Abstract
There is a great demand for using phytases to reduce phytate content in animal feed stuffs and food for human consumption. Industrial application of phytase requires efficient methods to immobilize the enzyme, yielding a biocatalyst with high activity and stability compared to the free enzyme. In the present study, phytase was immobilized on Sepabead EC-EP and then used in the biodegradation of soymilk phytate. The immobilized enzyme exhibited an activity of 0.1 U per g of carrier and activity yield of 70.83%. Optimum temperature and pH for the immobilized enzyme were 55°C and 5.5, respectively. Both the enzymes were stable between pH 3.0–8.0 and below 70°C. Kinetic parameters(Km and Vmax) and the usability of the immobilized enzyme were determined. The immobilized enzyme hydrolyzed 65% of soymilk phytate in 8 h at 60°C, as compared with 56% hydrolysis observed for the native enzyme over the same period of time.
INTRODUCTION
Phytate is the principle storage form of phosphorus comprising 1–5% by weight in cereals, legumes, oil seeds, and nuts. It may have benefical and/or deleterious effects on human and animal nutrition. Phytate and inositol intermediates have been implicated in blood glucose response, lowering of cholesterol, and triglycerides, in tumor formation, in the treatment of Parkinson's disease, Alzheimer's disease, multiple sclerosis, anticancer properties, and preventive effects against heart diseases. The typical negative effect known is the binding of divalent cations such as magnesium, calcium, zinc, and iron forming insoluble complexes, hence reducing their bioavailability Citation[24], Citation[30]. Legumes provide a large amount of protein, carbohydrates, dietary fiber, minerals, and water-soluble vitamins in human diets. In some areas of the world, where the predominant diet pattern is vegetarian or animal meat is only available in small amounts, legumes provide the major source of proteins. Therefore, legumes can be considered as foods with health benefits, but their phytate contents can limit the availability of minerals Citation[7]. A large portion of the world's population ingests a high level of phytate from the plant staple foods, suffering from iron and zinc deficiences. In addition, infant soy formula and other soy protein products also contain high levels of phytate Citation[19]. The monogastric animals, including man, are unable to utilize phytate phosphorus either due to lack of or insufficient amount of phytate degrading enzymes in their gastrointestinal tract Citation[10], Citation[31].
Phytases are a class of phosphatases that catalyze the sequential hydrolysis of phytate to less phosphorylated inositol phosphates and, in some cases, to inositol Citation[21], Citation[23]. Adding phytases in food for human consumption may diminish anti-nutritional effects of phytate and makes phytases very important for biotechnological applications, especially for the reduction of phytate in food and feedstuffs Citation[17]. Theoretically, an “ideal” phytase should be catalytically efficient, proteolysis resistant, thermostable, and cheap Citation[19].
The advantages of immobilized enzymes over soluble enzymes arise from their enhanced stability and ease of separation from the reaction media, leading to significant savings in enzyme consumption Citation[4]. For the industrial development of biocatalytic process an effective immobilization method is commonly required to allow the reuse of enzymes or continuous processing. Covalent immobilization has the advantage of forming strong and stable linkages between the enzyme and the carrier that result in robust biocatalysts Citation[28].
Epoxy-activated supports have been proposed as very efficient materials for the immobilization of proteins at the industrial scale for different reasons: e.g. high stability of the groups at neutral pH values even in wet conditions, commercial supports can be stored for long periods of time, high stability of the enzyme-support bonds, possibility of performing a final blocking of the remaining groups, and possibility of achieving stabilization of the enzymes via multipoint covalent attachment. Immobilization of proteins on commercial epoxy supports follows a two-step mechanism: first, the enzyme is hydrophobically adsorbed on a fairly hydrophobic support (e.g., Eupergit, Sepabeads) at very high ionic strength, and then, the covalent reaction between the enzyme and the support proceeds Citation[20], Citation[26]. Sepabeads EC are polymethacrylate-based carriers for enzyme immobilization. The series Sepabead EC-EP are epoxy activated, with high reactive group density. The chemistry for attachment of the enzyme to the support is straightforward. Compared with other epoxy acrylic polymers, Sepabeads EC-EP possess a high mechanical stability and do not swell in water. Furthermore, the raw materials applied for the production of these supports are included in the EU list of resins for the processing of foodstuffs Citation[9], Citation[14].
