Abstract
Context: Spinal cord injury or disease (SCI/D) leads to unchanged low-density lipoprotein and cholesterol, very low high-density lipoprotein a form of dyslipidemia and physical inactivity which combine to increase risk of morbidity and mortality from cardiometabolic disease. Herein, we describe the selection of structure, process and outcome indicators for adults in the first 18 months post-SCI/D rehabilitation admission.
Methods: A Pan-Canadian Cardiometabolic Health Working Group was formed to develop a construct definition. Cardiometabolic risk factors were summarized in a Driver diagram. Release of the Paralyzed Veterans of America “Identification and Management of Cardiometabolic Risk after Spinal Cord Injury” and the International Scientific Exercise Guidelines: “Evidence-based scientific exercise guidelines for adults with spinal cord injury”, informed the group’s focus on prevention strategies to advance this Domain of rehabilitation admission.
Results: The structure indicator identifies during rehabilitation the presence of appropriate time and resources for physical exercise prescription. Process indicators are lipid profile assessment at rehabilitation admission and documented exercise prescriptions prior to discharge. The outcome indicators track patient’s knowledge retention regarding exercise prescription at discharge, current exercise adherence and lipid status 18 months after rehabilitation discharge.
Conclusion: Routine national implementation of these indicators at the specified time points will enhance efforts to detect dyslipidemia and assure routine participation in endurance exercise. These indicators align with international initiatives to improve cardiometabolic health through interventions targeting modifiable risk factors specifically endurance exercising and optimal lipid profiles, crucial to augmenting cardiometabolic health after SCI/D.
Introduction
Spinal cord injury or disease (SCI/D) results in complex changes in a person’s physical health including their motor, sensory and autonomic functions, and their functional independence. The vast majority of these changes may be attributed to disruption of descending neural pathways that regulate the individual’s cardiorespiratory function, digestion, voiding, sex organs and thermoregulation.Citation1 During the chronic stage of SCI, cardiometabolic and cardiovascular disease risk factors become more prevalent. In this context, cardiometabolic disease refers to a clustering of interrelated risk factors that promote the development of atherosclerotic vascular disease and Type 2 diabetes mellitus.Citation2 The disease is comprised of maladaptive cardiovascular, renal, metabolic, pro-thrombotic and inflammatory pathologies, and has four component risks: obesity, insulin resistance, hypertension and dyslipidemia (characterized as low high-density lipoprotein cholesterol and elevated triglycerides). Cardiovascular disease refers conditions that involve narrowed or blocked blood vessels that result in myocardial ischemia and infarction, angina or stroke,Citation2 and is one of the leading causes of death in the chronic SCI population, contributing to approximately 46% of all deaths.Citation3 It is estimated that 30–50% of people living with a chronic SCI have cardiovascular disease.Citation2 Frequent reports of silent ischemia and sudden cardiac death after SCI/DCitation4,Citation5 highlight the need for interventions to reduce cardiometabolic disease precursors.
Prevalent risk factors for the accelerated development of cardiometabolic disease after SCI include: physical inactivity, low high-density lipoprotein, type II diabetes, hypertension, visceral adiposity, and elevated pro-atherogenic inflammation.Citation6–9 Thus, prevention strategies to mitigate cardiometabolic disease risk prior to disease expression should be a fundamental tenant of SCI/D rehabilitation. Although implementing appropriate interventions is a logical priority, little is known about how well the national SCI/D rehabilitation care system is performing in this regard. Currently, the National Rehabilitation Reporting System is limited to demographic and impairment descriptors, rehabilitation length of stay and changes in Functional Independence Measure scores from rehabilitation admission to discharge. Quality of care indicators are widely used to identify trends, inform priority setting and policy formulation, and monitor rehabilitation programs and care processes. Indicator data can further inform comparisons across different health care settings and ensure continuous quality improvement (i.e. benchmarking).Citation10,Citation11 Indicators can measure the structure, process or outcome of health care services and their evaluation can facilitate the sustainability of a high-quality health care delivery system that is based on evidence-informed programs and services.Citation12 National data describing care (i.e. indicator data) are a much-needed system barometer to enable future equitable and optimal care for persons living with SCI/D in Canada.
The type and severity of cardiometabolic dysfunction after SCI/D are specific to the individual’s impairment and the extent of the associated compromise of the sympathetic and parasympathetic nervous systems. Generally speaking, the higher the neurological level (i.e. motor and sensory level) the more severe the dysfunction. It is, however, important to note that the neurological level of injury does not always equate to the autonomic level of injury,Citation13 as the descending pathways controlling these respective functions travel in anatomically distinct areas of the spinal cord. Therefore, individuals that have the highest neurological level (i.e. tetraplegia) and most complete autonomic injury exhibit the most severe hypotension (i.e. lower baseline arterial blood pressure), and autonomic dysreflexia (a sudden increase in systolic blood pressure 30 mmHg above baseline in response to noxious stimuli below the level of injuryCitation14,Citation15). Concurrent poor diet and physical inactivityCitation16 result in a 79% prevalence of dyslipidemia within the first year post-injury (e.g. reduced levels of high-density lipoprotein cholesterolCitation17,Citation18 and elevated levels of low-density lipoprotein cholesterolCitation19).
