ABSTRACT
Background: Stroke is a common cause of long-term disability worldwide, and an increasing number of persons affected by stroke are of working age. In addition to physical impairments, a majority of patients reportedly suffer cognitive impairments after stroke. Reduced cognitive function may hinder poststroke return to work (RTW); however, most studies of this relationship have assessed cognitive function months after the stroke.
Objectives: The current study aims to investigate the degree of post-stroke RTW, and whether very early cognitive function screening can predict RTW after a stroke.
Methods: This study included 145 persons treated for stroke at 18–63 years of age at a large university hospital in Sweden between 2011 and 2016. Data were retrieved from the GOTVED database. Within 36–48 h after hospital admission, cognitive function was screened using the Montreal Cognitive Assessment (MoCA). Full and partial RTW were assessed based on the Swedish Social Insurance Agency’s register. Logistic regression was performed to analyze the potential predictors of RTW at 6 months and 18 months.
Results: Neither global cognitive function nor executive function at 36–48 h after stroke predicted any degree of RTW at 6 or 18 months. Male sex, lower stroke severity, and not being on sick leave prior to stroke were significant predictors of RTW.
Conclusions: Screening for cognitive impairments at 36–48-h post stroke is apparently too early for predicting RTW, and thus cannot be the sole basis for discharge planning after stroke. Additional research is needed to further analyze cognitive function early after stroke and RTW.
Introduction
The total stroke incidence in developed countries is decreasingCitation1; however, there is an increasing incidence of stroke within the working-age population.Citation2,Citation3 In Sweden, approximately one out of five strokes occur in persons of working age,Citation3 and this proportion is even higher globally.Citation4 Stroke can have diverse consequences, including physical impairments, cognitive impairments (e.g. attention, language, and executive ability),Citation5 and psychological problems (anxiety and depression),Citation6 which often cause long-lasting disability.
The high incidence of stroke among working-age persons raises the importance of considering stroke patients’ return to work (RTW). At the societal level, RTW is economically important, with productivity loss accounting for a considerable portion of the total stroke-related costs.Citation7 Furthermore, a person’s RTW positively affects their well-being and life satisfaction.Citation8 Post-stroke RTW rates vary widely, ranging from 7% to 75% in a review.Citation9 Another review identified several factors as RTW determinants, including age, social factors, and neurological deficits,Citation10 and reports that cognitive impairments may have a greater influence on RTW than physical impairments.Citation10 Young patients generally have a good functional outcome in the modified Rankin Scale (mRS) after stroke; however, a substantial proportion of these patients still cannot RTW. This might be related to deficits not captured by the mRS, such as psychological problems and cognitive impairments.Citation11
Although the prevalence varies, several studies indicate that the majority of stroke patients suffer from cognitive impairment after stroke.Citation12,Citation13 Notably, cognitive deficits are often silent and can be overlooked without specific screening, leaving some affected patients with unmet needs regarding cognition.Citation14 The Montreal Cognitive Assessment (MoCA) is a commonly used screening instrument for cognitive function at stroke units and it is quick and thereby feasible in the acute setting. The MoCA is reportedly more sensitive for detecting cognitive impairments than the Mini-Mental State Examination, and can satisfactory differentiate between different levels of cognitive function.Citation15
Previous studies show that RTW is significantly associated with cognitive function, as assessed with MoCA at 2 months,Citation16 3 months or 6 months following stroke.Citation17,Citation18 Apart from general cognitive function, the executive function has separately also been strongly correlated to RTW as it is often required in the workplace when performing complex functional tasks.Citation17 Kauranen et al.Citation19 assessed cognitive function early after stroke (8 days) and found that different types of cognitive deficits were associated with the inability to RTW within 6 months after stroke.Citation19 In Sweden, the decreasing hospital stay after stroke necessitates that most assessments be performed within the first days after stroke. To enable this, the screening for, e.g. cognitive function needs to be easy to perform and time efficient. It is presently unclear whether screening for cognitive function very early after a stroke can predict RTW.