Soybean, a concentrated source of isoflavones, has received considerable attention for its potential role in preventing and treating cancer and osteoporosis. Moreover, soymilk is considered as a low-cost substitute for cow's milk in developing countries as a nutritive supplement for lactose-intolerant infants and children Citation[10], Citation[27].
In the present investigation, an active phytase was isolated and partially purified from avocado at first and then immobilized on Sepabead EC-EP. The soluble and immobilized phytases were chemically characterized. For this aim, some parameters affecting the enzyme activity (pH, temperature, substrate concentration, and effector concentration) were searched and the stability tests (pH, thermal, storage, operational) were done. Beside these, usability of the immobilized enzyme was also tested for the hydrolysis of phytate in soymilk and compared with the soluble enzyme.
MATERIALS AND METHODS
Materials
Sepabead EC-EP was kindly provided by Resindion S.R.L. (Mitsubishi Chemical Co., Milan, Italy). Avocado and soy flour were obtained from local markets. All other chemicals and reagents were of the highest available purity and used as purchased. All the solutions were prepared in deionized water.
Phytase Activity Determination
Phytase activity was determined by measuring the amount of liberated inorganic phosphate from sodium phytate Citation[15]. The assay mixture consisted of 0.4 ml of 2 mM Na-phytate (in 0.1 M pH 5.0 acetate buffer) and 0.1 ml of soluble enzyme solution. For the immobilized enzyme, assay mixture consisted of 0.4 ml of Na-phytate, 100 mg of immobilized enzyme, and 0.1 ml of 0.1 M acetate buffer (pH 5.0). After incubation for 30 min at 37°C, the reaction was stopped by adding 0.5 ml of 15% (w/v) trichloroacetic acid. Reaction mixture centrifugated at 10000 rpm for 10 min. The liberated phosphate was determined spectrophotometrically at 660 nm using the method as reported previously Citation[6].
One unit (1U) of phytase activity was defined as 1 µmol inorganic phosphate released per min under conditions explained above.
Protein Determination
The total proteins originally taken for immobilization and proteins present in the supernatants (unbound proteins) after immobilization were estimated by the method of Bradford Citation[1] using bovine serum albumin as a standard. The amount of bound protein was found by subtracting the unbound protein from total protein.
Analysis of Phytate
Phytate content of soymilk was measured by the modified Latta and Eskin method Citation[18]. 0.1 ml sample was taken out from the reactor and then the reaction was stopped by adding 0.2 ml of 3% (w/v) trichloroacetic acid. After dilution with bidistilled water in a ratio 1:5 (depending on phytate content), 0.5 ml of Wade reactive (0.03% FeCl3. 6 H2O and 0.3% sulphosalisilic acid in bidistilled water) was added. The sample centrifugated at 4000 rpm for 2 min and then phytate content measured spectophotometrically at 500 nm.
Extraction of Phytase from Avocado
Avocado was separated from the outer covering and then ground in a blender in ice-cold with 0.1 M sodium acetate buffer (pH 5.0). Homogenate was filtered from a fine muslin. The cell debris was removed by centrifugation at 10000 rpm, at 4°C for 10 min, and then treated to 80% ammonium sulfate saturation. The precipitate was collected by centrifugation at 10000 rpm for 10 min, suspended in 100 mM acetate buffer (pH 5.0), and then dialyzed against 20 mM acetate buffer (pH 5.0). The dialysed enzyme was analyzed for phytase activity.
Immobilization of Phytase on Sepabead EC-EP
Sepabead EC-EP (10 g) was previously washed with distilled water, suspended in 60 ml of 0.1 M acetate buffer (pH 5.5), and then 7.5 ml of enzyme solution was added. The reaction mixture was first incubated for 18 h at room temperature with roller shaking and then incubated at room temperature for another 24 h without shaking. After this reaction period, the immobilized enzyme conjugate was separated by filtration. In order to remove unbound proteins, it was washed with 40 ml of 20 mM acetate buffer (pH 5.5). To completely block the residual epoxy groups, the enzyme derivative was incubated in 3 M glycine solution for 24 h at 4°C and then stored at 4°C in fresh buffer until use.
Physico-chemical Properties of Free and Immobilized Phytase
Effect of temperature on phytase activity and stability
In order to determine the temperature profiles for the free and immobilized phytase, activity assays were carried out over the temperature range of 4–80°C. The thermal stabilities of free and immobilized enzyme were determined by measuring the residual activity of the enzyme exposed to different temperatures in acetate buffer (0.1 M, pH 5.0) for 30 min with continious shaking. After desired incubation periods, enzyme aliquots were withdrawn and assayed at optimal assay conditions to determine the residual phytase activities.