Exercise is the primary rehabilitation intervention to reduce cardiometabolic disease incidence in the SCI/D population. Voluntary upper limb active aerobic exercise interventions are used with the aim of improving cardiometabolic fitness, glucose metabolism, and lipid profiles after SCI/D. The effectiveness of exercise may be limited by the level and severity of injury and adherence to SCI-specific physical activity guidelines. The 2018 International Scientific Exercise Guidelines: “Evidence-based scientific exercise guidelines for adults with spinal cord injury”, developed by Martin Ginis et al.Citation20 states that “at least 20 minutes of moderate to vigorous intensity aerobic exercise two times per week AND three sets of strength exercises for each major functioning muscle group, at a moderate to vigorous intensity, two times per week” are necessary to improve cardiorespiratory fitness and muscle strength. Cardiometabolic health benefits are achieved through “at least 30 minutes of moderate to vigorous intensity aerobic exercise three times per week”,Citation20 a volume of exercise which is lower than the recommendations from the Consortium for Spinal Cord Medicine Clinical Practice GuidelinesCitation21,Citation22 and the Exercise and Sports Science AustraliaCitation23 guidelines: 150 min per week, up to five days per week. Although cardiometabolic risk profiles are well documented after SCI/D, routine processes to detect and mitigate cardiometabolic risk during SCI/D rehabilitation and then in the transition to community living are urgently required.
The SCI Rehabilitation Care High Performance Indicators or “SCI-High Project” is a bold endeavor to develop/select, implement and evaluate consensus derived quality of care indicators for select Domains of rehabilitation prioritized by clinicians, researchers and individuals living with chronic SCI/D by 2020. The SCI-High indicators are measures intended to inform how well a health system is performing in terms of structures and processes of care and provide insight into the associated patient outcomes. This report describes the processes involved in the selection, development and implementation of structure, process and outcome indicators related to the Cardiometabolic Health Domain from rehabilitation admission to 18 months thereafter. These processes are part of a common methodology,Citation24 used to derive indicators for 11 prioritized Domains of SCI/D rehabilitationCitation25 within the SCI-High Project.
Methods
A detailed description of the overall SCI-High Project methods and process for identifying Cardiometabolic Health as a priority Domain for SCI/D rehabilitation care are described in related manuscripts in this issue,Citation24,Citation25 respectively. In addition to the SCI-High Project team (www.sci-high.ca), an External Advisory Committee, and National Data Strategy Committee supported the global project goals and provided oversight regarding the context for implementing all of the planned indicators.
The approach to developing the Cardiometabolic Health indicators followed a modified but similar approach to that described by Mainz et al.Citation10 which included the following planning and development phases: (a) formation and organization of the national and local Working Groups;Citation24 (b) defining and refining the key Domain-specific construct; (c) providing an overview of existing evidence and practice to develop and interpret a Driver diagram; (d) selecting indicators; and (e) pilot testing and refinement of the Domain-specific structure, process and outcome indicators. Throughout these processes, a facilitated discussion occurred amongst the Cardiometabolic Health Working Group and the SCI-High Project team to utilize relevant expertise on the topic, while assuring the broader alignment of goals across the other 10 Domain Working Groups (as appropriate).
Cardiometabolic Health Working Group
Experts in cardiometabolic health were invited to participate in the SCI-High Project as members of the Domain-specific Working Group based on their lived experience, practical or empirical knowledge of: rehabilitation, cardiovascular health, and/or health service delivery. The group was composed of a cardiac rehabilitation specialist, kinesiologists, a neurologist, a physical therapist, a community educator, physiatrists and a stakeholder routinely engaged in physical exercise activities. The Working Group met six times via conference call over two months, totaling eight hours of discussions when developing the indicators and for an additional three hours thereafter, to refine the indicators and discuss report preparation. Additionally, outside of the formal meetings, individual members of the working group completed their own independent review of the prepared materials, or shared resources and practice standards, with one another, or conducted independent evaluations of the proposed indicators.
