartilage was obviously less than in the co-culture and positive control groups. Conclusions: Cartilage cells can provide a microenvironment for cartilage formation to some extent, and also effectively induce BMSC to differentiate into cartilage cells and form tissue-engineered cartilage in vitro.
INTRODUCTION
Cartilage defects or damage are a constant problem in surgical clinics. Cartilage tissue engineering may provide a new method for replacing defective or damaged cartilage Citation[1]. However, the scarcity of cartilage sources severely inhibits the application of cartilage tissue engineering in clinics. Auto-cartilage cells are limited in non-functional areas, and cartilage collection causes great damage to the human body Citation[2]. In addition, cartilage allografts are vulnerable to immunological rejection, and they cannot survive in the human body for long.
In recent years, research has focused on the differentiation of bone marrow stromal cells (BMSC) into cartilage cells, and many growth factors have been applied to induce BMSC to differentiate into cartilage cells Citation[3], Citation[4]. The main problem with this method is that a large amount of growth factor is needed, making the procedure too expensive for large-scale production of cartilage. An early study indicated that it is very important for BMSC to differentiate into cartilage cells and chondrificates Citation[5]. In this study, we investigated whether it is feasible to use the chondrogenic microenvironment provided by cartilage cells to construct cartilage tissues in vitro using bone marrow stromal cells. The cartilage tissues obtained in this way were compared with cartilage tissues constructed on their own in vitro.
MATERIALS AND METHODS
Preparation and Culture of BMSC
The femur and tibia from SD rats were collected under sterile conditions. The marrow cavity was flushed with Dulbeco's modified eagle's medium (DMEM). Bone marrow was centrifuged and inoculated using published methods for culturing bone marrow Citation[6]. DMEM supplemented with 10% calf serum was added (Gibco, Grand Island, NY, USA), and the mixture was directly inoculated into a culture dish at a concentration of 6×105 nucleated cells/cm2. Cells (P0) were cultured at 37°C with 50 ml/L CO2 and 100% saturated humidity. When 90% of the cells in the P0 culture had fused, digestion passage was performed with 0.25% trypsin (containing 0.02% EDTA) and the resulting cultures were marked as P1. The next passage was performed after cell fusion, and cultures were marked P2. Subsequent passages were performed as described above.
Preparation and Culture of Cartilage Cells
Rib cartilage was collected from both sides of the SD rats under sterile conditions, cut into tissue blocks of 1 mm3, then digested with 0.2% type II collagenase for 8-12 h. The solution was filtered, centrifuged, and washed, and cells were counted. Cells were then resuspended in DMEM containing 10% calf serum, and inoculated into a culture dish at 1×106 cells/60 mm. Cells were cultured at 37°C in a 5% CO2 atmosphere with 100% saturated humidity. After the cells grew to the point of fusion, they were digested and collected.
Induction of BMSC Differentiation into Cartilage Cells
Cellular supernatant was collected from P1, filtered and sterilized, and stored at 4°C. When 80% of the P1 BMSC had fused, the cells were digested and collected, and inoculated into a culture dish at 1.5×104 cells/cm2. A glass slide was placed in the culture dish prior to inoculation, and the dish was stored overnight with DMEM containing 10% calf serum. After the cells had attached, the culture solution was aspirated and the supernatant from cartilage cells culture was added to induction differentiation. This was the induction group. The non-induction group consisted of BMSC cultured without the addition of cartilage cells. Half of the medium was changed daily, and the slide was taken out after seven days of culture. The glass slide was fixed with 95% cold acetone, and subjected to immunohistochemistry. The rest of the cells were collected in a culture dish for use in RT-PCR analysis.
Preparation of PLA/PGA Compound Materials
PGA (10 mg) was uniformly loaded into the prepared silica gel column (internal diameter, 9 mm; height, 3 mm), 0.3 ml of PLA (1% in dichloromethane) was added dropwise onto the PGA fibers, and fibers were removed after they had dried naturally, which were prepared into a compound with the PLA/PGA scaffold (diameter 9mm, height 3mm). The compound material was immersed in 75% ethanol for 30 min, washed twice with PBS, and dried and stored for later use.
Experimental Grouping and Culture In Vitro
The experiment consisted of four groups. In Group A, BMSC and cartilage cells were mixed evenly in the ratio 8:2, and inoculated into PLA/PGA scaffold at a final concentration of 5.0×107 cells/ml. In Group B, pure cartilage cells of the same concentration were inoculated. Group C contained pure BMSC of the same concentration. In Group D, cartilage cells were inoculated to a final concentration of 1.0×107 cells/ml; this group served as the control. All the groups were analyzed during the eighth week of culture in vitro.