The current study aims to investigate the degree of RTW after a stroke, and whether screening cognitive function very early after stroke can predict RTW at different time-points. Both global cognitive function (short-term memory, visuospatial abilities, executive functions, attention/concentration/working memory, language, and orientation) and executive functions separately will be examined. This information will enable improved post-stroke rehabilitation planning, potentially enhancing the possibilities of RTW.
Materials and methods
Study design/settings
This is a prospective cohort study and the STROBE guidelines were followed when applicable. The study period began at stroke onset for each participant (2011–2016) and ended 18 months after stroke (2012–2017). The inclusion hospital is the biggest hospital in the west of Sweden. It is a centralized trauma hospital with availability to neurosurgery, thrombectomy, and thrombolysis.
Participants
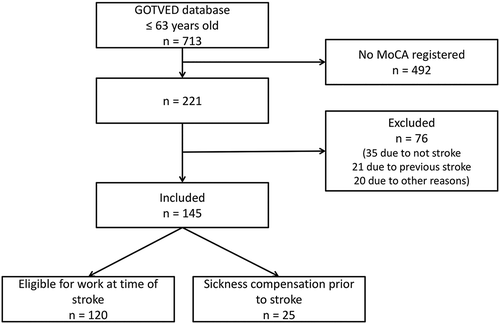
Study data were retrospectively retrieved from the Gothenburg Very Early Supported Discharge (GOTVED) studyCitation20 database, which includes persons with suspected stroke who were admitted to an acute stroke unit at Sahlgrenska University Hospital, Sahlgrenska, in Gothenburg, Sweden, between May 2011 and April 2016. Persons were only included in the current study if it was their first stroke, they were 18–63 years of age at the time of stroke, and the database included complete data on the MoCA within 36–48 h of admission. Cases of transient ischemic attack or subarachnoid hemorrhage were not included.
Measurements
Data from the acute phase were extracted from medical records and the National Swedish Stroke Register (Riksstroke). Date of death within the study period was collected from medical records, and participants deceased before follow-up were excluded before RTW analyses. At hospital admission, stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS), for which the score ranges from 0 to 42 with a lower score representing a better neurological function.Citation21 A score of 0–2 was considered to indicate a very mild stroke, and a score of >2 a mild to severe stroke.
Within 36–48 h after hospital admission, an occupational therapist screened patients for cognitive function using the MoCA. The occupational therapists had prior training in cognitive testing and use the MoCA in their daily work. MoCA (a screening tool with good validity/reliabilityCitation22) range from 0 to 30 with a higher score representing a better cognitive function. A score of ≥26 is considered to indicate normal global cognitive function, while a score of <26 indicates impaired global cognitive function.Citation22 The MoCA can be divided into six domains: short-term memory, visuospatial abilities, executive functions, attention/concentration/working memory, language, and orientation.Citation22 In addition to the total score, executive function from the MoCA was also analyzed separately. It is tested using an alternation task adapted from the trail-making task, a phonemic fluency task, and a two-item verbal abstraction task, with a possible total score of 4. In the current study, a score of 4 was considered to indicate normal executive function, and ≤3 impaired executive function. The cut-off values for impaired/normal ability in the other MoCA domains were based on clinical reasoning.
Work status
From the Swedish Social Insurance Agency, a number of days on sickness compensation (early retirement) or sickness benefit (sick leave) during the 12 months prior to stroke and up to 18 months post-stroke were retrieved. Participants who received early retirement for at least 50% of the 12 months prior to stroke were excluded from RTW analyses. Receiving at least 50% sickness benefit for ≥14 days during the year before stroke was considered as pre-stroke sick leave. Full RTW was defined as no longer being registered with any degree of sickness benefit or sickness compensation, and not dying within a month of non-registration. Partial RTW was defined as no longer being registered with more than 50% sickness benefit or sickness compensation, and not dying within a month of non-registration. RTW was assessed at 6 months (180 days) and 18 months (550 days) after stroke.
