Abstract
The aim of this study was to fabricate type II collagen fibrils with surface modified by long-chain hyaluronic acid. Monomeric type II collagen was isolated from bovine articular cartilage and reconstituted into collagen fibrils followed by a reaction with EDC (1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide)-activated long-chain hyaluronic acid. The existence of the hyaluronan molecules on the fibrillar surface was confirmed by the specific bindings of gold nanoparticles labeled with wheat germ agglutinin. The topographic pattern of type II collagen fibrils revealed by AFM scanning changed significantly after the surface coupling of hyaluronic acid. Beneath the hyaluronan, the characteristic D-bandings of the reconstituted collagen fibrils remained intact as shown by the results of TEM observation. The collagen fibrils became more resistant to collagenase digestion after surface coupling of hyaluronic acid as compared with that without hyaluronic acid immobilization. In addition, human mesenchymal stem cells encapsulated and cultured within the matrix of HA-collagen fibrils have a higher proliferation rate than cells grown within the unmodified type II collagen fibrils. The newly synthesized material of HA-collagen II fibrils has a great potential for use in constructing scaffold for tissue repair.
INTRODUCTION
The scaffolding materials determine the fate of tissue engineering bioconstruct by directing cellular processes depending on their structural and biochemical properties. There are several advantages of utilizing natural polymers to construct tissue engineering scaffolds, such as excellent biocompatibility and functional stimulation.
The fibrils of collagens that are prevalent in the human connective tissues support the growth of a wide variety of cells, and their structure imparts favorable properties such as mechanical strength Citation[1]. In cartilage and other soft tissues, collagen fibers provide the mechanical strength of the ECM (extracellar matrix) framework. Several specific sequences of amino acids in collagen molecules have been proven to stimulate cell adhesion and migration and, in addition, serve as the motifs for signal transduction Citation[2].
Hyaluronic acid (HA) is one of the essential polysaccharides that can furnish cells with a suitable growing environment. HA interacts with cells via its motifs binding to CD44 and RHAMM receptors. HA is known to play a pivotal role instimulating cell adhesion, migration, and proliferation Citation[3]. Potential benefits of HA as a scaffold material for articular cartilage tissue engineering could be realized from the observations that HA stimulated chondrocyte expression of ECM molecules in vitroCitation[4]. Soluble HA has been used in clinical applications including ocular surgery, visco-supplementation for arthritis, and wound healing Citation[5]. However, the poor mechanical properties and rapid degradation of the uncrosslinked soluble HA molecules limit many potential clinical applications.
We have previously used collagen to encapsulate mesenchymal stem cells (MSCs) and proved that the presence of collagen benefited the differentiation of MSCs Citation[6], Citation[7]. Tissue scaffolds containing collagen co-immobilized with HA were also constructed and the chondrogenesis was found to be enhanced by using this HA/collagen scaffold Citation[8]. These solid form scaffolds were not suitable as the matrix for encapsulate cells to construct chondrospheres. In order to extend the half life of hyaluronic acid in tissues, hybrid molecules were synthesized by utilizing EDC (1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) as an avtivation agent of coupling HA onto collagen fibril surface. The resulting HA-collagen hybrid fibrils were characterized and their use as cell carriers was demonstrated.
MATERIALS AND METHODS
Materials
The sodium salt of hyaluronic acid (molecular weight: 1,800 k Da) was purchased from Fluka (St. Louis, MO, USA). Collagenase and wheat germ agglutinin (WGA, from Triticum vulgaris) were purchased from Sigma (St. Louis, MO, USA). Type II collagen was purified from calf articular cartilage using a procedure described by Kuo, S. M. et al. Citation[9]. The human mesenchymal stem cell line was originally cultured from the bone marrow of a 61-year-old female donor and transduced with human papilloma virus E6E7 genes through retroviral vector at passage 3 Citation[8].
Reconstitution and Surface Modification of Type II Collagen
Type II collagen fibrils were prepared by reconstitution of monomeric collagen (0.2 mg/ml) at 37oC for 48 hrs prior to collecting by centrifugation (17,600 g).
HA (57µg, 29µg or 5.7µg) was dissolved in double de-ionized H2O containing 0.4M NaCl followed by the addition of EDC (final concentration of 50 mM) and then solution pH adjusted to 4.5. This EDC solution was passed through a 0.45-µm filter and then mixed with 50 mg of the reconstituted collagen II fibrils. The coupling reaction was conducted at 37oC for 24 hours and then terminated by centrifugation at 17,600g. The surface-modified reconstituted collagen fibrils thus obtained were collected and diluted with phosphate buffered saline (PBS).