Analysis
Determination of type II collagen
After seven days of induction, slides were taken out and immunohistochemical staining of type II collagen was performed on the induction group and the non-induction group. Cartilage cells were used as the positive control. The expression of type II collagen in all groups was examined using rabbit anti-mouse type II collagen monoclonal antibody (diluted 1:100) and the PV two-step immunohistochemistry kit (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd).
RT-PCR analysis
Trizol was used to extract total mRNA from all groups, and reverse transcription was performed to obtain cDNA according to the manufacturer's instructions (Shanghai Bioengineering Co., Ltd.). Col II and aggrecan primer sequences were designed and synthesized: upstream Col II primer, 5’-GTCCAGATGACTTTCCTCCGTC-3’; Col II downstream primer, 5’-TGTCCACACCAAATT CCTGATC-3 (PCR product: 325 bp); aggrecan upstream primer, 5’-GGAATCCCT AGCTGCTTAGCAG-3; aggrecan downstream primer, 5’-GAGTCATTGGAGCGAAGGTTC-3’ (PCR product: 458 bp). PCR conditions were as follows: for Col II, 95°C for 3 min, then 30 cycles of 95°C for 30 sec, 64°C for 30 sec, 72°C for 1.5 min, then 72°C for 10 min; for aggrecan, 95°C for 3 min, then 30 cycles of 95°C for 30 sec, 60°C for 30 sec, 72°C for 1.5 min, then 72°C for 10 min. PCR products were analyzed by electrophoresis in 1% agarose gels.
General observations
The in vitro cultures were observed for changes in size, shape, quality, and color of the cell-material compound, and results were compared between groups.
Determination of wet weight
After eight weeks of cell culture, the wet weight of new tissue in each group was determined, and results were compared between groups.
Histological determination
The newly formed tissues were fixed with 10% formaldehyde for 24 h, embedded in paraffin, sliced and subjected to staining with HE, Safranin O, Massion, or Alcian blue.
Determination by immunohistochemistry
The expression of type II collagen in all groups was examined using rabbit anti-mouse type II collagen monoclonal antibody (diluted 1:100) and the PV two-step immunohistochemistry kit (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd).
Determination of GAG content
Alcian blue staining was used to determine GAG content in all samples, and the results for different groups were compared.
RESULTS
Determination of Type II Collagen
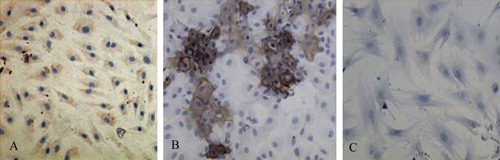
In the induction group, type II collagen immunohistochemistry showed that cells with cytoplasm stained brown were positive for type II collagen (A), whereas no cells in the non-induction group tested positive for type II collagen (B).
RT-PCR
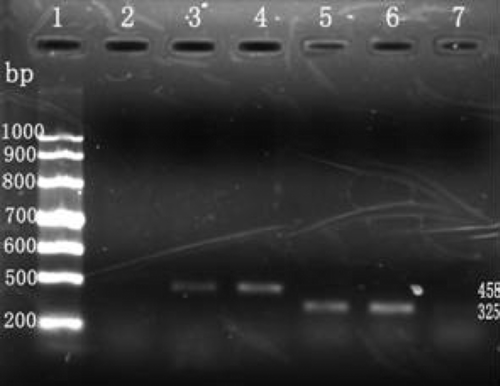
After the inducer cells were amplified by RT-PCR, two distinctive bands could be observed, one between 300 and 500 bp and another migrating near this region. These results suggest that the induced cells express both type II collagen and aggrecan mRNA, whereas no such expression was observed in the control group ().
Figure 2. Detection of collagen type II and aggrecan mRNA expression by RT-PCR. 1: RNA marker; 2: Col II expression of BMSCs without induction; 3: Col II expression of induced cells; 4: Col II expression of chontrocytes; 5: aggrecan expression of induced cells; 6: aggrecan expression of chontrocytes; 7: aggrecan expression of BMSCs without induction.
