ABSTRACT
Introduction
This longitudinal study aimed to explore the impacts of adopting a healthy lifestyle on self-reported physical and mental health outcomes among Australian females who are living with stroke.
Methods
The study utilized data retrieved from the Australian Longitudinal Study on Women’s Health’s 1946–51 cohort (from survey 5 conducted in 2007 to survey 9 conducted in 2019), focusing on 531 female stroke survivors. The dependent variables for this study were self-reported physical and mental health status, whereas the independent variables were lifestyle behaviors, including physical activity, smoking, alcohol consumption, and supplement use. Generalized Estimating Equation models were employed to assess the longitudinal associations between a dependent variable and the independent and confounding variables.
Results
The average age of the participants was 58.1 (SD = 1.4) years in survey 5 and 70.5 years in survey 9. The longitudinal analyses revealed that stroke survivors who engaged in moderate/high levels of physical activity had significantly better physical and mental health status than their inactive or sedentary counterparts. Besides, current smokers had significantly poorer physical and mental health status than nonsmokers. In addition, risky/high-risk alcohol consumers had significantly poorer mental health status compared to no/low-risk alcohol consumers.
Conclusions
Our findings suggest that post-stroke individuals can improve their physical and mental health by maintaining a healthy lifestyle. Specifically, targeted and appropriate programs and strategies are needed to promote physical activity and reduce smoking and alcohol consumption in female stroke survivors in order to optimize their overall health and quality of life.
Introduction
As a leading cause of long-term neurological and functional disability as well as adult mortality, stroke has become a significant global public health concern.Citation1,Citation2 More than half of stroke survivors are unable to regain their functional independence, and even those who attain functional independence continue to experience considerable physical and emotional difficulties.Citation3 Health status, including both physical and mental health, is significantly lower in stroke survivors compared to the age-matched general people.Citation4–7 The number of individuals living with stroke is increasing due to the aging population and advancements in acute stroke treatment.Citation8 Currently, the global population of stroke survivors exceeds 101 million, with females comprising approximately 56% of this population.Citation9
Previous studies have shown that several factors, including age, body mass index, functional status, stroke severity, comorbidities, degree of dependency, and social support, are associated with the self-reported physical and mental health status of post-stroke individuals.Citation10–19 Also, female stroke survivors are found to have substantially lower levels of physical and/or mental health than male stroke survivors.Citation12–15,Citation17,Citation18 Compared to their male counterparts, female stroke survivors exhibit poorer functional recovery, a diminished ability to engage in daily activities, and a higher prevalence of mental disorders.Citation12,Citation17–21 As such, it is important that future research focus on improving the physical and mental health of female stroke survivors.Citation12,Citation13,Citation15,Citation17–19
A number of international stroke guidelines recommend that post-stroke individuals should engage in self-management practices such as adopting a healthy lifestyle.Citation22–26 While physical activity, alcohol consumption, and smoking are well-established components of lifestyle behavior, the use of dietary supplements is also often regarded as an important aspect of maintaining health.Citation22,Citation27,Citation28 Stroke survivors frequently encounter nutritional deficiencies due to several reasons, such as dysphagia, changes in appetite, and the adverse effects of medications.Citation28–31 Dietary supplements can help address these deficiencies by providing essential vitamins and minerals that are crucial for recovery and overall health.Citation22,Citation32–34 Maintaining a healthy lifestyle, including engaging in adequate physical activity, refraining from smoking, reducing or abstaining from alcohol consumption, and taking nutritional supplements, can improve post-stroke symptoms management, functional ability, neurological recovery, prevention of secondary strokes, cardiovascular outcome, survival rate, and overall rehabilitation outcome.Citation22,Citation32–42
Given the immense health care burden caused by stroke on females, attention should be given to research exploring the impacts of adopting key healthy lifestyle behaviors on physical and mental health outcomes among women living with stroke. To date, only limited and inconclusive evidence is available, which is primarily based on cross-sectional or randomized controlled trialsCitation15,Citation35,Citation39,Citation43–47 (with the exception of a single longitudinal study that examined the effects of exercise on self-reported health among stroke survivors with a 2-year follow-upCitation46). To directly address this gap, this longitudinal study aimed to explore the impacts of a healthy lifestyle on self-reported physical and mental health in female stroke survivors in Australia.
