Abstract
Context
More than half of all spinal cord injuries (SCI) occur at the cervical level leading to loss of upper limb function, restricted activity and reduced independence. Several technologies have been developed to assist with upper limb functions in the SCI population.
Objective
There is no clear clinical consensus on the effectiveness of the current assistive technologies for the cervical SCI population, hence this study reviews the literature in the years between 1999 and 2019.
Methods
A systematic review was performed on the state-of-the-art assistive technology that supports and improves the function of impaired upper limbs in cervical SCI populations. Combinations of terms, covering assistive technology, SCI, and upper limb, were used in the search, which resulted in a total of 1770 articles. Data extractions were performed on the selected studies which involved summarizing details on the assistive technologies, characteristics of study participants, outcome measures, and improved upper limb functions when using the device.
Results
A total of 24 articles were found and grouped into five categories, including neuroprostheses (invasive and non-invasive), orthotic devices, hybrid systems, robots, and arm supports. Only a few selected studies comprehensively reported characteristics of the participants. There was a wide range of outcome measures and all studies reported improvements in upper limb function with the devices.
Conclusions
This study highlighted that assistive technologies can improve functions of the upper limbs in SCI patients. It was challenging to draw generalizable conclusions because of factors, such as heterogeneity of recruited participants, a wide range of outcome measures, and the different technologies employed.
Introduction
Each year in the UK, 1000 people sustain a traumatic spinal cord injury, and in total 40,000 people live with a spinal cord injury (SCI).Citation1–3 This number is higher in the United States, where approximately 294,000 (range 250,000–368,000) individuals live with SCI and each year around 17,810 new SCI cases are reported.Citation4–6 More than half of all cases of SCI occur at the cervical level leading to loss of hand and upper limb function.Citation6,Citation7 This complex impairment results in restricted activity and independence, hence significantly compromising wellbeing and quality of life.Citation8,Citation9 This life-changing injury remains a particular challenge to modern society as there is no cure. However, technological systems have been developed to restore some upper limb function for individuals with tetraplegia due to SCI including systems with neuroprostheses, orthotics, robots, and hybrid devices.
Individuals affected by high-level SCI see restoration of upper limb functions as a high priority.Citation10 Increased motor function in the hand and arms for this population can be achieved by surgical interventions or by assistive technologies.Citation11,Citation12 Unlike therapeutic technologies, which seek to improve physical impairments, assistive technologies are designed to assist with the performance of specific tasks for the user and intended for use when neurological recovery has reached a plateau. There has been ongoing research and development on assistive technologies for tetraplegia in the last 20 years. There is no clear clinical consensus on the effectiveness of the current assistive technologies for the cervical SCI population; therefore, we decided to review the literature for the years between 1999 and 2019.
The aim of this study was to systematically review the state-of-the-art assistive technology that supports and improves function of impaired upper limbs in people with cervical SCI. In addition, clinical outcomes, resulting from the implementation of such technologies, have been reviewed. To fulfill the aim of the study, we set out two main objectives and they were to:
Describe the assistive technology, with a focus on devices that interface with the upper limbs; and
Describe the outcome measures used when testing the efficacy of the technologies.
Methods
Search strategy
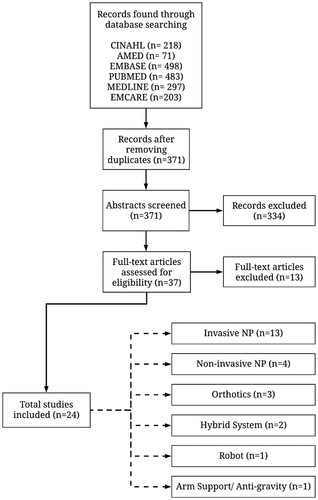
An electronic search of databases, including (CINAHL, AMED, EMBASE, PUBMED, MEDLINE, EMCARE) from 1999 to 2019, was performed. Initially, three categories essential to assess assistive technologies for clinical purposes were established: clinical condition, type of technology, and affected body part. Combinations of search terms within the three categories were used, sometimes with truncation, to capture all possible variations (). Two examples of search strategies are shown in the supplementary materials (Example S1 and S2). In addition to the electronic search of the databases, the reference lists of relevant publications were checked.