Combination of Cell Material and Culture
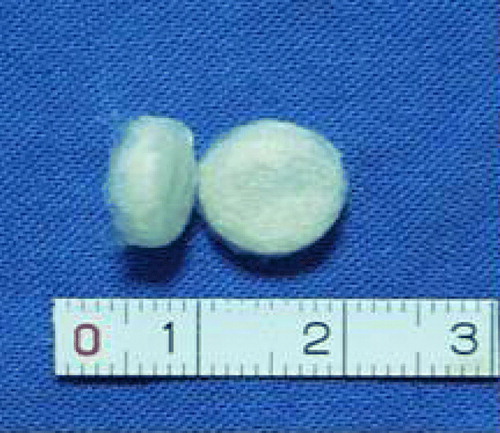
After the PGA/PLA scaffold was dried and removed from the silica gel model, the column shape was maintained (), and the material showed good hydrophilicity following sterilization with ethanol and washing with BPS. The cells adhered tightly to the bracket after inoculation ().
General Observations
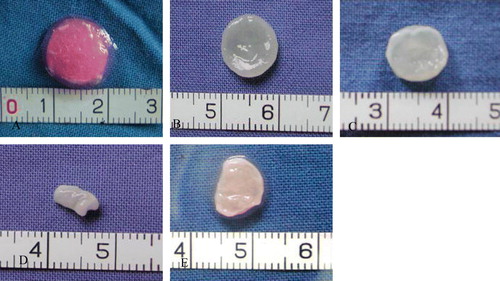
The morphology of the initial cell-material compound is shown in A. After eight weeks of culture in vitro, the compounds in group A (co-culture group, B) and group B (positive control, pure cartilage cells at the same concentration, C) showed the same diameter and thickness as the original material, a normal shape, a white ivory appearance, and elasticity. In group C (less concentrated cartilage cell group, E), the structure was relatively loose, the whole compound structure was visibly shrunken and deformed, and it showed no elasticity.
Histological Examination
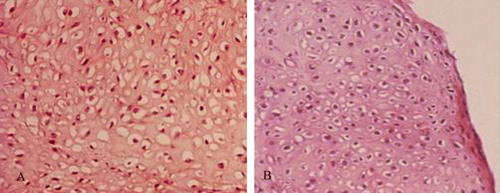
HE staining showed that by the eighth week, the histological characteristics of the co-culture group (A) and positive control group (B) had been established. All of the cultures contained mature and continuously linked cartilage tissues, and many mature cartilage lacunae could also be observed. The scaffold materials had been degraded, and no fiber-like tissues had formed. The BMSC group (C) mainly showed fibrous tissue, the structure was very loose, and a small amount of cartilage-like tissue had formed along the edges of the culture. Although the cartilage lacunae were not distinctive, the color of the extracellular matrix was similar to that of cartilage. The less concentrated cartilage cell group (D) showed mature cartilage tissues along the edges, but the succession of cartilage was poor, and the tissue density and amount of newly generated cartilage were clearly lower than in the culture group.
Figure 6. Histology of specimens in all groups cultured in vitro for 8 weeks. A: co-culture group; B: Chondrocyte group; C: BMSC group; D: Low chondrocytes group.
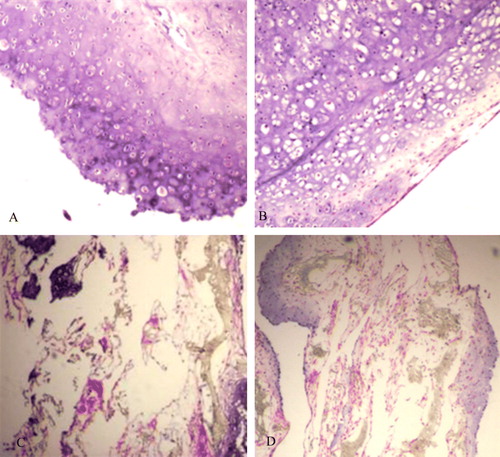
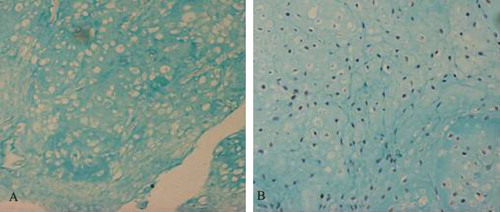
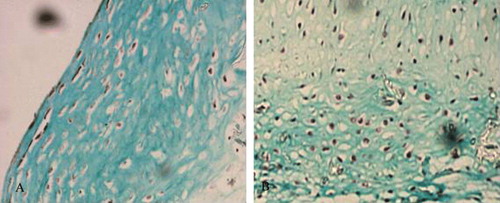
The other stains provided additional information. Safranin O staining revealed no obvious difference between the co-culture group (A) and the positive control group (B). The extracellular matrix was stained deep red and many vacuole-like cartilage lacunae were visible. Massion staining revealed a large number of green collagen sediments in the co-culture group (A) and the positive control group (B). The nuclei were stained black, and many mature cartilage lacunae were visible. Alcian blue staining showed no obvious difference between the co-culture group (A) and the cartilage cell group at the same concentration (B). Cartilage lacunae were obvious, and the extracellular matrix was stained blue and reflected active secretion by the cultured cells.