Driver diagram and construct definition
The selection of Cardiometabolic Health as a Domain of interest for developing indicators emerged from the environmental scan (E-Scan) consensus-building activity designed to select the broader set of Domains.Citation26 This process involved a systematic search to collect information about SCI/D rehabilitation care related to Cardiometabolic Health and factors that influence the outcome of rehabilitation interventions and a scoping synthesis of the acquired data. Medline and Embase databases were searched using the terms “cardiometabolic health”, “spinal cord injury”, or both. Non-English manuscripts and inappropriate study designs were excluded. This information was used to create a preliminary Driver diagram to illustrate known drivers or factors that impact cardiometabolic health among individuals with SCI/D (). A Driver diagram is a visual display of a high-level quality improvement goal, and a set of underpinning factors. The tool helped to visually organize change concepts as the Working Group discerned “what changes can we make, that will result in goal attainment”. The branches in red within the final Driver diagram represent the main areas that were the focus for development of the indicators based on experts’ opinions. The impairment branch of the Driver diagram was common to all 11 Domains ().
Figure 1 Cardiometabolic Health Driver diagram. UEMS: upper-extremity motor score; LEMS: lower-extremity motor score; NLI: neurological level of injury; AIS: ASIA Impairment Scale; HR: heart rate; BP: blood pressure; ABP: arterial blood pressure; FES: functional electrical stimulation; LDL: low-density lipoprotein; HDL: highdensity lipoprotein; TG: triglycerides; TC: total cholesterol. *Serum testosterone, growth hormone & renin-angiotensin-aldosterone system.
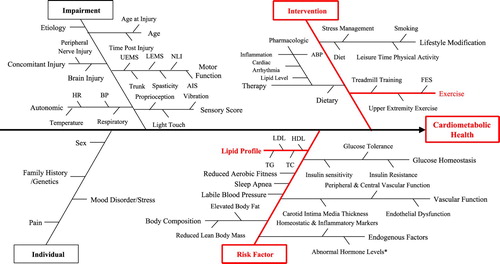
Following review of the literature and available guidelines, discussion and refinement of the Driver diagram, the group agreed that endocrine-metabolic disorders (i.e. lipid profile) and physical inactivity were the two main drivers most likely to advance SCI/D rehabilitation care in the near term (project context). This decision also aligned with cardiometabolic disease surveillance and treatment guidelines following SCI/D, which emphasizes preventive care for individuals who are overweight/obese, or who have serologic evidence of insulin resistance or dyslipidemia and hypertension, and or physical deconditioning on clinical examination.Citation22 Based on this discussion, the following construct definition was created:
“The cardiovascular system is responsible for the transport of oxygen-rich blood and energy supply throughout the body and is controlled on a beat-by-beat basis by the autonomic nervous system. After SCI/D, disruption of autonomic control, changes in metabolic profile, and inactivity combine to alter the functioning of the cardiovascular system at rest, and especially during exercise. Optimal cardiovascular health can be maintained or achieved through establishing appropriate health behaviors (i.e. physical activity and diet) and health interventions (i.e. treated total cholesterol and blood glucose levels) to mitigate dysautonomia, cardiometabolic risk and reduce cardiovascular morbidity and mortality.”
The Working Group also recognized that other potent cardiometabolic risk factors such as detection and management of diabetes,Citation29 hypertension and maintaining an optimal waist circumferenceCitation30 can play important roles on promoting cardiometabolic health during SCI rehabilitation. However, the Working Group chose to focus on physical exercise prescription and management of lipid profiles over other risk factors such as diabetes/hypertension recognition and management which are well embedded in primary care, as the recommendations for management do not differ for those with SCI/D versus the general population. Whereas, there are important nuances regarding management of dyslipidemia and inactivity after SCI/D that are unique from the general population.
Selection of indicators
The Cardiometabolic Health Working Group was responsible for selecting and developing at least one structure, one process and one outcome indicator related to the Domain construct. The leaders stipulated that the developed indicators must be relevant, concise and feasible (10 minutes or less to implement), and aligned in their aim across the structure, process and outcome indicators in order to achieve a single substantive advance in SCI/D rehabilitation care. The Working Group was advised that they could use established measurement tools or developing their own (i.e. questionnaires, data collection sheets, laboratory exams, and medical record data), depending on the requirements and feasibility of a given indicator.
Indicator piloting
The described indicators are intended for patients with a motor level below C6 [C6-T12 American Spinal Injury Association (ASIA) Impairment Scale (AIS) A-D] recognizing that these indicators may not address the needs of those with higher motor levels.Citation31 Patients with injuries above C6 were excluded due to their complete or partial inability to perform meaningful voluntary endurance exercise that includes large muscle groups.
