ABSTRACT
Background
One of the main challenges after stroke is gait recovery. To provide patients with an individualized rehabilitation program, it is helpful to have real-life objective evaluations at baseline and at regular follow-ups to adjust the program and verify potential improvements.
Objectives
To evaluate the accuracy and reliability of a fully stand-alone system of connected insoles (FeetMe® Monitor) against a widely used clinical walkway system (GAITRite®).
Methods
Twenty-nine subjects with a stroke that occurred >6 months prior participated in the study. Their comfortable gait over three 8-m trials was evaluated by four raters, on Day 1 and Day 7, using simultaneously FeetMe® Monitor and GAITRite®. Velocity, stride length, cadence, stance, and swing duration were calculated on both sides over three sequences of gait: one single stride, 8 m, and three 8-m trials pooled. The Intra-class Correlation Coefficient (ICC) and the Bland–Altman plot evaluated the construct validity (inter-device) and the reliability (test–retest and inter-rater) of FeetMe® Monitor.
Results
Through all gait analysis sequences, the inter-device ICCs were >0.95 for velocity, stride length, and cadence. Ranges of inter-device ICCs were [0.77–0.94] for stance duration for both limbs, and for swing duration [0.32–0.57] on the non-paretic side and [0.75–0.90] on the paretic side. Test–retest and inter-rater ICCs for all parameters were >0.73 for one single stride, >0.88 for 8-m trials and >0.94 for three 8-m trials.
Conclusion
FeetMe® Monitor is an accurate and reliable system for measurement of gait velocity, stride length, cadence, and stance duration in chronic hemiparesis.
Introduction
Stroke is the first cause of acquired handicap in adults. Citation1,Citation2 Each year, it is estimated that 11.9 million people suffer a stroke worldwide. Citation3 Seventy percent of stroke survivors experience persistent motor impairments directly affecting gait function and limiting daily activities. Citation4 Many spatial and temporal gait parameters are altered in patients with chronic hemiparesis, such as reduced speed, cadence, stride length, and increased stride time. Citation5–7
Regaining the ability to walk outdoors is an important goal for individuals who have experienced a stroke and it is often a primary focus of the rehabilitation care. Citation8–14 Gait recovery is correlated with better independence in daily living activities, Citation15–18 quality of life, Citation19–21 and a decrease of morbi-mortality. Citation22,Citation23 To achieve this goal, an individualized rehabilitation program based on a comprehensive gait evaluation is requested. To be optimal, a comprehensive gait evaluation at baseline and at regular follow-ups is necessary. However, the current validated and approved systems for such an evaluation (Electronic walkway systems, Motion capture systems) are expensive, time-consuming and require engineers to analyze the data such that a very small subset of patients can benefit them. Moreover, they can only evaluate patients in the laboratory setting, thus in an environment that may not reflect real life.
To overcome these limitations, a number of devices are currently developed to evaluate different parameters such as the daily number of steps (pedometers) or gait speed and walking distance (accelerometers). Citation24–27 In patients with stroke, some pedometers estimate the number of steps with good accuracy and mean distance with fair accuracy. Citation28 Yet, they do not provide data on gait patterns or on the intensity of motor activity. Accelerometers offer reliable raw information that must be computed to obtain gait parameters. Citation28,Citation29 However, their reliability and accuracy are significatively influenced by their location with better measurement when positioned at distal parts of the lower limbs. Citation29,Citation30 Moreover, the existing devices with an accelerometer have limited memory or require a mobile phone for permanent data transfer, thus limiting their use to few hours a day. Interfaces with mobile phones or computers allow post-processing or real-time feedback of accelerometers-based systems but may request to constantly carry a phone.
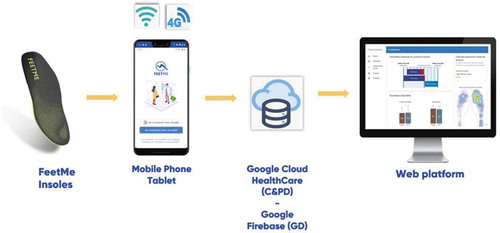
FeetMe® Monitor insole (FeetMe, Paris, France) is a new wearable medical device (class Im) combining plantar pressure sensors, accelerometers, and gyroscopes. An embedded microcontroller computes real-time temporal and spatial gait parameters. These insoles can be used as a stand-alone device, without a smartphone connection, and gait parameters can be stored up to 13 days dependent on walking activities. Citation31 To our knowledge, this is the first fully autonomous device that computes temporal and spatial gait parameters with no external computing sources.