Effect of pH on phytase activity and stability
The influence of pH on activity of free and immobilized phytase was studied by varying the pH from 2.5 to 9.0 at 37°C. The buffers (0.1 M) used were Glycine/HCl (pH 2.5–3.5), Na-acetate/Acetic acid (pH 4.0–6.5) and Tris/HCl (pH 7.0–9.0). In order to determine pH stability, the free and immobilized enzymes were incubated in above buffers for 3 h at 4°C and then the remaining activities were assayed under standard activity assay conditions.
Effect of substrate concentration
The study of the substrate concentration influence in the enzymatic activity was carried out, determining the initial rates of the hydrolysis reaction, with the initial concentration of sodium phytate ranging from 0.5 to 10 mM, in a temperature range of 37°C. Kinetic parameters include the maximum reaction rate (Vmax) of the enzymatic reaction and the Michaelis-Menten constant (Km) where the latter defines the affinity of enzyme towards its substrate. These parameters were obtained from Lineweaver-Burk plot, which is a plot of 1/V against 1/[S] for systems obeying the Michaelis-Menten equation. The graph being linear can be extrapolated anywhere approximating to a saturating substrate concentration, even if no experiment has been performed, and from the extrapolated graph the values of Km and Vmax can be determined.
Reusability
Reusability experiments were performed at 37°C under batch opreration mode. After incubation, immobilized enzyme containing support was removed from the reaction medium and washed three times with acetate buffer (0.1 M, pH 5.0). Activity was determined in the same manner as in the enzyme assay.
Storage stability
The free and immobilized phytase were stored in acetate buffer solution (100 mM, pH 5.0) at 4°C. The storage stability of the free and immobilized phytase was tested by determining the remaining activity of immobilized enzyme periodically during 8 months of incubation at 4°C.
Effect of effector concentration
The influence of various effectors (FeCl3.6H2O, citric acid, CaCl2, MgCl2.6H2O, CuSO4.5H2O, Tartarat, Na2MoO4.2H2O, NaF, ZnSO4.7H2O, MnCl2.4H2O) in concentrations of 0.1–2.5 mM on enzyme activity was investigated by preincubating the phytase with different compounds for 10 min at room temperature. Residual activity was calculated against control.
Biodegradation of Soymilk Phytate
Preperation of soymilk
Dried soybeans obtained from a local market were ground to flour and defatted with n-hexane in a ratio of 1:1 (w/v). The fat-free soybean flour was suspended in 10 volume of distilled water and heated to boiling. Undissolved residue was seperated from soymilk by centrifugation for 5 min at 5000 rpm. The supernatant containing soymilk was stored at 4°C for a short period until further use and diluted 3 times before usage Citation[22].
Phytate degradation in batch stirred-tank reactor
Hydrolysis of soymilk phytate was carried out in batch stirred-tank reactor at 60°C using free and Sepabead EC-EP immobilized phytase. Reservoir components were magnetically stirred. The initial level of phytate was determined prior to incubation and then the aliquots were withdrawn at different time intervals. By measuring the phytic acid content of samples, hydrolysis% was calculated. All treatments were again carried out in triplicate.
RESULTS AND DISCUSSION
Enzymes are biocatalysts, having some excellent properties such as high activity, selectivity, and specificity that make them advantageous compared to chemical ones. Enzyme immobilization can be defined as the fixation of the biocatalysts to insoluble solid supports. There are many advantages to attaching enzymes to a solid support. The major reasons are: (a) multiple or repetitive use of a single batch of enzymes; (b) the ability to stop the reaction rapidly by removing the enzyme from the reaction solution or vice versa; (c) immobilization did have a significant effect on stabilizing the enzyme activity against the denaturing effects; (d) the product is not contaminated with the enzyme, which is especially useful in the food and pharmaceutical industries; and (e) helping in easy recovery of the enzymes from the reaction solutions and separation of the enzymes from substrates and products Citation[29].