Table 1 Included and excluded terms used for electronically searching databases.
Study selection
Initially, duplicate, low-level of evidence (for example articles with excluded terms), and irrelevant articles were discarded. Subsequently, the remaining articles were assessed based on their title and abstract, and 10% of these articles were blindly re-assessed by another reviewer. With the 10% of article re-assessment we found little difference of opinion, hence giving us confidence in the selected articles. Agreement was reached by discussion and reasoning in case of discrepancies. Following abstract and title screening, full texts of the articles were reviewed for final screening.
Data extraction
The main categories for data extraction were type of assistive technology and its description, study participants, outlines of outcome measure, and functional ability with and without assistive technology. This information was used to summarize the efficacy of the current assistive technology for the upper limb in populations with tetraplegia.
Results
Study selection
The literature search in CINAHL, AMED, EMBASE, PUBMED, MEDLINE, and EMCARE yielded 218, 71, 498, 483, 297, and 203 studies, respectively. Following the initial study selection process, 371 studies were found. Subsequently, the abstracts of these studies were screened by searching for the predefined inclusion and exclusion terms (). Abstract screening yielded 37 studies. The 37 studies were further assessed for inclusion in the current study by reading the full text of the articles while looking for contents relevant to assistive technologies for the upper limb in cervical SCI population, and a clear report on outcome measures. The full-text assessment resulted in selecting a total of 24 studies for the analysis in this paper (). Of the 24 selected studies, 13 were identified as case studies or series,Citation13–25 two as clinical trials,Citation26,Citation27 one as a clinical study,Citation28 and eight as cohort studies.Citation29–36
Data extraction
Identified assistive technologies
In this study, assistive technologies for restoring upper limb function in populations with spinal cord injury were categorized as follows:
Neuroprosthesis (invasiveCitation20–26,Citation29–34 and non-invasive Citation18,Citation19,Citation27,Citation28) is a system where muscles are stimulated by small electrical currents to generate motor functions;
Orthosis is a non-invasive supportive device which assists with optimum use of remaining motor control;Citation16,Citation35,Citation36
Hybrid system is a combination of multiple technologies such as neuroprosthesis and orthosis,Citation13,Citation14 or powered orthosis;
Robot is a non-invasive device generating functional movements without the need for users to have any residual motor control,Citation17 and;
Antigravity arm support is an add-on device to other assistive technologies.Citation15
Table 2 Descriptive summary of assistive devices in the selected studies. Abbreviations: FES, Functional Electrical Stimulation; BCI, Brain–Computer Interface; IST-12, 12-channel implantable stimulator-telemeter; MES, Myoelectric Signals; EMG, Electromyographic.
Study participants
Characteristics of the participants recruited into each study are summarized in . Not all of the selected studies comprehensively reported characteristics of their participants, for example two studies did not report participants’ sex,Citation14,Citation28 two studies did not report participants’ age,Citation16,Citation31 and five studies did not report time between injury and participant recruitment.Citation14,Citation20,Citation28,Citation31,Citation34 In twenty-two studies, the neurological level of the injury ranged from C4 to C8, and two were above C3. The time since injury varied widely (range from 3 months to 62 years) with no particular pattern or correlation to the assistive devices in the selected studies.
Table 3 Descriptive summary of study participants in the selected studies. Abbreviations: M, Male; F, Female; NR, Not Reported.