The feasibility of some of the indicators was tested within an outpatient clinic setting at a tertiary SCI/D rehabilitation hospital using rapid cycles of Plan-Do-Study-Act.Citation32 These short-term quality improvement testing cycles allow for quick qualitative evaluations, feedback and refinement of tools or processes related to quality of care. Successive patients attending routine follow-up appointments were approached by a trained evaluator part of the Cardiometabolic Health Working Group to complete the questionnaire. Assistance was provided in physically completing the paper and pen questionnaire if individuals could not complete it themselves (i.e. individual had impaired hand function). Feedback received from patients and shared with the Working Group resulted in refinements to the questionnaire and a new testing cycles. The main focus of the Plan-Do-Study-Act cycles for the Cardiometabolic Health Domain was to pilot the feasibility of the questionnaires designed to collect indicator data regarding physical activity education (intermediary outcome indicator – inpatients; ) and adherence (final outcome indicator – outpatients; ) relative to SCI/D-specific exercise guidelines. In addition, barriers to questionnaire implementation were noted.
Table 1 Intermediary outcome indicator assessment form for individuals living with SCI/D.
Table 2 Final outcome indicator questionnaire.
Results
Indicator description
The selection and refinement of structure, process and outcome indicators related to Cardiometabolic Health was based on the information summarized from the Driver diagram depicted in , and the construct definition. summarizes the numerator and denominators, type of indicator and timing for measurement of each indicator selected by the Cardiometabolic Health Working Group. The group elected to add a second process indicator, an intermediary outcome indicator and a second final outcome indicator.
Table 3 Selected structure, process and outcome indicators for the Cardiometabolic Health Domain.
The assessment of the structure indicator will be based on submission of the related care map or care paradigm and a program self-evaluation of: “Does your rehabilitation program provide SCI/D-specific aerobic exercise prescription following the current guidelines proposed by Martin Ginis et al. 2018? Yes or No.”
Physical exercise intensity of moderate to vigorous should follow the recommendations of the Physical Activity Recall Assessment for People with Spinal Cord Injury (PARA-SCI).Citation33,Citation34 Moderate intensity is described as “Includes physical activities that require some physical effort. You should feel like you are working somewhat hard but you should feel like you can keep going for a long time”. Vigorous intensity is described as “Includes physical activities that require a lot of physical effort. You should feel like you are working really hard (almost at your maximum) and can only do the activity for a short time before getting tired. These activities can be exhausting”.
Indicator piloting
A trained evaluator conducted preliminary interviews in an outpatient clinic setting at an SCI/D rehabilitation hospital to evaluate and refine the indicator related questionnaires. The pilot data on cardiometabolic health related to physical exercise education and adherence includes results from 31 individuals with SCI/D that completed the questionnaire. Questionnaires were completed within an average of two minutes. Noted feasibility issues and challenges experienced included: (1) the impact of cognitive and motor deficits on the ability to self-administer the questionnaire; (2) inability to understand certain aspects of the questionnaire (i.e. clarifications needed on the timeframe for the questions being asked); (3) inability to recall if they were provided physical exercise education (i.e. due to duration of post-injury); (4) discrepancy between exercise education provided and current physical activity (e.g. helping individuals to understand that we were asking and what they were doing, as opposed to what they had been taught to do); (5) difficulties distinguishing aerobic exercise from strength training; and (6) the questionnaire was not applicable to the current physical ability status (e.g. due to frequent secondary health conditions, other comorbidities, age, and neurological level of injury) nor their willingness to adhere to the guidelines.
During the Working Group’s activities, routine testing of lipid profiles was incorporated into routine admission bloodwork at some centers. Thus, the investigative team elected not to pilot this assessment at rehabilitation admission. According to the Guidelines for Identification and Management of Cardiometabolic Risk after Spinal Cord Injury (or Paralyzed Veterans of America Guideline),Citation2 dyslipidemia can be identified as total plasma triglycerides levels equal or above 150 mg/dL (1.7 mmol/L) and reduced high-density lipoprotein [women: < 50 mg/dL (1.29 mmol/L); men: < 40 mg/dL (1.03 mmol/L)]. We had concurrently anticipated questions from clinicians regarding what to do once dyslipidemia is detected and planned to create a decision support tool to assist clinicians with the management of dyslipidemia at rehabilitation admission or 18 months post-injury; however, during our group deliberations an evidence-informed decision support tool was published by Dr Nash and colleaguesCitation35 and the Working Group elected to use this to inform provider practice behavior during indicator implementation. The recognition and management of dyslipidemia for individuals with SCI/D is unique when compared to the general population. Given that the group perceived value in endorsing a single care paradigm for the SCI/D community, the Nash et al.Citation35 paradigms for appropriate management of dyslipidemia (yes or no – 18-month outcome indicator) was adopted as the desired standard of care for interpretation of the 18-month outcome indicator.
Discussion
The structure, process and outcome indicators selected and developed by the SCI-High Cardiometabolic Health Working Group are intended to facilitate prevention of cardiometabolic disease through routine: (1) evaluation of lipid profiles at rehabilitation admission and 18 months post-injury and (2) education, training and subsequent adherence of individuals with SCI/D to SCI/D-specific exercise guidelines.