In the present study, phytase from avocado was immobilized covalently on Sepabead EC-EP and then characterized. Phytases catalyze the hydrolysis of phytate, the major storage form of phosphorus in the plant kingdom. They are widely distributed in nature in plants and microorganisms. Phytases are also of interest for producing defined breakdown products of phytate and may find applications in the processing of foods with improved nutritional value, health benefits, and retained sensory properties Citation[11], Citation[21]. Isolation, immobilization, and accurate characterization of phytate-degrading enzymes from plant source can facilitate obtaining effective enzymes for reducing phytate content during food processing Citation[7], Citation[12]. After the extraction of phytase from avocado, an enzyme preparate (0.14 U/ml, 0.58 mg/ml) was obtained and used for the immobilization studies. The immobilization results are given in . As is seen from the table, the yield of phytase immobilization varied significantly with the amount of loaded protein. The immobilized enzyme retained 70.83% of original specific activity with 79.31% of protein binding capacity. The activity yield of the enzyme was defined as:
Table 1. Immobilization of avocado phytase on Sepabead EC-EP
Sepabeads EC-EP is an epoxy-acrylic support with very good properties used to initially immobilize proteins via the epoxy chemistry. Sepabeads is a very interesting support for industrial applications such as immobilized biocatalysts because it has some excellent mechanical properties that allow its use in stirred tanks or bed reactors Citation[8], Citation[9], Citation[14].
Biochemical Properties of Free and Immobilized Phytase
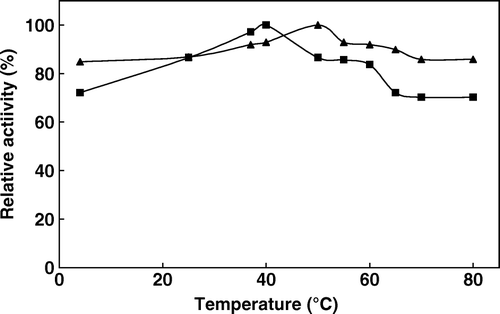
We attempt to examine the biochemical properties of the immobilized avocado phytase and compare them with those of the free enzymes. Temperature is known to enhance enzyme-catalyzed reactions. The temperature dependence of the phytate hydrolysis reaction catalyzed by free and immobilized phytase was studied in the interval from 4°C to 80°C and the results are shown in . The maximum activity was observed at 55°C for both forms of the enzyme. These results compare well with the previous reports. In general, plant phytases show high activity in the temperature range of 50–70°C and usually have an optimum temperature of 60°C Citation[2], Citation[30]. The calculated values of activation energy (Ea) obtained from Arrhenius plots (log activity versus 1/T) were equal to 8.9 kj mol−1 and 10.7 kj mol−1 for free and immobilized enzymes, respectively. These activation energy values indicate a lower sensitivity to temperature. The thermal stability of the immobilized enzyme is one of the most important criteria with respect to applications. Thermal stability experiments were carried out with free and immobilized enzymes, which were incubated in the absence of substrate at various temperatures, and the results are shown in . Free enzyme shows 92% activity at 60°C while it decreases to 86% at 80°C. Meanwhile, immobilized enzyme shows 84% activity at 60°C and the activity decreases to 70% at 80°C. The activity of the immobilized enzyme, especially in a covalently bound system, is more resistant than that of the soluble form against heat and denaturing agents. If the thermal stability of an enzyme was enhanced by immobilization, the potential utilization of such enzymes would be extensive. In principle, the thermal stability of an immobilized enzyme can be enhanced, diminished, or unchanged relative to free counterparts, and several examples of each kind have been previously reported. Thermal stability is a particularly important issue because food processing and preparation commonly involve exposure to elevated temparature Citation[13].
Figure 1. The effect of temperature on the activity of free (▴) and immobilized (▪) phytase activity (activities were assayed at indicated temperatures by using 2 mM sodium phytate prepared in 0.1 M acetate buffer at pH 5.0).
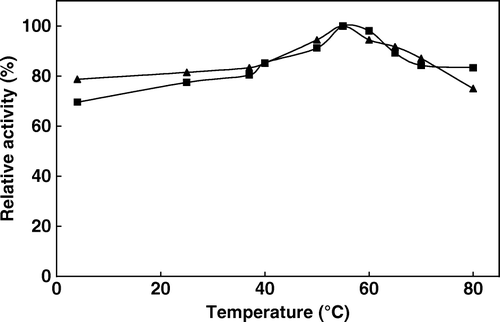
Figure 2. Thermal stability of free (▴) and immobilized (▪) phytase (after incubation at indicated temperatures activities were assayed at 37°C by using 2 mM sodium phytate prepared in 0.1 M acetate buffer at pH 5.0).