Detection of HA on Collagen Fibril Surface
The existence of HA molecules on the surface of collagen fibril could be detected by utilizing nano-gold particles coated with wheat germ agglutinin (WGA), which have a specific affinity binding with HA. About 0.2 mg HA-collagen fibril was suspended in 1ml phosphate buffered saline and then incubated with 10µg Au-WGA for 0.5 hours at 37oC. The collagen fibrils were collected by centrifugation at 17,600g and then washed by de-ionized H2O six times to remove nonspecific binding of Au-WGA.
Surface Scanning by Atomic Force Microscopy (AFM)
The surface topography of the collagen fibrils was analyzed by atomic force microscopic (AFM) scanning. The HA-collagen and collagen fibrils were suspended in de-ionized H2O, dripped upon mica. After standing for 10 minutes, the specimen was blown-dried by N2 gas and then scanned with BioScope® AFM (VEECO, NY, USA) operating in the tapping mode using silicon nitride cantilevers. Images were taken at a scan rate of 0.3∼0.8 Hz over a range of 1-2.5 µm2.
Degradation by Collagenase
Collagen and HA-collagen fibrils were collected by centrifugation and resuspended in PBS to obtain a final concentration of 0.3 mg/ml. The degradation test was initiated by adding collagenase 0.03 mg/ml and digested at 37oC. After 1, 3, and 6 hours, the solutions were centrifuged at 17,600g for 10 minutes. The protein concentration of the degraded collagen in the collected supernatant was determined by Lowry method.
Cell Culture
The KP-hMSC (an immortalized cell line derived from human bone marrow mesenchymal stem cells) was cultured as described Citation[8]. Approximately 6×105/ml inoculated cells were cultured in Dulbecco's modified Eagle's medium with low glucose (DMEM-LG; GIBCO, Grand Island, NY, USA) containing 100U/ml penicillin, 10mg/mL streptomycin, 0.25 mg/mL amphotericin B, and 10% fetal bovine serum (Hyclone, Logan, UT, USA) at 37oC under 5% CO2/95% air. The medium was changed every three days. Cells were subcultured by trypsinization and split at a ratio of 1:3 every week.
The cultured KP-hMSCs (about 6×104 cells) were trypsinized, suspended in 0.15 mL medium, and then mixed with 0.15 mL of cultured medium containing fibrillar collagen or HA-collagen (0.02 mg/mL). The cells/collagen mixtures were incubated at 37oC for 3 hours and then centrifuged at 400 g for 5 minutes to form the pellet. Cells were harvested after various culture periods and the cellular activity was measured by MTT (3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyl tetrazolium Bromide). Briefly, 0.03 mL MTT reagent was added to each pellet and incubated for 3 hours. After centrifugation, the supernatant was then removed and incubated with 0.3 mL dimethylsulphoxide for 30 minutes. Absorbance at 570 nm was recorded using an enzyme-linked immunosorbent assay plate reader.
RESULTS AND DISCUSSION
Over the past years, the tissue scaffolds of HA/collagen were usually fabricated by mixing collagen with HA followed by a cross-linking reaction. The scaffolds thus obtained are most likely inhomogeneous and can not be well characterized. In this study, the strategy was to construct the collagen fibrils with surface modified by hyaluronic acid. These HA-collagen fibrils could be used as the starting materials for fabrication of cells-containing bioconstruct.