Methods
This study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies.Citation48 The study utilized data retrieved from the Australian Longitudinal Study on Women’s Health (ALSWH).Citation49 The ALSWH commenced data collection in 1996, focusing on three distinct age groups of women: young (18–23 years/born 1973–1978), mid-age (45–50 years/born 1946–1951) and old (70–75 years/born 1921–1926) to track changes in women’s health over time, identify associated factors that may influence their health outcomes, and evaluate the effects of changes in policy and practice.Citation49 From the database of the Health Insurance Commission (currently known as Medicare Australia), the participants of the baseline surveys of the ALSWH study were selected using a random sampling method within each of the age groups (except for women residing in rural and remote areas, who were oversampled at a double rate than urban women).Citation50 The participants are broadly representative of the nationwide female population within the specified age groups.Citation50 Details about ALSWH can be found elsewhere.Citation50
This present study utilized the dataset of the ALSWH mid-age cohort, of which 13,714 women participated in the baseline survey in 1996.Citation49 The data from this cohort of participants was collected through mailed surveys at intervals of approximately three years. If required, proxy respondents – typically family members or close friends – were permitted to complete the survey on their behalf.Citation49 Proxy respondents are reliable and valid sources for assessing stroke-related health outcomes, including health-related quality of life.Citation51,Citation52 This cohort has undergone a total of nine surveys so far, with participants aged between 45–50 years during survey 1 in 1996 and 68–73 years during survey 9 in 2019.Citation53 Due to the availability and consistency of the variables associated with a healthy lifestyle, the present study employed data from surveys 5 through to 9. The questionnaire item “In the Past 3 years, have you been diagnosed with or treated for stroke” was utilized across all surveys to identify stroke patients. Several studies have examined the validity of self-reported stroke, providing support for the utilization of self-administered questionnaires in epidemiological research to determine the prevalence of stroke in population-based studies where hospital-recorded data are unavailable.Citation54–56 For instance, a study by Engstad et al.Citation54 found that self-reported stroke results closely correspond with medical records, exhibiting a positive predictive value of 79%, sensitivity of 80%, and specificity of 99%.
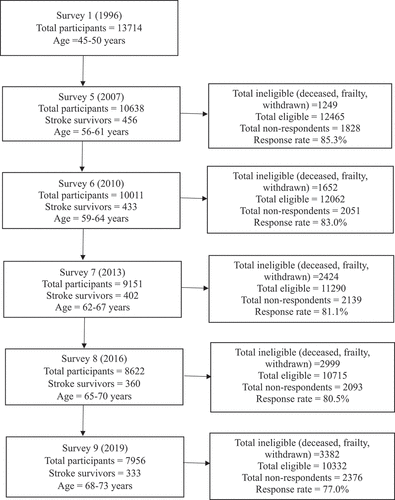
Attrition is common in longitudinal investigations.Citation57 illustrates the longitudinal progression of participant engagement, providing a detailed listing of participant retention rates and reasons for attrition at each follow-up interval over the course of the investigation. A total of 531 stroke survivors were identified in different surveys. Among them, 456 stroke survivors participated in survey 5 (2007); 433 stroke survivors participated in survey 6 (2010); 402 in survey 7 (2013); 360 in survey 8 (2016); and 333 in survey 9 (2019). This study is based on the information of those 531 stroke survivors.
Dependent variables
The dependent variables considered in this study were the self-reported physical and mental health status of the participants. The self-reported physical and mental health status was determined using the Physical Component Summary (PCS) scores and Mental Component Summary (MCS) scores obtained from the Short Form 36 Health Survey Questionnaire (SF-36).Citation58,Citation59 The SF-36 questionnaire comprises eight distinct multi-item scales consisting of a total of 36 items. These scales evaluate various aspects of an individual’s health, including physical function (PF), bodily pain (BP), role limitations due to physical health problems (RP), general health (GH), vitality (V), social functioning (SF), role limitations due to emotional problems (RE), and mental health (MH).Citation58,Citation59 Two summary measures of health status, namely PCS scores and MCS scores, are calculated using standard scoring algorithms based on the eight scales.Citation59,Citation60 The PCS score is comprised of PF, BP, RP, and GH, whereas the MCS score is comprised of V, SF, RE, and MH.Citation59,Citation60 PCS and MCS scores range from 0 to 100 (mean = 50, standard deviation = 10), with higher scores indicating better health.Citation59,Citation60 The SF-36 is commonly used to measure self-reported physical and mental health status in various populations. This questionnaire has also been shown to have high reliability and validity for use with stroke survivors.Citation61,Citation62
Independent variables
The independent variables for this study were physical activity, smoking, alcohol consumption, and supplement use. The Active Australia Survey questionnaire was utilized to measure physical activity levels,Citation63 whose validity and reliability have been demonstrated to be acceptable.Citation64 Participants reported the frequency and amount of time they spent (that lasted ≥10-minute periods) engaging in brisk walking, moderate physical activities (such as social tennis, moderate exercise classes, dancing, recreational swimming), and vigorous physical activities (that caused them to breathe harder or puff and pant such as aerobics, vigorous cycling, running, competitive sports, swimming) during the preceding week. Physical activity score was measured using metabolic equivalent (MET) minutes per week, which was calculated using the formula: (3.0* walking minutes + 4.0* moderate activities minutes + 7.5* vigorous activities minutes).Citation64 Based on total MET.minutes per week, physical activity was categorized as inactive/sedentary (<600) or moderate/high (≥600), where moderate/high physical activity is equivalent to the recommended physical activities for Australian adults (≥150 min/week of moderate activities).Citation64
The participants were asked to report the number of standard alcoholic drinks they usually consumed daily. The risk associated with alcohol consumption was categorized as: no/low-risk (≤14 drinks/week) and risky/high-risk (>14 drinks/week).Citation65 The smoking status was determined by asking participants how often they currently smoke cigarettes or any tobacco products. Supplement use was determined by asking participants if they had consumed vitamins/minerals during the 12-month period preceding the survey.