Figure 7. Massion strain of some specimens in vitro for 8 weeks. A: co-culture group; B: Chondrocyte group.
Immunohistochemistry
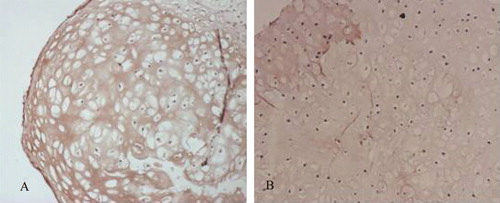
Type II collagen immunohistochemistry staining in the eighth week indicated that in the co-culture and cartilage cell groups, strong positive reactions (brown yellow) occurred in the areas surrounding cartilage lacunae, indicating robust secretions of cartilage protein-specific type II collagen. In the group with the lower cartilage cell concentration, strong reactions were observed only in local areas, while there was only a weak expression of type II collagen along the compound borders in the BMSC group ().
Determination of Wet Weight
Except for groups C and D, significant differences (multi-sample t test) existed between all combinations of other groups (P<0.05). Group D, which served as the control to the group with low cartilage cell concentration, had a wet weight that was approximately 30%-50% of that of group B.
Determination of GAG Content in Newly Generated Cartilage
Except for groups C and D (multi-sample t test), significant differences existed between all pairings of the other groups (P<0.05).
DISCUSSION
Many studies have demonstrated that BMSC can express large amounts of cartilage extracellular matrix after being jointly induced with dexamethasone and transforming growth factor β1 (TGFβ1). After the induced BMSC and PGA/PLA scaffolds are implanted into the affected joint, the articulation injury can be completely restored. When the same cells are injected subcutaneously into animals, fibrous tissues form with obvious vascularization Citation[5], Citation[7], Citation[8]. These results suggest that the chondrogenic microenvironment is potent for inducing the differentiation of BMSC into cartilage cells and the formation of cartilage tissues.
The results of this study indicate that the supernatant from cartilage cell cultures can induce BMSC to transform into cartilage cells. Immunohistochemical examination of type II collagen after seven days of induction showed that the cartilage cells had secreted an abundance of cartilage-specific type II collagen into the extracellular matrix. RT-PCR was used to detect the expression of type II collagen and aggrecan mRNA in cells before and after the experiment. The results indicate that type II collagen and aggrecan mRNA are expressed in the induction group, but not in the control group. These results strongly suggest that BMSC differentiated into cartilage cells after induction.
We co-cultured 20% cartilage and various amounts of BMSC on 3D scaffolding, leading to the formation of mature cartilage tissue by the eighth week of culture. Compared to the newly-generated cartilage produced by pure cultures of cartilage cells at the same concentration as in the co-cultures, the newly produced cartilage cells in the co-cultures all maintained the primary shape and size of the scaffold and a certain level of elasticity. Many mature cartilage lacunae were observed in histological and immunohistochemical examinations, and cells secreted abundant extracellular matrix showing the characteristics of cartilage-specific matrix. Our measurements found that both the wet weight and GAG levels in the co-culture group reached 70-80% of the levels of the positive control group.
In contrast, in the control group little or no cartilage formed when the biological materials were inoculated with only BMSC or only cartilage cells at a lower concentration than in the co-culture group. These results indicate that, without induction by cartilage, BMSC have little tendency to form mature cartilage in vitro. Furthermore, when a small number of cartilage cells is used to induce the BMSC, complete cartilage tissues cannot be constructed in vitro. In the co-culture group, the quality and quantity of newly produced cartilage were all significantly higher than in the low-concentration cartilage cell group; the two groups had the same numbers of cartilage cells, but the co-culture group also had BMSC. This demonstrates that a small relative number of cartilage cells in vitro (20%) can induce a large proportion of BMSC (80%) to mature into cartilage cells and form cartilage tissue. This result also provides robust confirmation that cartilage cells can induce BMSC to differentiate into cartilage cells and form mature cartilage tissues. This will reduce the amount of cartilage cells needed in the construction of cartilage and obviates the need for expensive growth factors.