Statistical methods
Statistical analyses were performed using IBM SPSS 22. The significance level was set at p ≤ 0.05. Chi2 test was used to compare differences between groups of categorical variables. Potential predictors of RTW were analyzed using logistic regression with dependent variables of full RTW and partial RTW at 6 months and 18 months, each in different models. For each of the outcomes, two different sets of independent variables were used, and the selection of these was based on clinical reasoning by the authors with many years of experience working with persons with stroke. The first set of predictors consisted of the dichotomized global cognitive function score, the dichotomized NIHSS score, age, sex, and pre-stroke sick leave. The second set of predictors consisted of the dichotomized executive function score, the dichotomized NIHSS, age, sex, and pre-stroke sick leave. Spearman correlation tests between independent variables were performed, and correlations of <0.5 were accepted for inclusion in the regression models. To test the models’ goodness of fit and accuracy, Hosmer and Lemeshow test, ROC curves, and Nagelkerke R2 were used.
Ethical considerations
The study follows the Declaration of Helsinki and the Regional ethical review board (EPN) in Gothenburg has approved the current study in 2011 (Dnr: 042–11), two amendments in 2017 (T392-17 and T966-17) and one amendment in 2018 (T540-18). The participants in the current study are anonymized and non-identifiable. According to the Swedish Inspection Board, data that are handled in the frame of national quality registers is an exception from the general rule of informed consent because it allows improvement of the quality of care and treatment that is of general interest.
Results
Characteristics
A total of 145 participants were included (). The 145 participants did not significantly differ from the 492 excluded persons with missing MoCA with regards to age (p = .511) or sex (p = .385).
presents patient characteristics during the acute phase of stroke. The median age was 54 years, and 57.2% were men. Most were classified as very mild stroke (75.9%) according to the NIHSS at hospital admission. The stroke severity had improved already on day 2 after stroke, with 93.4% classified as very mild according to the NIHSS. The mean MoCA score on day 2 was 25.8 (SD 2.95).
Table 1. Characteristics from the acute phase.
RTW
Of the 145 participants, 25 were registered for pre-stroke sickness compensation. Thus, 120 participants were eligible for work at the time of stroke. No participant died within the 6-month follow-up, but one died during the 18-month follow-up. Among the participants eligible for work at the time of stroke, 44 (36.7%) achieved full RTW and 70 (58.3%) partial RTW within the first 6 months; and 77 (64.7%) achieved full RTW and 90 (75.6%) partial RTW within the whole 18-month follow-up.
Compared to the participants who did not RTW, those who achieved full RTW at 18 months post-stroke had significantly less impaired visuospatial abilities in the MoCA (p = .048), . These groups did not significantly differ in any other MoCA domains. Furthermore, language (p = .769) and extinction/inattention (p = .113) assessed with the NIHSS did not significantly differ between the RTW and no RTW groups.
Table 2. MoCA domain scores in participants with full RTW versus no RTW at 18 months.
Predictors of RTW
Neither impaired global cognitive function nor impaired executive function predicted full RTW at any time-point (). Male sex was a significant predictor of full RTW at 6 months and at 18 months. Furthermore, having a more severe stroke (as measured with NIHSS) was a significant unfavorable predictor of RTW in the regression model that included executive function, but not in the model including global cognitive function.
Table 3. Predictors of full RTW at 6 and 18 months.
Impaired global cognitive function and impaired executive function did not predict partial RTW during follow-up (). Partial RTW at 6 months was significantly predicted by male sex, and lower stroke severity. Furthermore, being on pre-stroke sick leave was a significant predictor of lower RTW in the regression model containing an executive function, but not in the model containing global cognitive function. Higher stroke severity was a significant predictor of lower RTW at 18 months.
Table 4. Predictors of partial RTW at 6 and 18 months.
Discussion
The present results showed that very early screening of global cognitive function and executive function did not predict any degree of RTW at 6 or 18 months after stroke. Significant predictors of RTW included male sex, having a very mild stroke, and not being on pre-stroke sick leave. These findings are important for guiding post-stroke screenings of cognitive function, and its prediction of RTW.
At 18 months post-stroke, 65% of the participants achieved full RTW and 76% partial RTW. Previous studies show lower RTW rates at similar time-points.Citation23,Citation24 Within the first 6 months after stroke, 37% of the participants achieved full RTW and 58% partial RTW. Kauranen et alCitation19 reported a 41% rate of partial RTW at 6 months post-stroke, which gives the conclusion that the current study seems to have a relatively high frequency of RTW after stroke. RTW rates may differ among studies due to different social insurance systems, as well as variations in the importance of work and income for the affected persons. Furthermore, the current study had a large proportion of very mild stroke, which could be an explanation for the high RTW.