Confounding variables
Age, body mass index (BMI), marital status, the number of comorbidities, and year of survey were included as confounding variables in the regression models. Marital status was categorized as: married/de facto and widowed/divorced/separated/never married. The BMI was computed using the formula kg/m,2 utilizing self-reported measurements of height and weight. According to the WHO, the classification of BMI was as follows: underweight/normal (<25.0 kg/m2), overweight (25.0–30.0 kg/m2), and obese (≥30.0 kg/m2).Citation66 The survey questionnaires asked about a range of doctor-diagnosed chronic conditions within the previous three years. The number of comorbidities calculated from the conditions: diabetes, arthritis, hypertension, heart disease, cancer, asthma, depression, anxiety, bronchitis, low iron level, and osteoporosis.
Statistical analysis
This study analyzed the longitudinal association between a dependent variable and the independent and confounding variables using Generalized Estimating Equation (GEE) models, with the Gaussian family and log link function specified. The initial multivariable GEE models incorporated variables whose corresponding crude odds ratio (OR) had a p-value less than 0.25. Subsequently, a backward stepwise regression model approach was employed to identify the most parsimonious model for each dependent variable. Following the completion of the model development procedure, the final models provide the estimates for the adjusted OR (AOR).
GEE can provide robust estimates in the presence of dropouts and missing points, assuming the data are missing completely at random (MCAR) or missing at random (MAR).Citation66–68 To address biases caused by non-random missing data (e.g. cases where participants dropped out due to mortality or severe health issues potentially related to poorer lifestyle choices) and ensure reliable estimates, we performed sensitivity analysis utilizing multiple imputation and weighted GEE with inverse probability weighting approaches,Citation66–69 and found that our adjusted analyses were robust, with potential attrition bias had no significant impact on the main associations. A p-value of <0.05 was deemed statistically significant. The statistical software Stata 17.0 was employed throughout the analysis procedures.
Ethical approval
The Human Research Ethics Committees at the University of Newcastle and the University of Queensland, Australia, granted ethics approval for ALSWH. The participants provided clear written consent to participate in the ALSWH. We received approval from the ALSWH Data Access Committee to use the de-identified ALSWH Core dataset for this study.
Results
A total of 531 stroke survivors participated in different surveys over the course of five survey periods (2007–2019). The demographic and health status characteristics of the participants are shown in . The average age of the participants was 58.1 (SD = 1.4) years in survey 5 (conducted in 2007) and 70.5 (SD = 1.5) years in survey 9 (conducted in 2019). Physical health (PCS) showed a declining trend from 43.1 (SD = 11.5) in 2007 to 39.6 (SD = 11.4) in 2019. Conversely, mental health showed an increasing trend from 48.4 (SD = 12.0) in 2007 to 50.3 (SD = 10.8) in 2016, and 50.0 (SD = 10.5) in 2019.
Table 1. Demographic and health status characteristics of study participants across five survey periods (2007–2019).
The prevalence of smoking among the participants was reported as 17.5% in the year 2007, which subsequently decreased to 7.6% in 2016 and 7.9% in 2019. The prevalence of risky/high-risk alcohol consumption showed a gradual decline from 10% in 2007 to 4.3% in 2019. The percentage of participants with moderate to high levels of physical activity was its highest level in 2010 (57.3%), and its level lowest in 2019 (47.4%). There was a consistent prevalence of supplement utilization across the five surveys, with a range of 77.0% to 79.4%. The prevalence of three or more comorbidities increased from 37.3% in 2007 to 47.5% in 2019.
The results of the two GEE models – one adjusted for age, marital status, and year of survey, and the other additionally adjusted for BMI and number of comorbidities – are presented in . To assess the potential mediating effects of BMI and number of comorbidities on health outcomes, we compared these two models. Using two distinct models, our analyses demonstrated that AORs for the associations between lifestyle factors and health outcomes showed minimal differences, suggesting that BMI and comorbidities are not mediators. However, after adjusting for age, marital status, BMI, number of comorbidities and year of survey, the findings illustrate that current smokers exhibited 7% poorer physical health (AOR: 0.93; 95% CI: 0.89, 0.96) and mental health (AOR: 0.93; 95% CI: 0.90, 0.96) than nonsmokers. Likewise, stroke survivors who were risky/high-risk alcohol consumers had 7% lower mental health (AOR: 0.93; 95% CI: 0.89, 0.97) status than stroke survivors who abstained from or had low-risk alcohol use. In contrast, stroke survivors who engaged in moderate or high levels of physical activity had 1.17 times (AOR: 1.17; 95% CI: 1.14, 1.20) and 1.05 times (AOR: 1.05; 95% CI: 1.02, 1.08) better physical and mental health, respectively compared to their inactive or sedentary counterparts. Supplement use was not significantly associated with either physical health or mental health.
Table 2. GEE model for determining the significant longitudinal predictors of physical and mental wellbeing.
Discussion
This is the first longitudinal study that provides in-depth analyses of the impacts of a healthy lifestyle on self-reported physical and mental health status in female stroke survivors in Australia. Our analyses of a nationally representative dataset of Australian women (aged 45–50 years at baseline) reveal some important findings. The study found that stroke survivors who engage in moderate/high levels of physical activity have better physical and mental health. Moreover, stroke survivors who smoke have poorer physical and mental health. Likewise, stroke survivors who consume alcohol at a risky/high-risk level have poorer mental health. The findings of our study may be important for policymakers, healthcare professionals, and researchers to make evidence-based decisions, develop public health initiatives, and conduct further research in this area.