Outcome measures
The outcome measures, adopted in the selected studies, covered a variety of the domains that comprise the framework of International Classification of Functioning, Disability and Health (ICF).Citation37 In total, there were 30 different outcome measures assessing body functions and structures, activity, and participation domains (Supplementary Materials Table S1). In the body functions and structure domain, outcome measures described joint movement, force generation, active and passive range of motion (ROM) through a number of standardized tests, such as Jebsen-Taylor-Hand-Function (JTHF) and Toronto Rehabilitation Institute Hand Function Test (TRI-HFT). In the activity domain, outcome measures were evaluated using a range of tests, including, Grasp-and-Release-Test (GRT), Activity of Daily Living (ADL), Action Research Arm Test (ARAT), Functional Independence Measure (FIM), and Spinal Cord Independence Measure (SCIM). In the participation domain, outcome measures assessed individuals when using the device in the community through tools and surveys, including the Craig Handicap Assessment and Reporting Tool (CHART). Only one study clearly reported on this domain, investigating social integration and occupation subscale,Citation30 and three studies carried out satisfaction surveys and participant questionnaires for using the device at home.Citation24,Citation33,Citation34
Study functional outcomes
All studies reported improvement in functional ability of the upper limb while using the assistive devices (Supplementary Materials Table S1). Studies on neuroprostheses, both invasive and non-invasive devices, showed increased hand function, grip and pinch strength, average range of movement in the upper limb, and improvement in ADLs.
In one study, the application of non-invasive neuroprostheses showed an immediate increase in hand function in 63% of their compliant subjects of whom 15% scored a clinically relevant change of 5.7 ARAT points.Citation28 Studies reported that grip strength was increased from 0.57N to 16.5N,Citation18 average range of movement in the forearm and wrist was increased by 9%,Citation27 and participants successfully performed at least three new ADL tasks.Citation18,Citation19,Citation27 Participants, who continuously used the non-invasive neuroprosthesis devices, showed a 75% higher performance of the ADL tasks.Citation27 Similarly, the effect of training with the device increased ARAT score by 2 points which is clinically important.Citation28 In addition, using non-invasive neuroprosthesis is thought to cause therapeutic effects and improve hand function.Citation28
Participants, with invasive neuroprostheses, had undergone invasive methods to implant the device. The implanted components of the device consist of epimysial and intramuscular electrodes, electrode leads, and electromyography recording electrodes.Citation22,Citation29,Citation30 Some studies combined corrective surgeries such as tendon transfer with invasive neuroprostheses to further improve upper limb function.Citation24,Citation30,Citation32,Citation33 Participants, using invasive neuroprostheses, were able to manipulate objects with varied size, surface, and weights.Citation18,Citation20,Citation24,Citation26,Citation29,Citation30,Citation32,Citation33 For example, GRT scores showed that 92% of participants improved the ability to manipulate objects,Citation29 participants at least doubled the number of objects manipulated or tasks performed,Citation30,Citation32,Citation33 and lateral and palmar grasp improved.Citation33 A study, combining assistive technology with corrective surgeries, such as arthrodesis, tendon transfers of muscles, and tendon synchronization, reported pinch force values at three stages (before intervention, after corrective surgery, and after surgery with assistive device).Citation30 Pinch force was increased from 4 N before to 12 N after corrective surgery and then to 19 N with device use, in other words pinch force was increased by 58% after surgery with device use.Citation30 Increase in pinch force, when using the device, was also reported in lateral, palmar, and finger grasps.Citation26,Citation32 The range of lateral pinch force with the device was 11.6 N to17 N,Citation22,Citation29,Citation32,Citation33 palmar pinch force was 6.5 N to 10.4 N,Citation29,Citation32,Citation33 and finger grasp was 14.7 N.Citation32 The improvement of grasp and release function and strength of grips contributed to the increased success of the ADL tasks. ADL tasks, reported in the selected studies, varied widely, some studies allowed participants to choose the ADL tasksCitation24,Citation30,Citation32 and others predefined an extensive list of the tasks.Citation13,Citation15,Citation18,Citation19,Citation22,Citation26,Citation27,Citation33,Citation34 The results from ADL tests showed that participants experienced reduced disability and increased independence when using the invasive-neuroprosthesis.