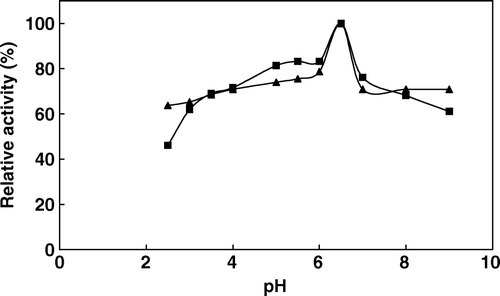
A comparative study between free and immobilized phytase was provided in terms of pH. The variation of the relative activity of the immobilized and free enzyme at different pH values is shown in , which indicates that their optimum pH values are pH 4.0 and pH 5.5, respectively. This shift is possibly due to the secondary interactions (e.g. ionic and polar interactions, hydrogen bonding) between the enzyme and polymeric matrix. The pH profiles of both enzymes were also broader in a pH range of 3.0–8.0. Similar observations upon immobilization of enzymes have been reported by other researchers. The pH optimum of the plant phytases changes in the range of pH 4.0–8.0 and usually between 4.0–5.6 [2, 12]. A rapid decline of the enzyme activity was observed on both sides of the pH optimum. The enzyme is virtually inactive below pH 3.0 and above pH 8.0. The pH stability of the enzyme was investigated by incubating the enzyme in buffers of varying pH for 3 h at 4°C and then determining the catalytic activity under standard activity assay conditions. As shown in , the immobilized phytase and the soluble phytase exhibited similar relative activities in the pH range of 3–8. The stability of most of the plant phytases decreased dramatically at pH values below pH 4.0 and above pH 7.5 Citation[13].
Figure 3. The effect of pH on the activity of free (▴) and immobilized (▪) phytase (activities were assayed at 37°C by using 2 mM sodium phytate prepared in appropriate buffer solution).
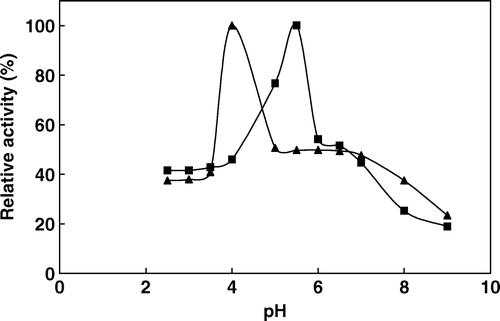
Figure 4. pH stability of free (▴) and immobilized (▪) phytase (after incubation at indicated pH's activities were assayed at 37°C by using 2 mM sodium phytate prepared in 0.1 M acetate buffer at pH 5.0).
The kinetic parameters of the free and immobilized phytase were also studied. These were determined from the Lineweaver-Burk graph obtained by plotting inverse values of substrate concentration against inverse values of initial reaction rates. The graph gave two straight lines in accordance with Michaelis-Menten equation governing enzyme catalyzed reactions. The Km and Vmax values were found to be 8.3 mM and 0.63 U/mg for free and 10 mM and 1 U/mg for immobilized enzymes, respectively. It was thus observed that the change in Km value of immobilized enzyme was about 1.2 times that of free enzyme. The physical meaning of the change implies that to achieve the same level of Vmax, immobilized enzyme requires around 1.2 times the amount of substrate concentration. The values for free enzyme obtained in this work are in agreement with those presented in the literature Citation[31]. An increase in the Km value is not an indication that the catalytic property of the enzyme is reduced as a result of immobilization, or its specificity for the substrate is effected. Immobilization restricts the kinetic movement of enzyme in the reaction medium while the free enzyme is not governed by such limitations. Thus, as a degree of freedom of enzyme immobilized to a matrix was reduced, its Km value increased.