Our results show that stroke survivors who adhere to the recommended level of physical activity (>150 minutes per week) have significantly better physical and mental health compared to those who do not meet the recommended threshold. A meta-analysis based on randomized controlled trialsCitation45 found beneficial effects of physical activity/exercise on physical and mental health among stroke survivors. There are numerous plausible health benefits associated with physical activity for post-stroke individuals.Citation35,Citation36,Citation38,Citation39,Citation44–46,Citation70 For example, adequate physical activity can improve functional recovery, muscle strength, motor movement, brain recovery, cardiovascular health, mood, and social participation, as well as reduce the risk of stroke-related complications, stroke recurrence, hospitalization, and cognitive impairments among those living post-stroke.Citation35,Citation36,Citation38,Citation39,Citation44–46,Citation70 These benefits may explain why stroke survivors in our study who maintain the recommended level of the physical activity report better physical and mental health. Nevertheless, we observed that nearly half of the stroke survivors report physical inactivity/sedentary behavior throughout surveys 5–9, indicating that urgent attention is required to help introduce appropriate initiatives promoting the recommended amounts of physical activity in female stroke survivors.
The negative association between smoking and both physical and mental health among stroke survivors in our study may be explained as follows. First, stroke survivors who smoke may have had a higher prevalence of several conditions due to their smoking behavior, including fatigue, cardiovascular and respiratory issues, and mental/cognitive disorders.Citation70–74 In general, post-stroke individuals with a greater number of comorbidities report decreased levels of self-reported health and well-being.Citation75,Citation76 Second, as smoking is a significant predictor for stroke recurrence,Citation77 the prevalence of stroke recurrence may have been higher among stroke survivors who smoked than those stroke survivors who were nonsmokers. Recurrent stroke is associated with an increased risk of disability, which may adversely affect both physical and mental health.Citation78 In order to enhance the physical and mental health of female stroke survivors, our findings suggest that appropriate and effective strategies need to be implemented to reduce smoking rates in this population. Specifically, self-management educational programs have the potential to act as useful interventions in reducing smoking prevalence and improving well-being in post-stroke females.Citation79
Our study also demonstrates a significant association between risky/high-risk alcohol consumption and a diminished state of mental health among those living post-stroke. While there is a lack of comparable research specifically focused on stroke survivors, this finding aligns with studies conducted on the general population,Citation79–81 which show poor self-reported mental health among high-risk alcohol consumers. One possible explanation for the negative impact of alcohol consumption on self-reported mental health is that alcohol can disrupt neurotransmitter systems, resulting in mood changes, cognitive deficits, a greater tendency for suicidal thoughts, an increased risk of mental health disorders, and a worsening of pre-existing mental health conditions.Citation81–85 Alcohol consumption can exacerbate stroke symptoms and interfere with stroke medication;Citation22 these effects may also contribute to a lower state of mental health among risky/high-risk alcohol consumers in our study sample. Our findings further support the need for the implementation of targeted interventions to reduce risky alcohol consumption among female stroke survivors in an effort to improve their mental health.
Interestingly, our study did not observe a significant association between risky/high-risk alcohol consumption and physical health. A recent cross-sectional study conducted in Sri Lanka also found an insignificant association between alcohol use and physical health among stroke survivors.Citation43 It is important to interpret this finding cautiously and consider potential factors that may explain this result. One possible explanation is that the participants who continue risky/high-risk alcohol consumption over time may have a lower incidence of stroke-related physical disability and/or fewer medical conditions. However, risky/high-risk alcohol consumption can still have negative effects on physical health status over time. To gain a deeper understanding of the association between alcohol consumption and physical health, additional longitudinal research with extended follow-up is required.
Regarding the physical and mental health of female stroke survivors, our findings underscore the critical importance of maintaining a healthy lifestyle. However, it is important to consider these findings within the existing framework of secondary prevention support in Australia. The Australian Stroke Clinical Registry (AuSCR) identifies notable gaps in the provision of secondary prevention services, such as inadequate post-treatment support and limited access to rehabilitation programs, which may impede the achievement of optimal recovery, long-term health maintenance, and overall quality of life.Citation86,Citation87 Current clinical guidelines for stroke management, as outlined by the Stroke Foundation, recommend comprehensive lifestyle modifications, including engagement in physical activity, smoking cessation, abstinence from alcohol, and adherence to a proper nutrition.Citation22 Despite these recommendations, our study indicates that a considerable percentage of stroke survivors do not comply with the guidelines, emphasizing the necessity for additional support and resources to ensure that stroke survivors maintain a healthy lifestyle throughout their survivorship. Addressing these gaps through targeted secondary prevention initiatives and strict adherence to clinical guidelines could significantly enhance the quality of life and health status of stroke survivors.
There are several limitations that should be taken into consideration when drawing conclusions from our research outcomes. Firstly, it is important to note that the study relies on self-reported data provided by the participants, which could potentially be influenced by recall bias. Second, due to a lack of data, we were unable to control for some potential confounders like stroke severity,Citation17 functional status,Citation10,Citation12 and carer rolesCitation88 which may have some effects on the physical and mental health outcomes. Third, the participants of our study were middle-aged and older; therefore, the findings may not be generalizable to young female stroke survivors. Nevertheless, our study possesses several strengths, including the utilization of a nationally representative dataset collected over a 12-year period from a large sample of women in Australia. Another strength of this study lies in its ability to control for various demographic and health-related characteristics within the model, thereby strengthening the specific analysis. Additionally, the measures included in this study have been widely used and validated in comparable large population samples.