The participants in the current study seemed to have a relatively intact cognitive function at the time of screening. At 36–48 h after stroke, the mean MoCA score was 26% and 41% of participants had impaired global cognitive function. This is in agreement with the high frequency of very mild stroke in the current study. Previous studies have mainly assessed cognitive function during a later phase. However, one prior study reports a mean MoCA score of 19 during days 4–8,Citation25 and another shows a mean score of 18 within the first 3 months after stroke,Citation26 which are both clearly lower than the mean score in the current study.
RTW was not significantly predicted by global cognitive function screened using the MoCA at 36–48-h post-stroke, or by the extracted executive function. Previous studies show that RTW is related to cognitive function screened using MoCA at a later phase (2–6 months post-stroke).Citation16–Citation18 Furthermore, Kauranen et al.Citation19 reports that RTW is significantly associated with cognitive function assessed using different neuropsychological tests approximately at 1-week post-stroke. Previous findings that initial cognitive function predicts RTW prompted the suggestion that post-stroke cognitive screening may be important for treatment and rehabilitation planning, particularly to optimize the RTW.Citation27 However, the current study failed to present a cognitive screening that were able to predict RTW. The timing could be one explanation. Cognitive function reportedly follows complex trajectories after a stroke.Citation28 In the current study, cognition was measured at day 2–3 post-stroke. At this time, many things are happening to the patient; numerous examinations and treatments are performed, and the patient may be in crisis. These circumstances could result in an inaccurate evaluation of the actual cognitive ability.Citation29 Notably, if post-stroke hospital stays continue to shorten, it will be important with routine follow-up with sensitive assessment tools where cognitive impairment can be detected and appropriately managed. Furthermore, it has to be discussed and further investigated if MoCA is the best instrument to use in this population. Its time efficiency and simplicity makes the MoCA suitable for the aim of very early screening of cognitive function, however, it has some limitations. Overall, the current study participants had very mild stroke severity, and the majority of patients with stroke in Sweden generally suffer mild stroke today.Citation30 It is possible that the MoCA is unsuitable for use in routine screening with the purpose of predicting RTW in a population with very mild stroke. Neuropsychological testing, preferable at a later stage, might be more valuable for this purpose among patients suspected to have cognitive deficits. However, in most health-care systems, neuropsychological testing is too cost and time-consuming to use as screening for every person with stroke and not possible to perform within the short hospital stay. Therefore, the MoCA could be a useful screening instrument for determining which patients will likely benefit from more detailed neuropsychological testing to enable prediction of RTW.
Male sex was found to predict higher RTW rate compared to female. Sex was important in all models predicting full RTW, while it was only predicting partial RTW at 6 months follow-up, not at 18 months follow-up. Male sex is a frequently reported predictor of RTW after stroke in previous research,Citation9 although there are some conflicting results regarding this association.Citation31 Sex differences in RTW may be related to the fact that men are more commonly the primary source of income, while women have other social roles and responsibilities, as suggested in a Canadian review.Citation9 However, these differences are likely decreasing in today’s modern western society. According to the results in the current study, it seems like men achieved partial RTW faster than women, but this difference in partial RTW was no longer significant at the 18-month follow-up.
Higher stroke severity was a negative predictor of RTW in almost all of the regression models in the current study. Accordingly, stroke severity is one of the most consistently reported predictors of RTW in previous research.Citation10 Furthermore, pre-stroke sick leave was a negative predictor of partial RTW at 6 months, but not of partial RTW at 18 months or full RTW at any time-point. In a different population, we have previously showed that sick leave prior to stroke was associated with no RTW at 12 months post-stroke, and with longer time to RTW within 6 years post-stroke.Citation32 Previous and current findings may indicate that sick leave prior to stroke could be a barrier to RTW within the first time after stroke, but may not be as important in the long-term perspective. Among all the regression models, only a relatively modest variance in the outcome was explained by the model, suggesting that factors other than those included might be important for predicting RTW, for instance, work-related factors.