The aim of the present study was to validate the measurement of gait parameters, using FeetMe® Monitor, in comparison with the GAITRite® system which is the most widely used system in gait evaluation laboratories, as well as to assess test–retest and inter-rater reliability in patients with a chronic post-stroke.
Material and methods
Participants
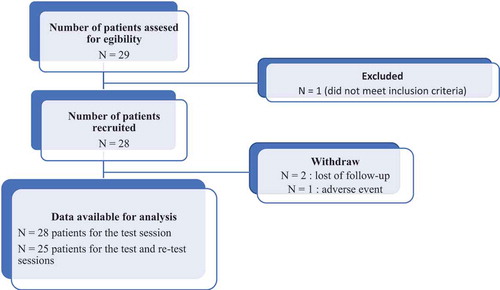
This prospective study was conducted in the Neurorehabilitation Department at University Hospital Center Henri Mondor at Créteil, France, between October 2018 and March 2019. The study was in accordance with the Declaration of Helsinki (2008), Good Clinical Practice guidelines, and local regulatory requirements (registration number, ID-RCB: 2018-A01388-46). This manuscript was written according to the STROBE Guidelines. Inclusion criteria were age ≥18 years, stable stroke-induced hemiparesis for over 6 months since stroke, ability to walk with shoes over 8 m without assistance and without walking aid device. The exclusion criterion was intercurrent medical condition preventing the patient from participating in two consecutive ambulation tests one week apart. Each subject provided written informed consent prior to participation in the study (see ).
Procedure
Each subject was evaluated simultaneously on the GAITRite® mat and wearing, in their shoes, the FeetMe® Monitor insoles (FeetMe, Paris, France). Each subject completed three 8-m runs of gait at a comfortable pace. These measures were repeated by four independent raters in random order, twice, 7 days apart (test and retest). The participants kept the same shoes at test and retest sessions. The insoles were placed in the subject’s shoes and calibrated by the raters. The calibration procedure consisted of putting the insoles in patient’s shoes and with FeetMe® Evaluation mobile application, click on a button to reset all the sensors' values to zero. Then, the patients put on his shoes and began measurements. Each subject was asked to stand in a defined area in front of the mat with both feet together. Then, the patient started walking on the mat while the rater started recording. The subject stopped with feet together right after the mat while the rater stopped recording on FeetMe® Monitor insoles. All the raters repeated the same procedure.
Patients were asked to complete a simple questionnaire evaluating the insoles comfort.
Data acquisition
FeetMe® Monitor (insole version FTM-MIN v2, firmware version: 2.5.4) is composed of an IMU (Inertial Measurement Unit), 19 related plantar pressure sensors, a battery, a BLE (Bluetooth Low Energy) emitter, and a storage unit. The IMU uses a combination of a triaxial accelerometer and triaxial gyroscopes. Paired to the 19 related pressure sensors, the IMU allows to segment each stride. Sixty spatiotemporal gait parameters were computed within an embedded algorithm. Then, collected data were stored within the insoles. Later, data were transferred to a secured web-platform, FeetMe® Mobility Dashboard, via FeetMe® Evaluation application (see ).
Figure 3. Inter-rater reliability at test and retest
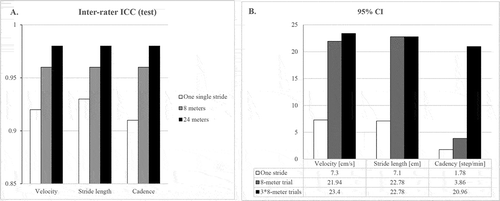
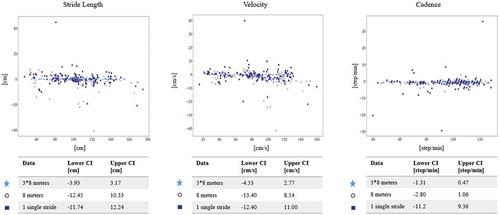
Figure 4. Bland–Altman plot between FeetMe® Monitor and GAITRite®
GAITRite® Platinum mat (dimensions, 0.61*7.92 m, sampling frequency 60 Hz, CIR Systems, Inc., Sparta, NJ, USA). The GAITRite system automates measuring temporal (timing) and spatial (distance) gait parameters via an electronic walkway connected to the serial port of a Windows® 95/98/ME personal computer. Encapsulated within the electronic walkway are sensor pads. Each sensor pad has an active area of 61 cm square and contains 2,304 sensors arranged in (48x48) grid pattern.