Conclusions
The present longitudinal study provides first specific insights into the impacts of maintaining a healthy lifestyle on the physical and mental health of female stroke survivors in Australia. The longitudinal analyses suggest that engaging in moderate-to-high amounts of physical activity and/or abstaining from smoking can positively affect self-reported physical and mental health and well-being among female stroke survivors. Similarly, none/low-risk alcohol consumption has been found to have a beneficial impact on the mental health of female stroke survivors. Our findings suggest that women living post-stroke could improve their physical and mental health by maintaining a healthy lifestyle. Targeted and appropriate programs and strategies may be needed to promote physical activity and reduce smoking and alcohol consumption in female stroke survivors in order to optimize their overall health and health-related quality of life.
STROBE_checklist_v4_cohort.doc
Download MS Word (87.5 KB)Acknowledgments
The research on which this paper is based was conducted as part of the Australian Longitudinal Study on Women’s Health by the University of Queensland and the University of Newcastle. We are grateful to the Australian Government Department of Health and Aged Care for funding and to the women who provided the survey data. In addition, MSR acknowledges the scholarships support of the University of Technology Sydney (UTS).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
ALSWH survey data are owned by the Australian Government Department of Health and due to the personal nature of the data collected, release by ALSWH is subject to strict contractual and ethical restrictions. Ethical review of ALSWH is by the Human Research Ethics Committees at The University of Queensland and The University of Newcastle. De-identified data are available to collaborating researchers where a formal request to make use of the material has been approved by the ALSWH Data Access Committee. The committee is receptive of requests for datasets required to replicate results. Information on applying for ALSWH data is available from https://alswh.org.au/for-data-users/applying-for-data/.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10749357.2024.2377517
Additional information
Funding
References
- Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820. doi:10.1016/S1474-4422(21)00252-0.
- Thayabaranathan T, Kim J, Cadilhac DA, Thrift AG, Donnan GA, Howard G, et al. Global stroke statistics 2022. Int J Stroke. 2022;17(9):946–956. doi:10.1177/17474930221123175.
- Bártlová S, Šedová L, Havierniková L, Hudáčková A, Dolák F, Sadílek P. Quality of life of post-stroke patients. Slov J Public Heal. 2022;61(2):101–108. doi:10.2478/sjph-2022-0014.
- Xie J, Wu EQ, Zheng ZJ, Croft JB, Greenlund KJ, Mensah GA, Labarthe DR, et al. Impact of stroke on health-related quality of life in the noninstitutionalized population in the United States. Stroke. 2006;37(10):2567–2572. doi:10.1161/01.STR.0000240506.34616.10.
- Kainz A, Meisinger C, Linseisen J, et al. Changes of health-related quality of life within the 1st year after stroke–results from a prospective stroke cohort study. Front Neurol. 2021;12:715313. doi:10.3389/fneur.2021.715313.
- Em S, Bozkurt M, Karakoc M, Çağlayan M, Akdeniz D, Oktayoğlu P, et al. Determining quality of life and associated factors in patients with stroke. Turk J Phys Med Rehabil. 2015;61(2):146–154. doi:10.5152/tftrd.2015.80090.
- Bello UM, Chutiyami M, Salihu D, Abdu SU, Tafida BA, Jabbo AA, et al. Quality of life of stroke survivors in Africa: a systematic review and meta-analysis. Qual Life Res. 2021;30(1):1–19. doi:10.1007/s11136-020-02591-6.
- Grefkes C, Fink GR. Recovery from stroke: current concepts and future perspectives. Neurol Res Pract. 2020;2(1):1–10. doi:10.1186/s42466-020-00060-6.
- Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29. doi:10.1177/17474930211065917.
- King RB. Quality of life after stroke. Stroke. 1996;27(9):1467–1472. doi:10.1161/01.STR.27.9.1467.
- Kim P, Warren S, Madill H, Hadley M. Quality of life of stroke survivors. Qual Life Res. 1999;8:293–301. doi:10.1023/A:1008927431300.
- Carod-Artal J, Egido JA, González JL, Varela de Seijas E. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke. 2000;31(12):2995–3000. doi:10.1161/01.STR.31.12.2995.
- Abubakar SA, Isezuo SA. Health related quality of life of stroke survivors: experience of a stroke unit. Int J Biomed Sci. 2012;8(3):183–187. doi:10.59566/IJBS.2012.8183.
- Larsen LP, Johnsen SP, Andersen G, Hjollund NH. Determinants of health status after stroke: a cohort study with repeated measurements. Clin Epidemiol. 2020;12:1269–1279. doi:10.2147/CLEP.S270249.
- Mei YX, Zhang ZX, Wu H, Hou J, Liu XT, Sang SX, et al. Health-related quality of life and its related factors in survivors of stroke in rural China: a large-scale cross-sectional study. Front Public Heal. 2022;10:810185. doi:10.3389/fpubh.2022.810185.
- Ramos-Lima MJM, de C BI, de LT, Braga-Neto P. Quality of life after stroke: impact of clinical and sociodemographic factors. Clinics. 2018;73:e418. doi:10.6061/clinics/2017/e418.