Pinch force in participants increased with the use of an orthosis, such that in one study pinch force with an orthosis was 14.3 times greater than without the device.Citation36 Another study found that an orthosis increased maximum voluntary contraction (MVC), resulting in an increase of lateral grip force (the force ranging between 4.7 N and 22.3 N).Citation16 Only one study looked at the effect of an orthosis on performing ADL tasks, and reported that a greater number of tasks were achieved with the device compared to without.Citation16
Studies on hybrid systems reported successful performance of GRT tasks,Citation13 and increased ability to manipulate objects using palmar grasp.Citation14
The only study evaluating the antigravity arm support device on its own showed that the device facilitates ADL tasks such as eating.Citation15 A combination of mobile arm supports with other assistive devices, such as neuroprostheses, to support the weight of an arm has been reported but not evaluated on their own.Citation22
Discussion
In this review, we defined a set of inclusion and exclusion criteria to systematically select original research articles, focusing on the state-of-the-art assistive technologies, which support and improve function of impaired upper limbs in cervical SCI populations. The objectives of the paper are fulfilled by describing the assistive technologies and the outcome measures used to assess them.
During study selection, it was noted that a larger number of recent studies focused on developing control systems to regulate assistive technologies for the upper limbs.Citation38–47 Similarly, several studies reported the use of rehabilitation technologies, such as training and therapeutic tools to restore function in the upper limb.Citation48–59 These studies were excluded in this review paper so that a comprehensive focus could be made on the efficacy of assistive technologies that offer ongoing support to the upper limb for restoring function in people with cervical-level SCI. As a result of the study selection, the assistive technologies developed, trialed, or used for restoring upper limb function were grouped into five categories, namely, neuroprostheses, orthoses, hybrid systems, robots, and antigravity arm supports. Some of the technologies described can be assigned to multiple categories. The Handmaster, for example, is a combination of a hand orthosis with surface electrodes and it is categorized under non-invasive neuroprosthesis.Citation18,Citation19 One reason for placing the Handmaster in the non-invasive neuroprosthesis category is because the studies investigated the neuroprosthesis more than the orthotic part of the device. The same reasoning was used for other devices that spanned categories, such as antigravity arm support devices and neuroprosthesis.Citation15,Citation22 However, devices with multiple technologies, such as those reported in,Citation13,Citation14 are classified as hybrid systems. A survey study, involving participants with SCI, reported that many of the participants were not aware of the current assistive technologies, hence they were not aware of available options that could improve their independence and quality of life.Citation60 It is possible that the lack of clear and accessible categories of assistive technologies for restoring the upper limb functions could have been a factor.
The incidences of SCIs vary across countries, regions, and cities. A study reviewing global prevalence of SCI highlighted that the highest SCI prevalence was in the US (Alaska), while the lowest prevalence was in France (Rhone-Alpes region).Citation61 Globally, there was a greater percentage of males with SCI than females.Citation61 The demographics of recruited participants in the selected studies showed a high male-to-female ratio, such as 11:1, 9:1, and 8:1,Citation31,Citation32,Citation36 and a wide age range between 19 and 54 years old. Others recruited either one participant or a relatively lower male-to-female ratio, such as a ratio below 5:1. In the UK, for the years between 1985 and 1988, male-to-female ratio for people, sustaining a spinal cord injury, was 3.8:1 with an average age of 35.5 and 46 years old for males and females, respectively.Citation62 No more recent consensus about the epidemiology of SCI in the UK was found, however, the demographics of SCI patients in the developed countries is believed to have changed in terms of age more than sex over the last 20 to 40 years.Citation6,Citation61,Citation63,Citation64 In the US, for the years between 1970 and 2015, the average age at injury increased from 29 to 43 years.Citation6 Similarly, in Scotland, for the years between 1994 and 2013, there was a notable increase of new SCI in the over 50-year old population and those with high level (C1–C4) tetraplegia.Citation63 With the exception of Japan, where SCI patients in their 70s are the largest age group,Citation65 a larger percentage of SCI patients are under the age of 30 in most countries.Citation61 The assistive technologies were population dependent and inclusion criteria for participant recruitment was focused more on injury level than age of participants or time since injury. Two of the selected papers recruited participants with C3 or higher levels of injury and the assistive technologies implemented were invasive neuroprostheses.Citation22,Citation25 Participants in these two studies had limited to no voluntary contraction in the upper or lower limbs, hence it is possible that the decision on the type of device for this population was based on practicality for device operations. Similarly, participants in studies investigated orthotic devices for SCI had the ability to extend their wrist against gravity.Citation16,Citation35,Citation36 It would be beneficial for future studies to outline reasons behind opting to use an assistive device, so that a library of different types of assistive devices and their suitability for different SCI populations can be established.