Exercise interventions after SCI are associated with improved arterial structure and function,Citation36 glucose homeostasisCitation37 and lipid profiles.Citation38 The quality of exercise interventions may depend on the availability of infrastructure, time and the expertise of the people trained to provide exercise prescription for this population.Citation39 The structure indicator was designed to quantify the availability of time and resources within a rehabilitation center dedicated to exercise prescription. The Cardiometabolic Health Working Group operated with the assumption that this structure indicator is the initial building block required to educate patients about how to exercise after the injury and regarding the importance of physical exercise to maintain or improve cardiovascular health.
It is well established that the SCI/D population has similar or higher cardiometabolic risk as non-injured populations. However, dyslipidemia resulting from physical deconditioning, sarcopenia, lower resting metabolic rate and poor diet play an important role in the increased cardiometabolic risk after SCI/D [see Nash et al.Citation40 for review]. The first process and the first final outcome indicators were selected to screen the lipid profile of inpatients at rehabilitation discharge and 18 months following admission, respectively. These will contribute to understanding the lipid profiles of individuals with SCI/D prior to and following receipt of educational and interventional strategies aiming to promote an active lifestyle. In the event that abnormal lipid values are noted at the time of collection of the intermediary and final lipid profile outcomes, the therapy intervention thresholds proposed by Baumann et al.Citation41 and the specific interventions proposed by Nash et al.Citation35 are the current recommendations from the Cardiometabolic Health guideline.
The recent publication of SCI-specific exercise guidelines for fitness and cardiometabolic healthCitation20 as well as guidelines for improving cardioendocrine disease surveillanceCitation22 were key documents to inform the Working Group when selecting the Cardiometabolic Health indicators. These guidelines provided evidence regarding of intensity, volume and frequency of exercise for people living with SCI; however, there are no mechanisms currently in place to measure aerobic exercise prescription, knowledge retention, and adherence for inpatients and outpatients. The indicators were intentionally designed to address this gap. The second process indicator was intended to measure the efficacy of educational interventions regarding exercise prescription aligned with current SCI/D exercise guidelines.
The first outcome indicator was developed to identify if inpatients can learn and retain the minimal frequency, intensity, and type of exercise required to maintain or improve cardiometabolic health. The second final outcome indicator focuses on measuring an outpatient’s adherence to the SCI/D exercise guidelines for aerobic exercise once they are living in the community. Although physical exercise is fundamental to reduce cardiometabolic disease risk in the SCI/D population, it is important to recognize that the proposed indicators will not capture data from individuals with all levels of impairment due to SCI/D.
In the last decade, the length of stay in inpatient rehabilitation has been declining.Citation42 Thus, individuals with SCI/D are entering the community having had less time to learn and establish positive health behaviors prior to discharge. Further, in the first year post-injury, more than 50% of individuals discharged with an SCI require re-hospitalization for a secondary health condition such as a urinary tract infection or tissue injury.Citation43 These events often disrupt established health behaviors to address urgent health issues. To further challenge the individual’s ability to sustain a focus on secondary health prevention, primary care providers and community settings are not well-equipped to support individuals with SCI/D and their impairment-related specialized needs.Citation44,Citation45 Condition-specific self-management skills defined by Barlow et al.Citation46 as “the ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a chronic condition” are essential to ensuring longevity and prevention of cardiometabolic disease after SCI/D. The enclosed indicators reflect both the provider and patient perspectives on the effectiveness of prevention strategies. The Cardiometabolic Health indicators are currently being implemented in the provinces of Ontario and Quebec to investigate the overall feasibility of in-hospital and community-based data collection and challenges associated with culture and language (English vs. French).
There are some potential limitations of the Cardiometabolic Health indicators, such as glucose control, questionnaire terminology, physical exercise recall, knowledge retention regarding appropriate physical exercise, and patient level of injury. The indicators are focused on tracing lipid profiles during rehabilitation and in the community, excluding other important markers related to glucose metabolism. The choice for lipid profiles relates to the fact that treatment of diabetes in SCI/D is not different from normal population and there are well established Canadian treatment guidelines. Complex questionnaire terminology may reduce completion rates in patients with reduced cognitive ability or English fluency. Self-reported physical activity is problematic in the SCI population and the proposed final outcome indicator will not be immune to this limitation.Citation47,Citation48 Although physical exercise recall can be problematic, it was shown to be feasible when using recall periods of 7 daysCitation49 or less.Citation50 Knowledge retention regarding the guidelines for physical exercise in the SCI population were noted as potentially problematic during the Plan-Do-Study-Act cycles. However, most patients that participated in the piloting were outpatients with varying duration of injury post-rehabilitation discharge. We anticipate that knowledge retention rates will be higher prior to rehabilitation discharge, up to 18 months post-discharge. Lastly, individuals with motor compete lesions above C6 will be excluded from the indicator data collection cohort, limiting the generalizability of our results.