Limitations
A large number of stroke patients were not eligible for this study due to missing MoCA screening. This carries a risk of selection bias. Dropout analysis did however not show any significant age or sex difference between the included participants and the patients excluded due to missing MoCA. Unfortunately, potential differences in stroke severity could not be analyzed due to missing data, but it is likely that a substantial part of the persons with missing MoCA turned out not to have an actual stroke. The majority of the included participants suffered a very mild stroke, which may also influence the results and lower the generalizability to other countries with higher stroke severity. Another limitation might be the definition of RTW. There may have been cases where participants stopped registering at the Social Insurance Agency, even if they were alive and had not actually RTW. There is a small chance that a person could instead receive social assistance from the Social Services, or simply end up outside the system. Furthermore, the current study did not consider what type of work the participants were returning to. The results might perhaps be different if cognitively demanding work and manually demanding work were analyzed separately.
Conclusions
Screening for global cognitive function or executive function at 36–48 h after stroke did not predict any degree of RTW at 6 or 18 months post-stroke. This screening time-point is probably too early, and cognitive testing at a later time-point is needed in order to plan for rehabilitation that can optimize the RTW. However, further research is needed about the association between early cognitive function and RTW after a stroke at working age.
Disclosure
No potential conflict of interest was reported by the author(s).;Promobilia
Acknowledgments
Thank you to the Riksstroke Collaboration http://www.riksstroke.org/eng/for providing data to the current study.
Data availability statement
The data that supports the findings of this study are available from the authors (contact Professor Katharina S. Sunnerhagen, email: [email protected]) upon reasonable request. Due to Swedish regulations https://www.epn.se/en/start/regulations/, the data can only be used for what has been approved by the ethical board, and therefore the data used in the current study is not publicly available.
Additional information
Funding
References
- Johnson CO, Nguyen M, Roth GA, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):439–458. doi:10.1016/S1474-4422(19)30034-1.
- Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79(17):1781–1787. doi:10.1212/WNL.0b013e318270401d.
- Rosengren A, Giang KW, Lappas G, Jern C, Toren K, Bjorck L. Twenty-four-year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke. 2013;44(9):2388–2393. doi:10.1161/STROKEAHA.113.001170.
- Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet. 2014;383(9913):245–254. doi:10.1016/s0140-6736(13)61953-4.
- Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2014;2:8.
- Broomfield NM, Quinn TJ, Abdul-Rahim AH, Walters MR, Evans JJ. Depression and anxiety symptoms post-stroke/TIA: Prevalence and associations in cross-sectional data from a regional stroke registry. BMC Neurol. 2014;14:1. doi:10.1186/s12883-014-0196-x.
- Persson J, Ferraz-Nunes J, Karlberg I. Economic burden of stroke in a large county in Sweden. BMC Health Serv Res. 2012;12:1. doi:10.1186/1472-6963-12-341.
- Vestling M, Tufvesson B, Iwarsson S. Indicators for return to work after stroke and the importance of work for subjective well-being and life satisfaction. J Rehabil Med. 2003;35:127–131.
- Edwards JD, Kapoor A, Linkewich E, Swartz RH. Return to work after young stroke: A systematic review. Int J Stroke. 2017;13:1747493017743059.
- Treger I, Shames J, Giaquinto S, Ring H. Return to work in stroke patients. Disabil Rehabil. 2007;29(17):1397–1403. doi:10.1080/09638280701314923.
- Knoflach M, Matosevic B, Rucker M, et al. Functional recovery after ischemic stroke–a matter of age: data from the Austrian stroke unit registry. Neurology. 2012;78(4):279–285. doi:10.1212/WNL.0b013e31824367ab.
- Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, de Haan EH, Kappelle LJ. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23(5–6):408–416. doi:10.1159/000101464.
- Jokinen H, Melkas S, Ylikoski R, et al. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol. 2015;22(9):1288–1294. doi:10.1111/ene.12743.
- Blackburn DJ, Bafadhel L, Randall M, Harkness KA. Cognitive screening in the acute stroke setting. Age Ageing. 2013;42(1):113–116. doi:10.1093/ageing/afs116.