The patient ambulates across the walkway, the system captures the geometry and the relative arrangement of each footfall as a function of time. The application software processes the raw data into footfall patterns and computes the temporal (timing) and spatial (distance) parameters. The software’s relational database stores tests individually under each patient and supports a variety of reports and analyses.
Statistical analysis
Five gait parameters (velocity, stride length, cadence, stance, and swing durations – swing and stance durations have been assessed for both paretic and non-paretic side) were measured using FeetMe® Monitor insoles and GAITRite® while the patients were performing 8 m on the mat. To assess the validity of FeetMe® Monitor measurements, two tests have been performed. Intra-class Correlation Coefficients (ICC(2.1), absolute agreement) were calculated on one single stride, one 8-m run, and three times 8-m run pooled together. Citation32 Bland–Altman plot with 95% Confidence Interval (CI) was calculated between gait parameters measured by FeetMe® Monitor and GAITRite® (inter-device comparison) at the first (test) and the second (retest) session for each rater. The test–retest reliability was evaluated using ICC between gait parameters measured in the two sessions performed one week apart. The inter-rater reliability was evaluated using ICC between gait parameters measured by the four raters at test and retest. The level of agreement was defined as excellent above 90%, good between 75% and 90%, moderate between 50% and 75% and poor below 50%. Citation32
Results
Twenty-nine patients with chronic hemiparesis were evaluated (age 59 ± 15, time since stroke, 67 ± 50 months, mean ± standard deviation, ). One patient has been excluded between test and retest because of a non-respect of inclusion criteria. Three patients stopped their participation to the study after the first test: two patients canceled their visit for the retest due to availability issues, one patient fell during the retest session while walking in the laboratory due to a heart condition. The data analysis, therefore, covered 28 subjects at the test session and 25 subjects at the retest session. The demography of the study in terms of age, foot size, height, weight, and paretic limb is provided in .
Table 1. Subjects’ demographics
Validity
Inter-device ICCs of velocity, stride length, and cadence were at least 0.95 in all gait sequences (one single stride, 8-m and three 8-m trials, ). Inter-device ICCs of stance duration were between 0.77 and 0.94 regardless of the limb. Yet, inter-device ICCs of swing duration were between 0.32 and 0.57 in the non-paretic side and between 0.75 and 0.90 in the paretic side.
Table 2. FeetMe® Monitor validity
Test–retest reliability
Test–retest ICCs of all parameters measured with FeetMe® Monitor were between 0.75 and 0.88 for one single stride, between 0.90 and 0.95 for 8 m and between 0.94 and 0.97 for three 8-m trial analysis (). Results regarding GAITRite® measurement are incorporated in .
Table 3. Test–retest reliability of FeetMe® Monitor and GAITRite®
Inter-rater reliability
Inter-rater ICCs for each parameter measured with FeetMe® Monitor were between 0.73 and 0.92 for one single stride, between 0.88 and 0.96 for 8 m and between 0.94 and 0.98 for three 8-m trial analysis distance and (). Results regarding GAITRite® measurement are incorporated in . In , reliability is increased on average by 12 ± 6% if the gait analysis is extended to 8 m and by 16 ± 9% when three 8-m trials are pooled together.
Table 4. Inter-rater reliability of FeetMe® Monitor and GAITRite®
Bland–Altman analysis
Bland–Altman plot of main averaged parameters – velocity, stride length, cadence – between GAITRite® and FeetMe® Monitor is presented in . The limits of agreement over one single stride were [−12.4, 11.0] cm/s for velocity, [−11.7, 12.2] cm for stride length, [−11.2, 9.4] step/min for cadence. The limits of agreement over 8-m gait analysis were [−13.4, 8.5] cm/s for velocity, [−12.5,10.3] cm for stride length, [−2.8, 1.1] step/min for cadence. When the results of the three 8 m evaluation tests are pooled together, the limits of agreement were [−4.5, 2.8] cm/s for velocity, [−3.9, 3.2] cm for stride length, [−1.3, 0.5] step/min for cadence, [−0.06, 0.12] s. In , the increase of precision compared with one stride reaches 6% for velocity, 5% for stride length and 82% for cadence when the patient is analyzed over 8 m, and 69% for velocity, 70% for stride length, and 91% for cadence over three 8-m trials.