Prior to adopting an assistive device, SCI patients go through a rehabilitation process which commences in the acute care setting and lasts for 6 to 12 weeks, during this time the focus is on patient’s neurological stability status, indirect complications, such as pressure ulcers, maintaining range of motion and preventing muscle atrophy.Citation66 Early rehabilitation is believed to prevent the development of joint contractures, especially contractures of elbow flexion and supination.Citation67 Therefore, identifying a suitable assistive technology to meet the needs of SCI patients at the early rehabilitation stage might improve the efficacy of the selected assistive device, hence enhance the patients’ quality of life. The literature showed limited focus on the relationship between the efficacy of assistive technologies and time since injury. It was reported that assistive technologies built for functional purposes have therapeutic effects, however, small to no significant correlation was reported between time since injury and functional outcomes as a result of the device use.Citation28 The selected literature assessed functional capabilities of the assistive devices through clinical outcome measures.
The outcome measures for assessing the assistive technologies were in the activity domain of the ICF. A limited number of the selected studies covered all three domains of the ICF; however, all covered the activity domain. All studies, except for three, followed the outcome measures identified by the Spinal Cord Injury Research Evidence (SCIRE) to assess the effect of device use on activities.Citation14,Citation21,Citation34 One of the three studies, investigating a hybrid system, assessed shoulder and scapular movements when using the device through the measurement of rotational speed, real-time angular variation, and real-time force. Although these measurements indicated an increased palmar grasp, the study did not clearly report the translation of the measurements and their relevance to ICF activity domain.Citation14 Similarly, a study on invasive neuroprosthesis reported an improved volitional control across a continuous wrist angle but did not test the effect of this increased ability on participant’s activities.Citation21 The third study focused on elbow extension using invasive neuroprosthesis, developed new evaluations to assess the device because at the time the existing tests did not evaluate specific functions.Citation34 They reported that specific information (i.e. interval data) to augment the muscle grade is needed because few gradations exist for a muscle that achieves full ROM and takes resistance. To obtain interval instead of ordinal data, they developed a technique of measuring the weight against gravity when participants were extending their elbows. Grip or pinch strength measurement was the most frequently used outcome measure after ADL tasks. Interestingly, this finding aligns with the choices professional practitioners make when selecting an outcome measure from SCIRE toolkit during their practice.Citation68 Professionals tend to choose the SCIM and FIM for assessing self-care and daily living, the GRT for assessing the upper limb functions, and the Quebec user evaluation of satisfaction and predisposition assessment for assessing the effect of assistive technology. The outcome measures reported in this review included the FIM, SCIM, GRT and Graded Redefined Assessment of Strength, Sensibility, and Prehension (GRASSP) and these were reported to be reliable and valid.Citation69–71 Whereas the Quadriplegia Index of Function (QIF) is suggested for use only in non-ambulatory tetraplegia and its validity has not been investigated sufficiently.Citation69 It is important to note that, unlike the FIM, the SCIM, GRT, GRASSP, and QIF were specifically designed for the SCI population. The FIM was designed to assess a broad range of disabling medical conditions, hence it might not specifically reflect on measures for SCI population. In addition, the selected papers in this review were limited by the lack of assessment on the efficacy of the assistive technologies during mobility. It is essential for future studies to assess assistive devices by looking at function, activity, and independence in the context of mobility from the ICF.