The Extent of HA Coupled to Collagen Fibrils
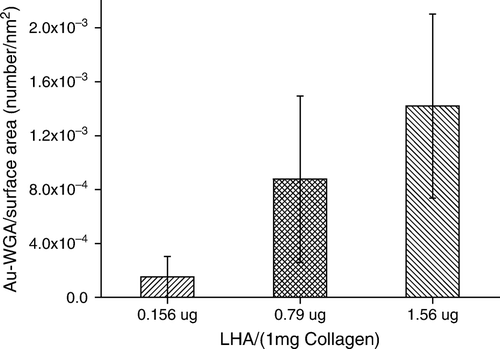
We have tried to measure the amounts of HA coupled to collagen fibrils by using the carbazole method, which was a common technique for determining the mass of uronic acid, but without success. Theoretically, 1 mg of collagen can only accommodate ∼1 µg of surface-coupled HA if one takes the hydrodynamic radius of HA into account (see discussion in detail). Due to the limited coupling sites available on the fibrillar surface, the amounts of immobilized HA were not enough to be measured using this carbazole wet method. The HA molecules on the fibril surface, however, could be detected under TEM after binding with wheat germ agglutinin-gold particles. As shown in , a significant number of WGA-gold particles were found bound to the fibril surface (b). Some of these nano particles located at a distance from the fibril surface (see arrow in the figure), indicating the binding sites on HA molecules instead of the polypeptides of collagen II. This specific WGA-gold nanoparticle binding to the unmodified collagen fibrils could not be found (a). A semi-quantitative analysis of the HA coupled was conducted by estimating the number of WGA-gold nanoparticles bound to the collagen fibrils. We have calculated the numbers of bound Au-WGA on the HA-modified-collagen by counting the nanoparticles on 100 TEM frames. The results show that the average numbers of bound Au-WGA per unit area increased with increasing feed ratio of HA to collagen in the reaction mixture (). Therefore, the extent of the HA coupling can be controlled by adjusting the mass ratio of HA to collagen.
Figure 1. Transmission electron microscopy of collagen and HA-collagen fibrils reacted with Au-WGA, which could be bound specifically onto HA. (a) collagen; (b) HA-Collagen. (X100K) (arrows: nano particles bound to HA at a distance from collagen fibrils).
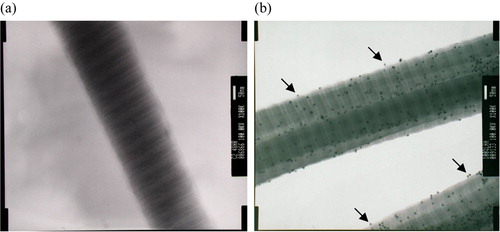
Figure 2. Effect of feed HA/collagen ratio on the extents of surface immobilization by HA. The extent of HA immobilized on the collagen II fibrillar surface was estimated indirectly by the number of Au-WGA nanoparticles bound to fibrils. The number of bound Au-WGA, calculated from 100 TEM photo frames, increase with increasing amounts of reactant HA in the feed.
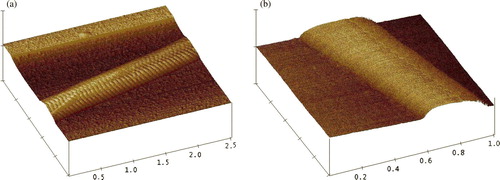
Topography of the Surface-modified Collagen Fibrils
The atomic force microscope is a useful tool to probe the roughness of material surface. The topography of collagen fibrils obtained by AFM scanning exhibited the surface pattern characteristic of the D-banding (a). After the coupling of HA, collagen fibrils became stickier and the D-banding pattern became obscure because the surface was covered by HA molecules (b). Note that under the surface-covered HA, the D-bandings remained intact as demonstrated by the results of the TEM study (b).
HA Increases the Resistance of the Collagen Fibrils to Collagenase Digestion
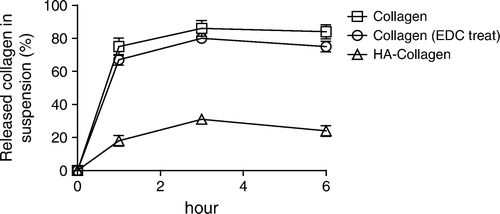
In vitro degradation test was performed by reacting fibrils with collagenase at 37oC for six hours. After centrifuging at 17,600g for 10 minutes, the degraded collagen was measured by Lowry method. The results showed that the degradation rate of collagen fibrils decreased after coupling with HA (). Therefore, the HA on the surface could protect collagen fibrils from enzyme degradation.
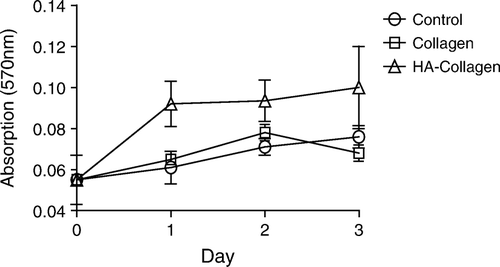
Proliferation of the Mensenchymal Stem Cells Enhanced by HA-collagen Fibrils
KP-hMSCs were mixed with the suspended fibrils of collagen or HA-collagen and then centrifuged to obtain a bioconstruct of cells-containing pellet. Cells in the matrix of HA-collagen fibrils have a higher proliferation rate as compared with that in collagen (). There was no significant difference between the two control groups (collagen and EDC-treated collagen fibrils). These results suggest that the HA-collagen fibrils provide a better growth environment for the human mesenchymal stem cells.