- Phan HT, Blizzard CL, Reeves MJ, Thrift AG, Cadilhac DA, Sturm J, et al. Sex differences in long-term quality of life among survivors after stroke in the INSTRUCT. Stroke. 2019;50(9):2299–2306. doi:10.1161/STROKEAHA.118.024437.
- Gargano JW, Reeves MJ. Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke. 2007;38(9):2541–2548. doi:10.1161/STROKEAHA.107.485482.
- Gall SL, Tran PL, Martin K, Blizzard L, Srikanth V. Sex differences in long-term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke. 2012;43(7):1982–1987. doi:10.1161/STROKEAHA.111.632547.
- Sue-Min L, Duncan PW, Dew P, Keighley J. Sex differences in stroke recovery. Prev Chronic Dis. 2005;2(3):A13. PMID: 15963315.
- Rexrode KM, Madsen TE, Ayx Y, Carcel C, Lichtman JH, Miller EC. The impact of sex and gender on stroke. Circ Res. 2022;130(4):512–528. doi:10.1161/CIRCRESAHA.121.319915.
- Stroke Foundation. Clinical Guidelines for Stroke Management. 2017. https://informme.org.au/guidelines/living-clinical-guidelines-for-stroke-management. Accessed November 8, 2023.
- Hebert D, Lindsay MP, McIntyre A, Kirton A, Rumney PG, Bagg S, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11(4):459–484. doi:10.1177/1747493016643553.
- Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364–e467. doi:10.1161/STR.0000000000000375.
- Intercollegiate Stroke Working Party. National clinical guideline for stroke. (First edition) Royal College of Physicians UK. 2016. https://www.rcplondon.ac.uk/guidelines-policy/stroke-guidelines. Accessed November 8, 2023.
- Gittler M, Davis AM. Guidelines for adult stroke rehabilitation and recovery. JAMA. 2018;319(8):820–821. doi:10.1001/jama.2017.22036.
- Reinert A, Rohrmann S, Becker N, Linseisen J. Lifestyle and diet in people using dietary supplements: a German cohort study. Eur J Nutr. 2007;46(3):165–173. doi:10.1007/s00394-007-0650-2.
- Smithard D, Weekes CE. Swallowing and nutritional complications. Manag Post-Stroke Complicat. 2015:99–155. doi:10.1007/978-3-319-17855-4_7.
- Scharver CH, Hammond CS, Goldstein LB. Post-stroke malnutrition and dysphagia. Handb Clin Nutr Aging. 2009:479–497. doi:10.1007/978-1-60327-385-5_24.
- Corrigan ML, Escuro AA, Celestin J, Kirby DF. Nutrition in the stroke patient. Nutr Clin Pract. 2011;26(3):242–252. doi:10.1177/0884533611405795.
- Perry L, Boaden E. Nutritional aspects of stroke care. Stroke Nurs. 2019:103–141. doi:10.1002/9781119581161.ch5.
- Zielińska-Nowak E, Cichon N, Saluk-Bijak J, Bijak M, Miller E. Nutritional supplements and neuroprotective diets and their potential clinical significance in post-stroke rehabilitation. Nutrients. 2021;13(8):2704. doi:10.3390/nu13082704.
- Ko S-H, Shin Y-I. Nutritional supplementation in stroke rehabilitation: a narrative review. Brain & Neurorehabil. 2022;15(1):e3. doi:10.12786/bn.2022.15.e3. PMID: 36743847.
- Dong J-Y, Iso H, Kitamura A, Tamakoshi A. Multivitamin use and risk of stroke mortality: the Japan collaborative cohort study. Stroke. 2015;46(5):1167–1172. doi:10.1161/STROKEAHA.114.008270.
- Pang MYC, Charlesworth SA, Lau RWK, Chung RCK. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovasc Dis. 2013;35(1):7–22. doi:10.1159/000346075.
- Buvarp D, Viktorisson A, Axelsson F, et al. Physical activity trajectories and functional recovery after acute stroke among adults in Sweden. JAMA Netw Open. 2023;6(5):e2310919. doi:10.1001/jamanetworkopen.2023.10919.
- Rahman MS, Peng W, Adams J, Sibbritt D. The use of self-management strategies for stroke rehabilitation: a scoping review. Top Stroke Rehabil. 2023;30(6):552–567. doi:10.1080/10749357.2022.2127651.
- Bailey RR. Lifestyle modification for secondary stroke prevention. Am J Lifestyle Med. 2018;12(2):140–147. doi:10.1177/1559827616633683.
- Saunders DH, Greig CA, Mead GE. Physical activity and exercise after stroke: review of multiple meaningful benefits. Stroke. 2014;45(12):3742–3747. doi:10.1161/STROKEAHA.114.004311.
- Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–2553. doi:10.1161/STR.0000000000000022.
- Towfighi A, Markovic D, Ovbiagele B. Impact of a healthy lifestyle on all-cause and cardiovascular mortality after stroke in the USA. J Neurol Neurosurg Psychiatry. 2012;83(2):146–151. doi:10.1136/jnnp-2011-300743.
- Epstein KA, Viscoli CM, Spence JD, Young LH, Inzucchi SE, Gorman M, et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology. 2017;89(16):1723–1729. doi:10.1212/WNL.0000000000004524.