There are challenges and limitations that come with utilizing assistive technologies for people with SCI. Loss of proprioception, for example, can make it challenging for people with SCI to adopt the aforementioned assistive technologies.Citation72 Other disadvantages of assistive technologies, such as invasive neuroprostheses, include risks associated with surgical operations and potentially additional surgeries to reposition migrated electrodes or replace failed hardware components. However, compared to non-invasive neuroprostheses’ stronger forces and better muscle selectivity can be achieved with invasive neuroprostheses because the stimulation electrode can be implanted closer to the motor nerve and in deeper muscles.Citation73–75 In addition, the orthosis, robots, hybrid systems, and antigravity arm supports are advantageous because of their non-invasive nature; however, they are disadvantaged by the difficulties with donning, doffing, and achieving selective muscle stimulation.Citation76 In addition to the generic disadvantages of these assistive devices, the technologies reported in the selected literature were limited, including the fabric-based soft robotic glove which could not generate adequate pinch grasp between the thumb and index finger due to a deficiency in their actuator design.Citation17 Furthermore, a study on grasp coordination with an invasive neuroprosthesis did not have an electrode to stimulate thenar muscle; therefore, they could not accurately measure the maximal palmar, lateral, and tip-to-tip grip force.Citation20 It is worth mentioning that achieving the upper limb movements with assistive devices alone can be challenging; therefore, a number of the studies reported a combination of surgical and technological interventions for improving upper limb functions.Citation24,Citation30,Citation32,Citation33 For example, corrective surgeries, such as tendon transfer, were performed to augment the system.Citation32 A study reported that smaller objects, such as pegs and wooden blocks, could be manipulated better with an active tenodesis grasp rather than with a transcutaneous functional electrical stimulation.Citation24 This is because the position of the object within the hand can be corrected more effectively and there is no time required for the interaction with the device. However, the electrical stimulation is advantageous and sometimes necessary to manipulate heavier or slippery objects. Assistive technologies, combined with corrective surgeries, could provide higher degrees of upper limb functionalities in tetraplegia.Citation33,Citation73 Further research is needed to investigate the efficacy of assistive technologies when they are integrated with corrective surgeries, such as tendonCitation77 or nerve transfer.Citation78
In conclusion, here we categorized the assistive technologies into five main cohorts, hence making the evidence base of current technology more accessible and identifiable for clinicians, users, researchers, and readers. There is evidence that the assistive technologies reported in this study can help people living with cervical SCI. Compared to the other technologies, a larger number of studies focused on the development of neuroprostheses two decades ago which was followed by much less interests in recent years. As a result, the application of neuroprostheses has been more extensively studied recently, hence future research is equipped to focus on developing user–control systems. There is an imbalance on how the efficacy of assistive technologies is assessed in relation to the three domains of the ICF. We recommend future studies on assistive technologies to follow the outcome measures identified by SCIRE and, when possible, equally address the three domains of the ICF in order to better quantify the effectiveness of assistive technologies. For example, future studies could focus on developing and following a methodology that would facilitate comparisons between different assistive devices.
Disclaimer statements
Contributors None.
Conflicts of interest None.
Supplemental Material
Download MS Word (39.1 KB)Additional information
Funding
References
- NHS England. NHS standard contract for spinal cord injuries (all ages). Redditch: NHS England; 2013. p. 1–70.
- Barr F. Preserving and developing the national spinal cord injury service: phase 2 – seeking the evidence. Milton Keynes: Spinal Injuries Association; 2009. p. 1–32.
- Gall A, Turner-Stokes L. Chronic spinal cord injury: management of patients in acute hospital settings. Clin Med (Lond). 2008 Feb;8(1):70–4.