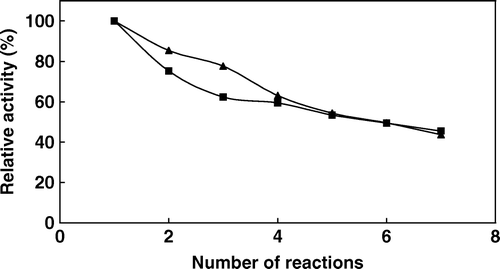
The reusability of the immobilized phytase is important for economical use of the enzyme in repeated batch or continious phytate hydrolysis. The stability of immobilized phytase on repeated use was examined by measuring the activity for the hydrolysis of Na-phytate at 37°C and 60°C (). As is seen from the figure, the immobilized enzyme retained 52% of its initial activity after 21 cycles of reuse at 37°C and 46% of its initial activity after 7 cycles of reuse at 60°C. The result shows that the phytase immobilized on Sepabead EC-EP can be easily recovered and used repeatedly, although significant loss of its activity at 60°C is unavoidable under the conditions of our experiment. No detachment of immobilized enzymes from support was observed. Such reusability is advantageous for the continuous use of the enzyme in industrial applications and also could significantly reduce the operation costs in practical applications.
Figure 5. Repeated use of immobilized phytase at 37°C (▴) and 60°C (▪) (activities were assayed at 37°C by using 2 mM sodium phytate prepared in 0.1 M acetate buffer at pH 5.0).
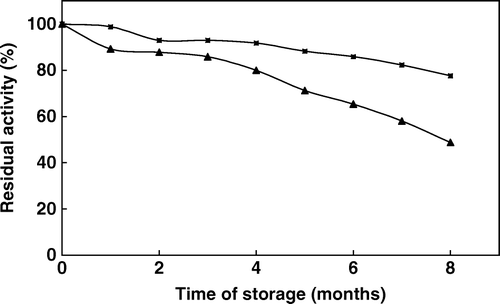
Storage stability is an important advantage of immobilized enzymes over the free enzymes, because free enzymes can lose their activities fairly quickly. To determine the change in activity of the phytase with time, both free and immobilized enzymes were stored at 4°C under the same conditions mentioned before and the activity measurements were carried out for a period of 8 months. By this period no enzyme release was observed. The results are shown in . Under the same storage conditions the free enzyme lost about 51% of its activity over a period of 8 months, whereas the immobilized enzyme lost about only 22% of its original activity over the same period of time. This decrease in enzyme activity was explained as a time-dependent natural loss in enzyme activity and this was prevented to significant degree upon immobilization. The result readily indicates that the immobilized phytase exhibits an improved stability over that of the free enzyme. Of the immobilization methods, fixation of enzyme molecules on a surface often gives rise to the highest stabilization effect on enzyme activities because the active conformation of the immobilized enzyme is stabilized by multipoint bond formation between the substrate and the enzyme molecules. The enhanced storage stability of the immobilized phytase makes it an excellent biocatalyst for phytate hydrolysis. Various reports can confirm that the storage stability of immobilized enzymes varies depending on the immobilization method applied and storage conditions Citation[25].
Figure 6. Storage stability of free (▴) and immobilized (▪) phytase (activities were assayed at 37°C by using 2 mM sodium phytate prepared in 0.1 M acetate buffer at pH 5.0).
The effects of various effectors on the activity of free and immobilized enzymes were also studied. The immobilized phytase is more resistant against to effectors than free enzyme (). Citric acid shows an activator effect especially at 0.1 mM concentration for both enzymes. The enzyme activity was not greatly inhibited by the other compounds. The addition of chelating agents together with phytase enzyme increased the solubility of mineral elements, except for potassium. The combined effect of phytase and citric acid increased the solubilities of Ca, Mg, Zn, and Mn in oat bran sample significantly. With the addition of 3% citric acid the total solubility of Mg and Mn icreased from 21 to 70% and from 6 to 54%, respectively. The increase in the solubility of Ca was also significant with a citric acid concentration of 1.0% Citation[5].
Table 2. The effects of some minerals and ions on free (a) and immobilized (b) enzymes
Biodegradation of Soymilk Phytate by Free and Immobilized Phytases
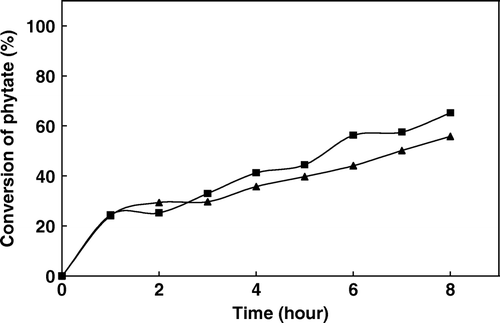
The hydrolysis of soymilk phytate by soluble and immobilized phytase was carried out in batch stirred-tank reactor at 60°C as mentioned in the Materials and Methods section. The initial level of phytate was determined prior to incubation and then the aliquots were withdrawn at different time intervals. By measuring the phytic acid content of samples, % hydrolysis was calculated. A time course of hydrolysis of phytate present in soy milk is shown in . As is seen from the figure, both soluble and immobilized enzymes hydrolysed the soymilk phytate. After 8 h incubation, soluble phytase led to 56% degradation whereas immobilized phytase resulted in 65% reduction in phytate in soymilk. The immobilized enzyme showed a beter percent of degradation. Compared to earlier reports Citation[3], Citation[16], the results obtained for Sepabead immobilized phytase treatment of soymilk are satisfactory.