Figure 5. Proliferation of KP-hMSCs within the pellet formed by the cells and fibrils of HA-collagen (or untreated collagen). The control group represents cells-pellet without fibrils.
In this paper, we have demonstrated that the surface immobilization of collagen fibrils with hyaluronic acid could be achieved by using the zero-length crosslinking agent EDC. The presence of HA molecules on the fibril surface was confirmed by the binding of WGA-gold nanoparticles and the AFM scanned topography of the HA-modified surface.
The immobilized HA can not be measured using the conventional carbazole method. This is not unexpected if we consider the limited immobilization sites available on the surface of the fibrils. The collagen fibrils are comprised of bundles of molecules packed by five triple-helix collagen, and the theoretically available surface of fibrillar collagen is dependent on the fibrillar diameter. Collagen fibril of 75 nm diameter is comprised of 17,575 molecules and the theoretically available surface is about 2.3×10−8 nm2 for 1 mg of collagen (Mw = 320 kDa, length = 280 nm/triple helix). The radius of gyration of long-chain hyaluronic acid (1,800 kDa) was estimated to be about 93 nm Citation[10]. Even with full coverage of HA (HA as sphere can only occupy ∼75% of the fibril surface), 1 mg of type II collagen fibrils can only accommodate about 1 µg of HA. Therefore, the amount of the coupled HA is less than the detection limit of the conventional carbazole assay Citation[12].
Although the HA-collagen fibrils contain only a small amount of HA, these HA molecules are in fact concentrated on the fibrillar surface where cells/matrix interaction takes place. Therefore, the newly developed hybrid molecules of HA and collagen provide an excellent environment of hyaluronic acid for the proliferation of mesnechymal stem cells. We expect that this HA-collagen hybrid fibrillar material can be utilized as the base material to construct a 3-D scaffold useful for tissue engineering application. Furthermore, various scaffolding material could be constructed with different HA grafting ratio by controlling the feed ratio of HA to collagen. The effect of HA grafting extent on the growth and differentiation of mesnechymal stem cells is currently underway.
CONCLUSION
We have demonstrated that the surface immobilization of collagen fibrils with hyaluronic acid could be achieved by using the zero-length crosslinking agent EDC. The newly developed hybrid molecules of HA and collagen provide an excellent environment for the proliferation of mesnechymal stem cells. We anticipate that this HA-collagen hybrid fibrillar material is useful for constructing 3-D scaffolds in tissue engineering applications.
Acknowledgements
This work was supported by a grant from National Science Council, ROC. NSC 94-2213-E010-006. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- White D.J., Puranen S., Johnson M.S., Heino J. Int J Biochem Cell Biol. 2004; 36: 1405–1410
- Staatz W.D., Fok K.F., Zutter M.M., et al. J Biol Chem. 1991; 266: 7363–7367
- Underhill C.B., Toole B.P. J Cell Physiol. 1982; 110: 123–128
- Ehlers E.M., Behrens P., Wunsch L., et al. Ann Anat. 2001; 183: 13–17
- Rosier R.N., O'Keefe R.J. Instr Course Lect. 2000; 49: 495–502
- Chang C.F., Lee M.W., Kuo P.Y., et al. J Biomed Mater Res. 2007; 80A: 466–474
- Shih Y.R., Chen C.N., Tsai S.W., et al. Stem Cells. 2006; 24: 2391–2397
- Tang S., Spector M. Biomed Mater. 2007; 2(3)S135–141
- Kuo S.M., Wang Y.J., Weng C.L., et al. J Mater Sci Mater Med. 2005; 16: 525–531
- Hung S.C., Yang D.M., Chang C.F., et al. Int J Cancer. 2004; 110: 313–319
- Scott D., Coleman P.J., Mason R.M., Levick J.R. J Physiol. 2000; 528: 609–618
- Marina C., Elisa L., Francesca M., et al. Carbohydrate Polymers 2003; 54: 59–61