Conclusions
Successful implementation of the indicators will characterize the cardiometabolic health status of individuals with SCI/D during the first 18 months following rehabilitation admission. These indicators will: (1) inform the availability of resources (i.e. infrastructure, staff and educational tools) related to exercise prescription; (2) identify the adherence to SCI/D-specific physical exercise guidelines dedicated to improve cardiometabolic health; and (3) screen patients regarding their lipid profile post-injury and in response to physical exercise routines. The implementation of these structure, process and outcome indicators is a first step towards providing equitable and optimal care related to the Cardiometabolic Health Domain after SCI/D.
Disclaimer statements
Disclosures Dr. Wiest acknowledges support from the Toronto Rehab Foundation. Dr. Julio C. Furlan receives salary support from the Wings for Life Spinal Cord Research Foundation. Dr. Bayley acknowledges support from the Saunderson Family Chair in Acquired Brain Injury Research, the Toronto Rehab Chair in SCI Rehabilitation, and the Toronto Rehab Foundation. Dr. Craven acknowledges support from the Toronto Rehab Foundation as the Toronto Rehabilitation Institute Chair in Spinal Cord Injury Rehabilitation and receipt of consulting fees from the Rick Hansen Institute. Dr. S. Mohammad Alavinia, Dr. David Ditor, Farnoosh Farahani, Dr. Masae Miyatani, Dr. Paul Oh, and Dr. Christopher West report no conflicts of interest.
Acknowledgements
The authors would like to acknowledge the time, energy and expertise of Heather Flett and Dr. Gaya Jeyathevan from Toronto Rehabilitation Institute – University Health Network, Dr. Audrey Hicks from McMaster University, and Dr. Sander Hitzig from St. John’s Rehab Research Program– Sunnybrook Research Institute throughout the indicator development process. As well we would like to thank Dr. Krista L. Best from the Centre for Interdisciplinary Research in Rehabilitation and Social Integration for translating the Cardiometabolic Health Questionnaires to French.
ORCID
Matheus J. Wiest http://orcid.org/0000-0003-1444-4828
Christopher West http://orcid.org/0000-0002-0815-4122
David Ditor http://orcid.org/0000-0001-8045-9433
Julio C. Furlan http://orcid.org/0000-0002-2038-0018
Masae Miyatani http://orcid.org/0000-0003-3802-7148
Farnoosh Farahani http://orcid.org/0000-0002-3937-7708
S. Mohammad Alavinia http://orcid.org/0000-0002-5503-9362
Paul I. Oh http://orcid.org/0000-0002-0603-6958
Mark T. Bayley http://orcid.org/0000-0001-7860-9463
B. Catharine Craven http://orcid.org/0000-0001-8234-6803
Additional information
Funding
References
- Sezer N, Akkus S, Ugurlu FG. Chronic complications of spinal cord injury. World J Orthop. 2015;6(1):24–33. doi: 10.5312/wjo.v6.i1.24
- Nash MS, Groah SL, Gater Jr. DR, Dyson-Hudson TA, Lieberman JA, Myers J, et al. Identification and management of cardiometabolic risk after spinal cord injury: clinical practice guideline for health care providers. Top Spinal Cord Inj Rehabil. 2018;24(4):379–423. doi: 10.1310/sci2404-379
- Whiteneck GG, Charlifue SW, Frankel HL, Fraser MH, Gardner BP, Gerhart KA, et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia. 1992;30(9):617–30.
- Miyatani M, Masani K, Moore C, Szeto M, Oh P, Craven C. Test-retest reliability of pulse wave velocity in individuals with chronic spinal cord injury. J Spinal Cord Med. 2012;35(5):400–405. doi: 10.1179/2045772312Y.0000000042
- Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC. Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med. 2009;32(1):72–8. doi: 10.1080/10790268.2009.11760755
- Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile – part I. J Spinal Cord Med. 2014;37(6):693–702. doi: 10.1179/2045772314Y.0000000245
- Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43(6):749–56. doi: 10.1016/0026-0495(94)90126-0
- Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia. 1992;30(10):697–703.