CONCLUSION
In this paper, we have shown the preparation of a very active and stable immobilized preparation of phytase from avocado. It is significant that this is the first report on immobilization of phytase on Sepabeads supports. The immobilization of phytase on Sepabead EC-EP is simple, easily scaleable, and its subsequent use is comparatively safe and cheap with durable enzyme activity. Based on the above results, the high mechanical stability and absence of swelling in water of these supports converts them into very interesting alternatives for different industrial applications, compared with other related carriers. The combined properties of the immobilized enzymes make them good candidates for industrial use in the hydrolysis of phytate in soy milk and other soy-based products.
Acknowledgements
This project has been funded by the Ege University Research Foundation under Project 2004 FEN 003. We especially thank Dr. P. Caimi (Resindion S.R.L.) for providing us Sepabead EC-EP polymers and for technical help. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal. Biochem. 1976; 72: 248–254
- Brinch-Pedersen H., Sørensen L.D., Bach-Holm P. Engineering crop plants: getting a handle on phosphate. Trends in Plant Sci. 2002; 7(3)118–125
- Brinch-Pedersen H., Borg S., Tauris B., Bach-Holm P. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J. Cereal Sci. 2007; 46: 308–326
- Cunha A.G., Fernàndez-Lorente G., Bevilaqua J.V., Destain J., Paiva L.M.C., Freire D.M.G., Fernàndez- Lafuente R., Guisán J.M. Immobilization of Yarrowia lipolytica lipase—a comparison of stability of physical adsorption and covalent attachment techniques. Appl. Biochem. Biotechnol. 2008; 146: 49–56
- Ekholm P., Virkki L., Ylinen M., Johansson L. The effect of phytic acid and some natural chelating agents on the solubility of mineral elements in oat bran. Food Chem. 2003; 80: 165–170
- Fiske C.H., Subbarow Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925; 66: 375–400
- Frias J., Doblado R., Antezana J.R., Vidal-Valverde C. Inositol phosphate degradation by the action of phytase enzyme in legume seeds. Food Chem. 2003; 81: 233–239
- Gallego F., Betancor L., Hidalgo A., Alonso N., Lorente G., Guisan J.M., Lafuente R. Preparation of a robust biocatalyst of D-amino acid oxidase on Sepabeads supports using the glutaraldehyde crosslinking method. Enzyme and Microb. Tech. 2005; 37: 750–756
- Ghazi I., Segura A.G., Fernandez-Arrojo L., Alcalde M., Yates M., Rojas-Cervantes M., Plou F.J., Ballesteros A. Immobilization of fructosyltransferase from Aspergillus aculeatus on epoxy activated Sepabeads EC for the synthesis of fructooligosaccharides. J. Mol. Cat. B: Enzymatic 2005; 35: 19–27
- Gote M., Umalkar H., Khan I., Khire J. Thermostable α-galactosidase from Bacillus stearothermophilus (NCIM 5146) and its application in the removal of flatulance causing factors from soymilk. Process Biochem. 2004; 39: 1723–1729
- Greiner R., Jany K.D., Alminger M.L. Identification and properties of myo-inositol hexakisphosphate phosphohydrolyses (phytases) from barley (Hordeum vulgare). J. Cereal Sci. 2000; 31: 127–139
- Greiner R., Muzquiz M., Burbano C., Cuadrado C., Pedrosa M.M., Goyoaga C. Purification and characterisation of a phytate-degrading enzyme from a germinated faba beans (Vicia faba var. Alameda). J. Agric. Food Chem. 2001; 49: 2234–2240
- Greiner R., Konietzny U. Phytase for food application. Food Technol. Biotechnol 2006; 44: 125–140
- Hilterhaus L., Minow B., Müler J., Berheide M., Quitmann H., Katzer M., Thum O., Antranikian G., Zeng A.P., Liese A. Practical application of different enzymes immobilized on sepabeads. Bioprocess Biosyst. Eng. 2008; 31: 163–171