- Ellepola S, Nadeesha N, Jayawickrama I, Wijesundara A, Karunathilaka N, Jayasekara P. Quality of life and physical activities of daily living among stroke survivors; cross‐sectional study. Nurs Open. 2022;9(3):1635–1642. doi:10.1002/nop2.1188.
- Aidar FJ, de Oliveira RJ, Silva AJ, de Matos DG, Carneiro AL, Garrido N, et al. The influence of the level of physical activity and human development in the quality of life in survivors of stroke. Health Qual Life Outcomes. 2011;9(1):89. doi:10.1186/1477-7525-9-89.
- Chen M-D, Rimmer JH. Effects of exercise on quality of life in stroke survivors: a meta-analysis. Stroke. 2011;42(3):832–837. doi:10.1161/STROKEAHA.110.607747.
- Hou L, Du X, Chen L, et al. Exercise and quality of life after first-ever ischaemic stroke: a two-year follow-up study. Int J Neurosci. 2018;128(6):540–548. doi:10.1080/00207454.2017.1400971.
- Chiba R, Tominaga S, Mikami K, Kitajima M, Urushizaka M, Tomisawa T, et al. Factors influencing quality of life in stroke patients: Focus on eating habits. J Stroke Cerebrovasc Dis. 2019;28(6):1623–1628. doi:10.1016/j.jstrokecerebrovasdis.2019.02.031.
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X.
- Australian Longitudinal Study on Women’s Health (ALSWH). Study design. https://alswh.org.au/for-data-users/study-design/. Accessed November 9, 2023.
- Lee C, Dobson AJ, Brown WJ, et al. Cohort profile: the Australian longitudinal study on women’s health. Int J Epidemiol. 2005;34(5):987–991. doi:10.1093/ije/dyi098.
- Lapin BR, Thompson NR, Schuster A, Honomichl R, Katzan IL. The validity of proxy responses on patient-reported outcome measures: are proxies a reliable alternative to stroke patients’ self-report? Qual Life Res. 2021;30(6):1735–1745. doi:10.1007/s11136-021-02758-9.
- Reimer C, Ali-Thompson S, Althawadi R, O’Brien N, Moran CN, Hickey A. Reliability of proxy reports on patient reported outcomes measures in stroke: an updated systematic review. J Stroke Cerebrovasc Dis. 2024;33(6):107700. doi:10.1016/j.jstrokecerebrovasdis.2024.107700.
- Australian Longitudinal Study on Women’s Health (ALSWH). Surveys. 2020. https://alswh.org.au/for-data-users/data-documentation/surveys/. Accessed November 9, 2023.
- Engstad T, Bønaa KH, Viitanen M. Validity of self-reported stroke: the tromsø study. Stroke. 2000;31(7):1602–1607. doi:10.1161/01.STR.31.7.1602.
- Jackson CA, Mishra GD, Tooth L, Byles J, Dobson A. Moderate agreement between self-reported stroke and hospital-recorded stroke in two cohorts of Australian women: a validation study. BMC Med Res Methodol. 2015;15(1):7. doi:10.1186/1471-2288-15-7.
- Jamrozik E, Hyde Z, Alfonso H, et al. Validity of self-reported versus hospital-coded diagnosis of stroke: a cross-sectional and longitudinal study. Cerebrovasc Dis. 2014;37(4):256–262. doi:10.1159/000358583.
- Gustavson K, von Soest T, Karevold E, Røysamb E. Attrition and generalizability in longitudinal studies: findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health. 2012;12(1):918. doi:10.1186/1471-2458-12-918.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi:10.1097/00005650-199206000-00002.
- Ware JE Jr, Kosinski M, Bayliss MS, Ca M, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the medical outcomes study. Med Care. 1995;33(4):AS264–AS279.
- Farivar SS, Cunningham WE, Hays RD. Correlated physical and mental health summary scores for the SF-36 and SF-12 health survey, V. 1. Health Qual Life Outcomes. 2007;5(1):54. doi:10.1186/1477-7525-5-54.
- Anderson C, Laubscher S, Burns R. Validation of the short form 36 (SF-36) health survey questionnaire among stroke patients. Stroke. 1996;27(10):1812–1816. doi:10.1161/01.STR.27.10.1812.
- Dorman P, Slattery J, Farrell B, Dennis M, Sandercock P. Qualitative comparison of the reliability of health status assessments with the EuroQol and SF-36 questionnaires after stroke. Stroke. 1998;29(1):63–68. doi:10.1161/01.STR.29.1.63.
- Australian Institute of Health and Welfare (AIHW). The active Australia survey: a guide and manual for implementation, analysis and reporting. Published. 2003. https://www.aihw.gov.au/reports/physical-activity/active-australia-survey/summary. Accessed November 9, 2023.
- Brown WJ, Burton NW, Marshall AL, Miller YD. Reliability and validity of a modified self‐administered version of the active Australia physical activity survey in a sample of mid‐age women. Aust N Z J Public Health. 2008;32(6):535–541. doi:10.1111/j.1753-6405.2008.00305.x.
- The National Health and Medical Research Council (NHMRC). Australian Guidelines to Reduce Health Risks from Drinking Alcohol. 2009. https://www.nhmrc.gov.au/sites/default/files/documents/reports/alcohol-harm-reduction-faq.pdf. Accessed November 9, 2023.