- Noller CM, Groah SL, Nash MS. Inflammatory stress effects on health and function after spinal cord injury. Top Spinal Cord Inj Rehabil. 2017;23(3):207–17. doi: 10.1310/sci2303-207
- Mainz J. Developing evidence-based clinical indicators: a state of the art methods primer. Int J Qual Health Care. 2003;15(Suppl 1):i5–11. doi: 10.1093/intqhc/mzg084
- Rubin HR, Pronovost P, Diette GB. The advantages and disadvantages of process-based measures of health care quality. Int J Qual Health Care. 2001;13(6):469–74. doi: 10.1093/intqhc/13.6.469
- Burns AS, Yee J, Flett HM, Guy K, Cournoyea N. Impact of benchmarking and clinical decision making tools on rehabilitation length of stay following spinal cord injury. Spinal Cord. 2013;51(2):165–9. doi: 10.1038/sc.2012.91
- West CR, Bellantoni A, Krassioukov AV. Cardiovascular function in individuals with incomplete spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil. 2013;19(4):267–78. doi: 10.1310/sci1904-267
- Berger MJ, Kimpinski K, Currie KD, Nouraei H, Sadeghi M, Krassioukov AV. Multi-domain assessment of autonomic function in Spinal cord injury using a modified autonomic reflex screen. J Neurotrauma. 2017;34(18):2624–33. doi: 10.1089/neu.2016.4888
- Berger MJ, Hubli M, Krassioukov AV. Sympathetic skin responses and autonomic dysfunction in spinal cord injury. J Neurotrauma. 2014;31(18):1531–9. doi: 10.1089/neu.2014.3373
- Bravo G, Guizar-Sahagun G, Ibarra A, Centurion D, Villalon CM. Cardiovascular alterations after spinal cord injury: an overview. Curr Med Chem Cardiovasc Hematol Agents. 2004;2(2):133–48. doi: 10.2174/1568016043477242
- Koyuncu E, Nakipoglu Yuzer GF, Yenigun D, Ozgirgin N. The analysis of serum lipid levels in patients with spinal cord injury. J Spinal Cord Med. 2017;40(5):567–72. doi: 10.1080/10790268.2016.1228286
- Vichiansiri R, Saengsuwan J, Manimmanakorn N, Patpiya S, Preeda A, Samerduen K, et al. The prevalence of dyslipidemia in patients with spinal cord lesion in Thailand. Cholesterol. 2012;847462.
- Benvenuto LJ, Krakoff LR. Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am J Hypertens. 2011;24(2):135–44. doi: 10.1038/ajh.2010.146
- Ginis KA M, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56(4):308–21. doi: 10.1038/s41393-017-0017-3
- Identification and management of cardiometabolic risk after spinal cord injury – clinical practice guideline for health care providers: paralyzed Veterans of America; 2018. Available from https://www.pva.org/CMSPages/GetFile.aspx?guid = f3c29b7e-e201-4392-b241-9933dc620e40.
- Nash MS, Bilzon JLJ. Guideline approaches for cardioendocrine disease surveillance and treatment following spinal cord injury. Curr Phys Med Rehabil Rep. 2018;6(4):264–276. doi: 10.1007/s40141-018-0203-z
- Tweedy SM, Beckman EM, Geraghty TJ, Theisen D, Perret C, Harvey LA, et al. Exercise and sports science Australia (ESSA) position statement on exercise and spinal cord injury. J Sci Med Sport. 2017;20(2):108–15. doi: 10.1016/j.jsams.2016.02.001
- Craven B, Alavinia S, Wiest M, Farahani F, Hitzig S, Flett H, et al. Methods for development of structure, process and outcome indicators for prioritized spinal cord injury rehabilitation domains: SCI-High Project. J Spinal Cord Med. 2019;42(Suppl 1):S51–S67.
- Alavinia S, Hitzig S, Farahani F, Flett H, Bayley M, Craven B. Prioritization of rehabilitation domains for establishing spinal cord injury high performance indicators using a modification of the hanlon method: SCI-High Project. J Spinal Cord Med. 2019;42(Suppl 1):S43–S50.
- Craven C, Balioussis C, Verrier MC, Hsieh JT, Cherban E, Rasheed A, et al. Using scoping review methods to describe current capacity and prescribe change in Canadian SCI rehabilitation service delivery. J Spinal Cord Med. 2012;35(5):392–9. doi: 10.1179/2045772312Y.0000000045
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31.
- Van der Scheer JW, Martin Ginis KA, Ditor DS, Goosey-Tolfrey VL, Hicks AL, West CR, et al. Effects of exercise on fitness and health of adults with spinal cord injury: a systematic review. Neurology. 2017;89(7):736–45. doi: 10.1212/WNL.0000000000004224
- Banerjea R, Sambamoorthi U, Weaver F, Maney M, Pogach LM, Findley T. Risk of stroke, heart attack, and diabetes complications among veterans with spinal cord injury. Arch Phys Med Rehabil. 2008;89(8):1448–53. doi: 10.1016/j.apmr.2007.12.047
- Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43(9):513–8. doi: 10.1038/sj.sc.3101744
- Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695
- Langley GL, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The improvement Guide: a practical approach to Enhancing Organizational Performance. 2nd edition. San Francisco: Jossey-Bass Publishers; 2009.