- Houde R.L., Alli I., Kermasha S. Purification and characterization of canola seed (Brassica sp.) phytase. J. Food Biochem. 1990; 14: 331–351
- Khare K.S., Jha K., Gupta M.N. Entrapment of wheat phytase in polyacrilamide gel and its application in soymilk phytate hydrolysis. Biotechnol. Applied Biochem. 1994; 19: 193–198
- Kyriakidis N.B., Galiotou-Panayotou M., Stavropoulou A., Athanasopoulos P. Increase in phytase avtivity and decrease in phytate during germination of four common legumes. Biotechnol. Letters 1998; 20(5)475–478
- Latta M., Eskin M. A simple and rapid colorimetric method for phytate determination. J. Agric. Food Chem. 1980; 28: 1313–1315
- Lei G.X., Porres J.M. Phytase enzymology, applications and biotechnology. Biotechnol. Letters 2003; 25: 1787–1794
- Mateo C., Torres R., Fernàndez-Lorente G., Ortiz C., Fuentes M., Hidalgo A., Lòpez-Gallego F., Abian O., Palomo J.M., Betancor L., Pessela B.C.C., Guisán J.M., Fernàndez-Lafuente R. Epoxy-amino groups: A new tool for improved immobilization of proteins by the epoxy method. Biomacromol. 2003; 4: 772–777
- Mehta B.D., Jog S.P., Johnson S.C., Murthy P.P.N. Lily polen alkaline phytase is a histidine phosphatase similar to mammalian multiple inositol polyphosphate phosphatase (MINPP). Phytochemistry 2006; 67: 1874–1886
- Mulimani V.H., Ramalingham. Enzymic hydrolysis of raffinose and stachyose present soymilk by crude α-galactosidase from Gibberella fujikuroi. Biochem. Mol. Biol. Int. 1995; 36: 897–905
- Mullaney E.J., Ullah A.H.J. The term phytase comprises several different classes of enzymes. Biochem. Biophys. Research Commun. 2003; 312: 179–184
- Okot-Kotber M., Yong K.J., Bagorogoza K., Liavoga A. Phytase activity in extracts of flour and bran wheat cultivars: enhanced extractability with β-glucanase and endo-xylanase. J. Cereal Sci. 2003; 38: 307–315
- Onal S., Telefoncu A. Preparation and properties of alpha-galactosidase chemically attached to activated chitin. Art. Cell Blood Subst. And Biotech. 2003; 31(3)339–355
- Pessela B.C.C., Mateo C., Caarascosa A.V., Vian A., Garcia J.L., Rivas G., Alfonso C., Guisán J.M., Fernàndez-Lafuente R. One step purification, covalent immobilization, and additional stabilization of a thermophilic poly-His-tagged β-galactosidase from Thermus sp. strain T2 by using novel heterofunctional chelate-epoxy sepabeads. Biomacromol. 2003; 4: 107–113
- Prashanth S.J., Mulimani V.H. Soymilk olgiosaccharide hydrolysis by Aspergillus oryzae α-galactosidase immobilized in calcium alginate. Process Biochem. 2004; 40(3,4)1199–1205
- Torres P., Datla A., Rajasekar V.W., Zambre S., Ashar T., Yates A., Rojas-Cervantes M.L., Caler-Rueda O., Barba V., Martinez M.J., Ballesteros A., Plou F.J. Characterization and application of a sterol esterase immobilized on polyacrylate epoxy-activated carriers (dilbeads). Catalysis Commun. 2008; 9: 539–545
- Türünç O., Kahraman M.V., Akdemir Z.S., Kayaman-ApohAn N., Güngör A. Immobilization of α-amylase onto cyclic carbonate bearing hybrid material. Food Chem. 2008; 112: 992–997
- Vats P., Banarjee U.C. Production studies and catalytic properties of phytases (myo-inositolhexakisphosphate phosphohydrolases): an overview. Enzyme and Microb. Technol. 2004; 35: 3–14
- Vats P., Banarjee U.C. Catalytic characterization of phytase (myo-inositolhexakisphosphate phosphohydrolase) from Aspergillus niger van Teighem: Glycosylation pattern, kinetics and molecular properties. Enzyme and Microb. Technol. 2006; 39: 596–600