Insoles comfort
Over the 28 subjects questioned, 75% found the insoles comfortable or extremely comfortable, 14% uncomfortable or extremely uncomfortable, and 11% did not complete this field due to lack of time at the end of the first session.
Discussion
A huge number of patients who experienced a stroke are leaving with various degrees of mobility impairment. Various therapeutic interventions aim at improving mobility, especially physiotherapy, rehabilitation, drugs (especially antispastic ones), and surgery. Gait evaluation is usually performed through clinical observation and use of scales (e.g. Barthel index, Modified Ashworth scale). In order to have a better evaluation of the evolution of gait following therapeutic interventions, some centers evaluate gait parameters via a pressure-sensitive mat. However, this is time-consuming and expensive so that it is difficult to generalize to all patients that would require it. FeetMe® Monitor insoles may be a more practical and affordable device.
This study shows that FeetMe® Monitor insoles, a real-time gait analysis device with embedded computation, have a good to excellent accuracy and reliability for velocity, cadence, stride length, and stance duration.
Validity of FeetMe® Monitor system
Spatio-temporal parameters
The validity of velocity, stride length, and cadence measurement using FeetMe® Monitor insoles was excellent (ICC > 0.95) in all the sequences of walk analyses, from one single stride.
Duration of gait cycle phases
The validity of stance duration measurement was good to excellent over one single stride, , or when pooling 3 times 8 m (ICC > 0.77) regardless of the side.
Regarding the swing phase, the paretic side was associated with a good agreement over 8 m (ICC, 0.75) and one single stride (ICC, 0.78) and an excellent agreement when the 3 evaluations were pooled together (ICC, 0.90). On the non-paretic side, the validity of swing duration measurement was poor over 8 m (ICC, 0.34) and one stride (ICC, 0.32) and moderate when the 3 evaluations were pooled together (ICC, 0.57). The increased agreement when gathering more data is a well-known phenomenon explained by the decrease of variance. Citation33 Usually, studies comparing various devices to the GAITRite® system pooled together 3 to 4 runs. Citation34 That suggests that the agreement of duration parameters can be considered as enough in both laboratory settings (in which the measurement could be repeated 3 times) and daily life when enough data are collected (at least 24 m) for the paretic side. Regarding, the non-paretic limb, a larger dataset may be necessary to increase the accuracy of the device. The difference may be related to the shorter time of swing duration in the non-paretic limb.
Reliability of FeetMe® Monitor insoles
Test–retest reliability was good (ICC>0.75) for one single stride and excellent (ICC>0.9) when the sample size of collected data increased whatever the gait parameter analyzed.
Interrater reliability was moderate to excellent (ICC, [0.73; 0.93]) on one single stride for all parameters and excellent (ICC, [0.88; 0.98]) when more data were pooled (8 m and 3 times 8 m). Those reliabilities were increased when retesting the patients, in favor of a learning process of the raters. The same distance-related effect was observed for the confidence interval.
Those results demonstrate that in clinical practice, measures can be repeated over time and performed by different raters.
Potential use of FeetMe® Monitor insoles in clinical practice
Gait is a key target for therapeutic intervention in numerous conditions. Ideally, all stroke survivors able to walk should be objectively and accurately evaluated in order to individualize their treatment, Citation35,Citation36 yet only a minority of patients are currently assessed using a quantitative gait analysis due to practical issues as described above.
FeetMe® Monitor insoles can be an affordable alternative to electronic walkways. Moreover, it can be used for home monitoring.
Potential use of FeetMe® Monitor insoles in research
FeetMe Monitor insoles can be potentially used alone or in combination with other gait evaluation devices to better understand the way patients are walking from lab conditions to daily activities. Moreover, it can be a surrogate marker to evaluate therapeutic interventions.
Limitations
Though those results are encouraging, further studies are needed to compare FeetMe Insoles to other devices such as motion capture systems or other portable devices embedding an accelerometer. Home use has not been evaluated. Yet, there is no current gold standard for home-monitoring device. The first validation of FeetMe® Monitor insoles on an urban area and on stairs has led to promising results on the velocity of healthy subjects with a range of error of [3.10–4.70] % compared to an odometer. Citation31
Conclusion
FeetMe® Monitor is an innovative system of spatio-temporal gait analysis. This could be a solution for a quantitative gait analysis outside the laboratory: as a home-monitoring system or as an evaluation system for clinicians in their offices. Although the results of validity and reliability were highly satisfactory, that system has the potential to be optimized to improve the computation of purely temporal metrics as swing duration.