Previous studies have shown that the chondrogenic microenvironment can strongly affect the differentiation of BMSC into cartilage, and that even without induction by growth factors, BMSC can form mature cartilage tissues in the affected joints Citation[8]. Therefore, detailed analysis of induction by the articulatory microenvironment will be helpful in understanding how cartilage cells induce BMSC to differentiate into cartilage. Recently, studies have demonstrated that co-culture of sheep MSCs with synovial cells30 or human MSCs with bovine chondrocytes Citation[11] can induce chondrogenesis. Similarly, human embryonic stem cells (hESCs) can be induced to differentiate to osteoblasts when co-cultured with bone-derived cells Citation[12] or to chondrocytes in the presence of limb bud cells, precursors of chondrocytes Citation[13]. This response is not confined to chondrocytes, because cardiomyocytes were generated when hESCs were co-cultured with visceral endodermal-like cells in serum-free cultures, and CD34+ hESCs efficiently produced hematopoietic cells when co-cultured with OP9 mouse stromal cells [Citation14]. These co-culture experiments all used stem cells: human embryonic or mesenchymal. In the articulatory environment, factors that affect cartilage differentiation include activation factors secreted by cartilage cells or the synovium into the synovial fluid, mechanical stimulation in the movements of the articulation, and the non-vascularized state around the articulation. Several studies have indicated that cartilage cells can secrete growth factors such as TGF-β1and IGF-1, which are commonly used to induce the differentiation of BMSC into cartilage cells. These findings together provide a basis for using cartilage cells to induce the differentiation of BMSC.
In general, the microenvironment provided by cartilage cells strongly induces the differentiation of BMSC, and the relevant inducing factors including diverse secreted soluble factors, extracellular matrix proteins, and cell surface molecules. If through further study we can completely reproduce the chondrogenic microenvironment in the joint, perhaps by providing mechanical stimulation with a biological reactor, we may be able to improve the degree of maturation and the mechanical characteristics of the cartilage constructed through co-culture in vitro.
Acknowledgement
Declaration of interest: The authors declare no conflicts of interest.
References
- Langer R., Vacanti J.P. Tissue engineering. Science 1993; 260: 920–926
- Rahfoth B., Weisser J., Sternkopf F., et al. Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthritis Cartilage 1998; 6(1)50–65
- Grigoriadis A.E., Heersche J.M., Aubin J.E., et al. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: Effect of dexamethasone. J Cell Biol 1988; 106: 2139–2151
- Pittenger M.F., Mackay A.M., Jaiswal S.C., et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411)143–147
- Zhou G.D., Wang X.Y., Miao C.L., et al. Repairing porcine knee joint osteochondral defects at non-weight bearing area by autologous BMSCs. Natl Med J China 2004; 84: 925–931
- Friedenstein A.J., Chailakyan R.K., Gerasimov U.V., et al. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet 1987; 20: 263–272
- Butnariu-Ephrat M., Robinson D., Mendes D.G., et al. Resurfacing of goat articular cartilage by chondrocytes derived from bone marrow. Clin Orthop 1996; 330: 234–243
- Im G.I., Kim D.Y., Shi J.H., et al. Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells fron bone marrow. J Bone Joine Surg Br 2001; 83: 289–294
- Pedrozo H.A., Schwartz Z., Gomez R., et al. Growth plate chondrocytes store latent transforming growth factor (TGF)-beta 1 in their matrix through latent TGF-beta 1 binding protein-1. J Cell Physiol 1998; 177: 343–354
- Bhaumick, B. Insulin-like growth factor secretion by culured chondrocyte cells: identification, characterization and ontongeny during cell differentiation. Regul Pept, 48: 113–122.
- Ahn S.E., Kim S., Park K.H., Moon S.H., Lee H.J., Kim G.J., Lee Y.J., Park K.H., Cha K.Y., Chung H.M. Primary bone-derived cells induce osteogenic differentiation without exogenous factors in human embryonic stem cells. Biochem. Biophys. Res. Commun. 2006; 340: 403
- Sui Y., Clarke T., Khillan J.S. Limb bud progenitor cells induce differentiation of pluripotent embryonic stem cells into chondrogenic lineage. Differentiation 2003; 71: 578
- Chen J., Wang C., Lu S., Wu J., Guo X., Duan C., Dong L., Song Y., Zhang J., Jing D., Wu L., Ding J., Li D. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 2005; 319: 429
- Passier R., Oostwaard D.W., Snapper J., Kloots J., Hassink R.J., Kuijk E., Roelen B., de la Piviere A.B., Mummery C. Increased cardiomyocyte differentiation from human embryonicstem cells in serum-free cultures. Stem Cells 2005; 23: 772