- Lasfargues JE, Custis D, Morrone F, Cars Well J, Nguyen T. A model for estimating spinal cord injury prevalence in the United States. Spinal Cord 1995 Feb;33(2):62–8.
- Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O’Connor KC, et al. Traumatic spinal cord injury in the United States, 1993–2012. JAMA 2015 Jun 9;313(22):2236–43.
- National Spinal Cord Injury Statistical Center. Facts and figures at a glance. [Internet]. University of Alabama at Birmingham; 2020 [cited 2020 Nov 15]. Available from https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%202020.pdf.
- Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007;30(4):319–30.
- Graupensperger S, Sweet SN, Evans MB. Multimorbidity of overweight and obesity alongside anxiety and depressive disorders in individuals with spinal cord injury. J Spinal Cord Med. 2018 Sep 5: 1–9. doi:10.1080/10790268.2018.1507801.
- Noonan VK, Fallah N, Park SE, Dumont FS, Leblond J, Cobb J, et al. Health care utilization in persons with traumatic spinal cord injury: the importance of multimorbidity and the impact on patient outcomes. Top Spinal Cord Inj Rehabil. 2014;20(4):289–301.
- Simpson LA, Eng JJ, Hsieh JTC, Wolfe DL. The health and life priorities of individuals with spinal cord injury: A systematic review. J Neurotrauma 2012 May 20;29(8):1548–55.
- House JH, Gwathmey FW, Lundsgaard DK. Restoration of strong grasp and lateral pinch in tetraplegia due to cervical spinal cord injury. J Hand Surg [Am]. 1976 Sep 1;1(2):152–9.
- Kamper DG. Restoration of hand function in stroke and spinal cord injury. In: Reinkensmeyer DJ, Dietz V, (ed.) Neurorehabilitation technology. Cham: Springer International Publishing; 2016. p. 311–31.
- Rohm M, Schneiders M, Muller C, Kreilinger A, Kaiser V, Muller-Putz GR, et al. Hybrid brain-computer interfaces and hybrid neuroprostheses for restoration of upper limb functions in individuals with high-level spinal cord injury. Artif Intell Med. 2013 Oct;59(2):133–42.
- Varoto R, Barbarini ES, Cliquet AJ. A hybrid system for upper limb movement restoration in quadriplegics. Artif Organs. 2008 Sep;32(9):725–9.
- Asai N, Kuroiwa S. Comparison of portable spring balancer with mobile arm support in aiding self-feeding in quadriplegic patients with high cervical injuries-rate of adaptation to both devices. J Phys Ther Sci 1999;11(1):11–7.
- King M, Verkaaik J, Nicholls A, Collins F. A wrist extension operated lateral key grip orthosis for people with tetraplegia. Technol Disabil. 2009;21:19–23.
- Cappello L, Meyer JT, Galloway KC, Peisner JD, Granberry R, Wagner DA, et al. Assisting hand function after spinal cord injury with a fabric-based soft robotic glove. J Neuroeng Rehabil. 2018 Jun 28;15(1):59.
- Alon G, McBride K. Persons with C5 or C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003 Jan;84(1):119–24.
- Snoek GJ, IJzerman MJ, in ‘t Groen FA, Stoffers TS, Zilvold G. Use of the NESS handmaster to restore handfunction in tetraplegia: clinical experiences in ten patients. Spinal Cord 2000 Apr;38(4):244–9.
- Bockbrader M, Annetta N, Friedenberg D, Schwemmer M, Skomrock N, Colachis S, et al. Clinically significant gains in skilful grasp coordination by an individual with tetraplegia using an implanted brain-computer interface with forearm transcutaneous muscle stimulation. Arch Phys Med Rehabil. 2019;100(7):1201–17.
- Friedenberg DA, Schwemmer MA, Landgraf AJ, Annetta NV, Bockbrader MA, Bouton CE, et al. Neuroprosthetic-enabled control of graded arm muscle contraction in a paralyzed human. Sci Rep. 2017 Aug 21;7(1):8386.