References
- World Health Organization . Neurological Disorders: Public Health Challenges . Geneva: World Health Organization; 2006.
- Mendis S. Stroke disability and rehabilitation of stroke: World Health Organization perspective. Int J Stroke . 2013 janv;8(1):3–4. doi:10.1111/j.1747-4949.2012.00969.x.
- Avan A , Digaleh H , Di Napoli M , et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the global burden of disease study 2017. BMC Med . 2019 déc;17(1):191. doi:10.1186/s12916-019-1397-3.
- Dugan Et EL , Combs-Miller SA. Physiological complexity of gait is decreased in individuals with chronic stroke. Comput Methods Biomech Biomed Engin . 2019;22(6):658–663. avr. doi:10.1080/10255842.2019.1578961.
- Balaban Et B , Tok F . Gait disturbances in patients with stroke. PM&R . 2014 juill;6(7):635–642. doi:10.1016/j.pmrj.2013.12.017.
- Beyaert C , Vasa Et R , Frykberg GE . Gait post-stroke: pathophysiology and rehabilitation strategies. Neurophysiol Clin Neurophysiol . 2015 nov;45(4–5):335. doi:10.1016/j.neucli.2015.09.005.
- Geiger M , Supiot A , Pradon D , Do M-C , Zory Et R , Roche N . Minimal detectable change of kinematic and spatiotemporal parameters in patients with chronic stroke across three sessions of gait analysis. Hum Mov Sci . 2019 avr.;64:101‑107. doi:10.1016/j.humov.2019.01.011.
- Jorgensen HS , Nakayama H , Raaschou HO , Olsen Et TS , Jergensen HS . Recovery of walking function in stroke patients: the copenhagen stroke study. Archives of Physical Medicine and RehabilitationVolume 76, Issue 1, January 1995, Pages 27-32 . doi:10.1016/S0003-9993(95)80038-7.
- Bohannon WJB , Horton RW . Importance of four variables of walking to patients with stroke. Int J Rehabil Res . 1991;14:246–250. doi:10.1097/00004356-199109000-00010.
- Bohannon Richard MB , Andrews W , Smith AW . Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res . 1988;11:181–184.
- Harris Et JE , Eng JJ . Goal priorities identified through client-centred measurement in individuals with chronic stroke. Physiother Can . 2007;56(3):171. doi:10.2310/6640.2004.00017.
- Shepherd RB . Exercise and training to optimize functional motor performance in stroke: driving neural reorganization? Neural Plast . 2001;8(1–2):121–129. doi:10.1155/NP.2001.121.
- Mauritz K-H . Gait training in hemiplegia. Eur J Neurol . 2002;9(1):23–29. dicussion 53-61. doi:10.1046/j.1468-1331.2002.0090s1023.x.
- Fulk GD , He Y , Boyne Et P , Dunning K . Predicting home and community walking activity poststroke. Stroke . 2017 févr.;48(2):406–411. doi:10.1161/STROKEAHA.116.015309.
- Moseley AM , Lanzarone S , Bosman JM , Van Loo MA , De Bie Et RA , Hassett L . Ecological validity of walking speed assessment after traumatic brain injury: A pilot study. J Head Trauma Rehabil . 2004;19(4):341–348. doi:10.1097/00001199-200407000-00008.
- Perry J , Garrett M , Gronley Et JK , Mulroy SJ . Classification of walking handicap in the stroke population. Stroke . 1995;26(6):982–989. doi:10.1161/01.STR.26.6.982.
- Lord SE , McPherson K , McNaughton HK , Rochester Et L , Weatherall M . Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil . 2004;85(2):234–239. doi:10.1016/j.apmr.2003.05.002.
- Khanittanuphong Et P , Tipchatyotin S . Correlation of the gait speed with the quality of life and the quality of life classified according to speed-based community ambulation in Thai stroke survivors. NeuroRehabilitation . 2017 juill;41(1):135–141. doi:10.3233/NRE-171465.
- Park Et J , Kim T-H . The effects of balance and gait function on quality of life of stroke patients. NeuroRehabilitation . 2019 févr;44(1):37–41. doi:10.3233/NRE-182467.