- World Health Organization (WHO). A healthy lifestyle - WHO recommendations. 2010. https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle—who-recommendations. Accessed November 9, 2023.
- Thabane L, Mbuagbaw L, Zhang S, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. 2013;13(1):92. doi:10.1186/1471-2288-13-92.
- Aloisio KM, Micali N, Swanson SA, Field A, Horton NJ. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J. 2014;14(4):863–883. doi:10.1177/1536867X1401400410.
- Raitanen J, Stenholm S, Tiainen K, Jylhä M, Nevalainen J. Longitudinal change in physical functioning and dropout due to death among the oldest old: a comparison of three methods of analysis. Eur J Ageing. 2020;17(2):207–216. doi:10.1007/s10433-019-00533-x.
- Saunders DH, Sanderson M, Hayes S, Johnson L, Kramer S, Carter DD, et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2020: CD003316. 2020(3). 10.1002/14651858.CD003316.pub7.
- West R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol Health. 2017;32(8):1018–1036. doi:10.1080/08870446.2017.1325890.
- Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9(1):285. doi:10.1186/1471-2458-9-285.
- Australian Government Department of Health and Aged Care. What are the effects of smoking and tobacco? 2022. https://www.health.gov.au/topics/smoking-and-tobacco/about-smoking-and-tobacco/what-are-the-effects-of-smoking-and-tobacco. Accessed November 21, 2023.
- Kahraman T, Ozdogar AT, Abasiyanik Z, et al. Associations between smoking and walking, fatigue, depression, and health-related quality of life in persons with multiple sclerosis. Acta Neurol Belg. 2021;121(5):1199–1206. doi:10.1007/s13760-020-01341-2.
- Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke. 2005;36(7):1480–1484. doi:10.1161/01.STR.0000170706.13595.4f.
- Heinze M, Lebherz L, Rimmele DL, Frese M, Jensen M, Barow E, et al. Higher comorbidity burden is associated with lower self-reported quality of life after stroke. Front Neurol. 2022;13:1023271. doi:10.3389/fneur.2022.1023271.
- Chen J, Li S, Zheng K, Wang H, Xie Y, Xu P, et al. Impact of smoking status on stroke recurrence. J Am Heart Assoc. 2019;8(8):e011696. doi:10.1161/JAHA.118.011696.
- Peng Y, Ngo L, Hay K, Alghamry A, Colebourne K, Ranasinghe I. Long-term survival, stroke recurrence, and life expectancy after an acute stroke in Australia and New Zealand from 2008–2017: a population-wide cohort study. Stroke. 2022;53(8):2538–2548. doi:10.1161/STROKEAHA.121.038155.
- Fryer CE, Luker JA, Mn M, Hillier SL. Self management programmes for quality of life in people with stroke. Stroke. 2016;47(12):e266–e267. doi:10.1161/STROKEAHA.116.015253.
- Okoro CA, Brewer RD, Naimi TS, Moriarty DG, Giles WH, Mokdad AH. Binge drinking and health-related quality of life: do popular perceptions match reality? Am J Prev Med. 2004;26(3):230–233. doi:10.1016/j.amepre.2003.10.022.
- Volk RJ, Cantor SB, Steinbauer JR, Cass AR. Alcohol use disorders, consumption patterns, and health‐related quality of life of primary care patients. Alcohol Clin Exp Res. 1997;21(5):899–905. doi:10.1111/j.1530-0277.1997.tb03855.x.
- Mathiesen EF, Nome S, Eisemann M, Richter J. Drinking patterns, psychological distress and quality of life in a Norwegian general population-based sample. Qual Life Res. 2012;21(9):1527–1536. doi:10.1007/s11136-011-0080-8.
- Ledden S, Moran P, Osborn D, Pitman A. Alcohol use and its association with suicide attempt, suicidal thoughts and non-suicidal self-harm in two successive, nationally representative English household samples. BJPsych Open. 2022;8(6):e192. doi:10.1192/bjo.2022.594.
- Wilsnack SC, Wilsnack RW, Kantor LW. Focus on: women and the costs of alcohol use. Alcohol Res Curr Rev. 2014;35(2):219–228. PMID: 24881330.
- Dimitrova-Shumkovska J, Krstanoski L, Veenman L. Diagnostic and therapeutic potential of TSPO studies regarding neurodegenerative diseases, psychiatric disorders, alcohol use disorders, traumatic brain injury, and stroke: an update. Cells. 2020;9(4):870. doi:10.3390/cells9040870.
- Mireault M, de Man AF. Suicidal ideation among the elderly: personal variables, stress and social support. Soc Behav Pers An Int J. 1996;24(4):385–392. doi:10.2224/sbp.1996.24.4.385.
- Cadilhac DA, Dalli LL, Morrison J, et al. The Australian stroke clinical registry annual report 2020. The Florey Institute of Neuroscience and Mental Health; 2021. https://auscr.com.au/wp-content/uploads/2022/12/auscr-2020-annual-report_final_v2.pdf. Accessed May 25, 2024.
- Lynch EB, Butt Z, Heinemann A, et al. A qualitative study of quality of life after stroke: the importance of social relationships. J Rehabil Med. 2008;40(7):518–523. doi:10.2340/16501977-0203.