- Pendlebury ST, Cuthbertson FC, Welch SJ, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by mini-mental state examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke. 2010;41(6):1290–1293. doi:10.1161/STROKEAHA.110.579888.
- van der Kemp J, Kruithof WJ, Nijboer TCW, CAM VB, van Heugten C, Visser-Meily JMA. Return to work after mild-to-moderate stroke: work satisfaction and predictive factors. Neuropsychol Rehabil. 2017;29:1–16.
- Fride Y, Adamit T, Maeir A, et al. What are the correlates of cognition and participation to return to work after first ever mild stroke. Top Stroke Rehabil. 2015;22(5):317–325. doi:10.1179/1074935714Z.0000000013.
- Schulz CH, Godwin KM, Hersch GI, Hyde LK, Irabor JJ, Ostwald SK. Return to work predictors of stroke survivors and their spousal caregivers. Work. 2017;57(1):111–124. doi:10.3233/WOR-172544.
- Kauranen T, Turunen K, Laari S, Mustanoja S, Baumann P, Poutiainen E. The severity of cognitive deficits predicts return to work after a first-ever ischaemic stroke. J Neurol Neurosurg Psychiatry. 2013;84(3):316–321. doi:10.1136/jnnp-2012-302629.
- Sunnerhagen KS, Danielsson A, Rafsten L, et al. Gothenburg very early supported discharge study (GOTVED) NCT01622205: a block randomized trial with superiority design of very early supported discharge for patients with stroke. BMC Neurol. 2013;13:66. doi:10.1186/1471-2377-13-202.
- Young FB, Weir CJ, Lees KR. Comparison of the National Institutes of Health Stroke Scale with disability outcome measures in acute stroke trials. Stroke. 2005;36(10):2187–2192. doi:10.1161/01.STR.0000181089.41324.70.
- Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x.
- Tanaka H, Toyonaga T, Hashimoto H. Functional and occupational characteristics predictive of a return to work within 18 months after stroke in Japan: implications for rehabilitation. Int Arch Occup Environ Health. 2014;87(4):445–453. doi:10.1007/s00420-013-0883-8.
- Hannerz H, Ferm L, Poulsen OM, Pedersen BH, Andersen LL. Enterprise size and return to work after stroke. J Occup Rehabil. 2012;22(4):456–461. doi:10.1007/s10926-012-9367-z.
- Jacquin A, Binquet C, Rouaud O, et al. Post-stroke cognitive impairment: high prevalence and determining factors in a cohort of mild stroke. J Alzheimers Dis. 2014;40(4):1029–1038. doi:10.3233/JAD-131580.
- Chan E, Khan S, Oliver R, Gill SK, Werring DJ, Cipolotti L. Underestimation of cognitive impairments by the Montreal Cognitive Assessment (MoCA) in an acute stroke unit population. J Neurol Sci. 2014;343(1–2):176–179. doi:10.1016/j.jns.2014.05.005.
- Arauz A. Return to work after stroke: the role of cognitive deficits. J Neurol Neurosurg Psychiatry. 2013;84(3):240. doi:10.1136/jnnp-2012-303328.
- Mijajlovic MD, Pavlovic A, Brainin M, et al. Post-stroke dementia - a comprehensive review. BMC Med. 2017;15(1):11. doi:10.1186/s12916-017-0779-7.
- Tornbom K, HC P, Lundalv J, Sunnerhagen KS. Self-assessed physical, cognitive, and emotional impact of stroke at 1 month: the importance of stroke severity and participation. J Stroke Cerebrovasc Dis. 2017;26(1):57–63. doi:10.1016/j.jstrokecerebrovasdis.2016.08.029.
- Riksstroke (the Swedish Stroke Register). Stroke och TIA - slutlig årsrapport från Riksstroke. 2017.
- Wang YC, Kapellusch J, Garg A. Important factors influencing the return to work after stroke. Work. 2014;47(4):553–559. doi:10.3233/WOR-131627.
- Westerlind E, Persson HC, Sunnerhagen KS. Return to work after a stroke in working age persons; a six-year follow up. PLoS One. 2017;12(1):e0169759. doi:10.1371/journal.pone.0169759.