- Madhavan S , Lim H , Sivaramakrishnan Et A , Iyer P . Effects of high intensity speed-based treadmill training on ambulatory function in people with chronic stroke: A preliminary study with long-term follow-up. Sci Rep . 2019 déc.;9(1):1985. doi:10.1038/s41598-018-37982-w.
- Schmid A , Duncan PW , Studenski S , et al. Improvements in speed-based gait classifications are meaningful. Stroke . 2007 juill;38(7):2096–2100. doi:10.1161/STROKEAHA.106.475921.
- Jorgensen HS , Nakayama H , Raaschou HO , Olsen Et TS , Jergensen HS . Recovery of walking function in stroke patients: the copenhagen stroke study. Arch Phys Med Rehabil . 1995;76(1):27–32.
- Peng L-N , Lu WH, Liang CK, et al. Functional outcomes, subsequent healthcare utilization, and mortality of stroke postacute care patients in Taiwan: a nationwide propensity score-matched study. J Am Med Dir Assoc . 2017 nov;18(11):990.e7-990.e12. doi:10.1016/j.jamda.2017.06.020.
- Macko RF , Haeuber E , Shaughnessy M , et al. Microprocessor-based ambulatory activity monitoring in stroke patients. Med Amp Sci Sports Amp Ex . 2002;34(3):394–399. doi:10.1097/00005768-200203000-00002.
- Sullivan JE , Espe LE , Kelly AM , Veilbig Et LE , Kwasny MJ . Feasibility and outcomes of a community-based, pedometer-monitored walking program in chronic stroke: a pilot study. Top Stroke Rehabil . 2014 mars;21(2):101–110. doi:10.1310/tsr2102-101.
- Vanroy C , Vissers D , Vanlandewijck Y , Feys H, Truijen S, Michielsen M, Cras P. Physical activity in chronic home-living and sub-acute hospitalized stroke patients using objective and self-reported measures. Top Stroke Rehabil . 2016 févr;23(2):98–105. doi:10.1080/10749357.2015.1116227.
- Coleman KL , Smith DG , Boone DA , Joseph Et AW , Del Aguila MA . Step activity monitor: long-term, continuous recording of ambulatory function. J Rehabil Res Dev . 1999;36(1):8–18.
- Zheng H , Black Et ND , Harris ND . Position-sensing technologies for movement analysis in stroke rehabilitation. Med Biol Eng Comput . 2005;43(4):413–420. doi:10.1007/BF02344720.
- Compagnat M , Batcho CS , David R , Vuillerme N, Salle JY, Daviet, JC, Mandigout, S. Validity of the walked distance estimated by wearable devices in stroke individuals. Sensors . 2019 ;19(11):2497. doi:10.3390/s19112497.
- Pacini Panebianco G , Bisi MC , Stagni Et R , Fantozzi S . Analysis of the performance of 17 algorithms from a systematic review: influence of sensor position, analysed variable and computational approach in gait timing estimation from IMU measurements. Gait Posture . 2018 oct;66:76–82. doi:10.1016/j.gaitpost.2018.08.025.
- Jacobs D , Hutin E , Farid L , Ferré S , Herreaz Et K , Gracies JM . Validation de la vitesse de marche mesurée par les semelles FeetMe Monitor ® avec le tapis GAITRite ® chez le sujet sain. 2018;7377.
- Koo Et TK , Li MY . A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med . 2016 juin;15(2):155–163. doi:10.1016/j.jcm.2016.02.012.
- Altman DG . Practical statistics for medical research. In: Practical statistics for medical research . Chapman & Hall; 1991; 33-35. CRC press.
- Jagos H , Pils K , Haller M , Wassermann C, Chhatwal C, Rafolt D, Rattay F. Mobile gait analysis via eSHOEs instrumented shoe insoles: a pilot study for validation against the gold standard GAITRite®. J Med Eng Technol . 2017 juill;41(5):375–386. doi:10.1080/03091902.2017.1320434.
- S.I.M.F.E.R. (Italian Society of Physical Medicine and Rehabilitation) . Guidelines for rehabilitation services in Italy. Eur Medicophysica . 2005 mars;41(1):95–109.
- Balasubramanian CK , Clark Et DJ , Fox EJ . Walking adaptability after a stroke and its assessment in clinical settings. Stroke Res Treat . 2014;2014:1‑21. doi:10.1155/2014/591013.