- Latimer KGA. Physical activity recall assessment for people with spinal cord injury (PARA-SCI), Administration and Scoring Manual.
- Ginis KA, Latimer AE, Hicks AL, Craven BC. Development and evaluation of an activity measure for people with spinal cord injury. Med Sci Sports Exercise. 2005;37(7):1099–111. doi: 10.1249/01.mss.0000170127.54394.eb
- Nash MS, Bilzon JLJ. Guideline approaches for cardioendocrine disease surveillance and treatment following spinal cord injury. Curr Phys Med Rehabil Rep. 2018;6(4):264–76. doi: 10.1007/s40141-018-0203-z
- Phillips AA, Cote AT, Warburton DE. A systematic review of exercise as a therapeutic intervention to improve arterial function in persons living with spinal cord injury. Spinal Cord. 2011;49(6):702–14. doi: 10.1038/sc.2010.193
- Jeon JY, Hettinga D, Steadward RD, Wheeler GD, Bell G, Harber V. Reduced plasma glucose and leptin after 12 weeks of functional electrical stimulation-rowing exercise training in spinal cord injury patients. Arch Phys Med Rehabil. 2010;91(12):1957–9. doi: 10.1016/j.apmr.2010.08.024
- El-Sayed MS, Younesian A. Lipid profiles are influenced by arm cranking exercise and training in individuals with spinal cord injury. Spinal Cord. 2005;43(5):299–305. doi: 10.1038/sj.sc.3101698
- Dijkers MP, Zanca JM. Factors complicating treatment sessions in spinal cord injury rehabilitation: nature, frequency, and consequences. Arch Phys Med Rehabil. 2013;94(4 Suppl):S115–24. doi: 10.1016/j.apmr.2012.11.047
- Nash MS, Tractenberg RE, Mendez AJ, David M, Ljungberg IH, Tinsley EA, et al. Cardiometabolic syndrome in people with spinal cord injury/disease: guideline-derived and nonguideline risk Components in a pooled sample. Arch Phys Med Rehabil. 2016;97(10):1696–705. doi: 10.1016/j.apmr.2016.07.002
- La Fountaine MF, Cirnigliaro CM, Hobson JC, Dyson-Hudson TA, Mc Kenna C, Kirshblum SC, et al. Establishing a threshold to predict risk of cardiovascular disease from the serum triglyceride and high-density lipoprotein concentrations in persons with spinal cord injury. Spinal Cord. 2018;56(11):1051–8. doi: 10.1038/s41393-018-0187-7
- Whiteneck G, Gassaway J, Dijkers M, Backus D, Charlifue S, Chen D, et al. The SCIRehab project: treatment time spent in SCI rehabilitation. inpatient treatment time across disciplines in spinal cord injury rehabilitation. J Spinal Cord Med. 2011;34(2):133–48. doi: 10.1179/107902611X12971826988011
- Guilcher SJ, Craven BC, Calzavara A, McColl MA, Jaglal SB. Is the emergency department an appropriate substitute for primary care for persons with traumatic spinal cord injury? Spinal Cord. 2013;51(3):202–8. doi: 10.1038/sc.2012.123
- Iezzoni LI, Davis RB, Soukup J, O'Day B. Quality dimensions that most concern people with physical and sensory disabilities. Arch Intern Med. 2003;163(17):2085–92. doi: 10.1001/archinte.163.17.2085
- Veltman A, Stewart DE, Tardif GS, Branigan M. Perceptions of primary healthcare services among people with physical disabilities – part 1: access issues. MedGenMed. 2001;3(2):18.
- Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48(2):177–87. doi: 10.1016/S0738-3991(02)00032-0
- Jörgensen S, Svedevall S, Magnusson L, Martin Ginis KA, Lexell J. Associations between leisure time physical activity and cardiovascular risk factors among older adults with long-term spinal cord injury. Spinal Cord. 2019. Epub ahead of print.
- Ma JK, McCracken LA, Voss C, Chan FHN, West CR, Martin Ginis KA. Physical activity measurement in people with spinal cord injury: comparison of accelerometry and self-report (the physical activity recall assessment for people with spinal cord injury). Disabil Rehabil. 2018: 1–7. doi: 10.1080/09638288.2018.1494213
- Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;83(2):193–200. doi: 10.1053/apmr.2002.27467
- Latimer AE, Ginis KA, Craven BC, Hicks AL. The physical activity recall assessment for people with spinal cord injury: validity. Med Sci Sports Exercise. 2006;38(2):208–16. doi: 10.1249/01.mss.0000183851.94261.d2