- Memberg WD, Polasek KH, Hart RL, Bryden AM, Kilgore KL, Nemunaitis GA, et al. Implanted neuroprosthesis for restoring arm and hand function in people with high level tetraplegia. Arch Phys Med Rehabil. 2014 Jun;95(6):1201–11.e1.
- Gan LS, Ravid E, Kowalczewski JA, Olson JL, Morhart M, Prochazka A. First permanent implant of nerve stimulation leads activated by surface electrodes, enabling hand grasp and release: the stimulus router neuroprosthesis. Neurorehabil Neural Repair 2012 May;26(4):335–43.
- Mangold S, Keller T, Curt A, Dietz V. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord 2005 Jan;43(1):1–13.
- Yu DT, Kirsch RF, Bryden AM, Memberg WD, Acosta AM. A neuroprosthesis for high tetraplegia. J Spinal Cord Med. 2001;24(2):109–13.
- Carroll S, Cooper C, Brown D, Sormann G, Flood S, Denison M. Australian experience with the Freehand system for restoring grasp in quadriplegia. Aust N Z J Surg. 2000 Aug;70(8):563–8.
- Popovic D, Stojanovic A, Pjanovic A, Radosavljevic S, Popovic M, Jovic S, et al. Clinical evaluation of the bionic glove. Arch Phys Med Rehabil. 1999 Mar;80(3):299–304.
- Thorsen R, Dalla Costa D, Chiaramonte S, Binda L, Beghi E, Redaelli T, et al. A noninvasive neuroprosthesis augments hand grasp force in individuals with cervical spinal cord injury: the functional and therapeutic effects. Sci World J. 2013;2013:836959.
- Kilgore KL, Bryden A, Keith MW, Hoyen HA, Hart RL, Nemunaitis GA, et al. Evolution of neuroprosthetic approaches to restoration of upper extremity function in spinal cord injury. Top Spinal Cord Inj Rehabil. 2018;24(3):252–64.
- Kilgore KL, Hoyen HA, Bryden AM, Hart RL, Keith MW, Peckham PH. An implanted upper-extremity neuroprosthesis using myoelectric control. J Hand Surg Am. 2008 Apr;33(4):539–50.
- Memberg WD, Crago PE, Keith MW. Restoration of elbow extension via functional electrical stimulation in individuals with tetraplegia. J Rehabil Res Dev. 2003 Dec;40(6):477–86.
- Taylor P, Esnouf J, Hobby J. The functional impact of the freehand system on tetraplegic hand function, clinical results. Spinal Cord 2002 Nov;40(11):560–6.
- Peckham PH, Keith MW, Kilgore KL, Grill JH, Wuolle KS, Thrope GB, et al. Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study. Arch Phys Med Rehabil. 2001 Oct;82(10):1380–8.
- Bryden AM, Memberg WD, Crago PE, Polacek L. Electrically stimulated elbow extension in persons with C5/C6 tetraplegia: a functional and physiological evaluation. Arch Phys Med Rehabil. 2000;81(1):80–8.
- Portnova AA, Mukherjee G, Peters KM, Yamane A, Steele KM. Design of a 3D-printed, open-source wrist-driven orthosis for individuals with spinal cord injury. PLoS One 2018;13(2):e0193106.
- Kang Y-S, Park Y-G, Lee B-S, Park H-S. Biomechanical evaluation of wrist-driven flexor hinge orthosis in persons with spinal cord injury. J Rehabil Res Dev. 2013;50(8):1129–38.
- World Health Organization. International classification of functioning, disability and health (ICF). Geneva: World Health Organization; 2001.
- Young D, Willett F, Memberg WD, Murphy B, Rezaii P, Walter B, et al. Closed-loop cortical control of virtual reach and posture using Cartesian and joint velocity commands. J Neural Eng. 2019;16(2):026011.
- Annetta NV, Friend J, Schimmoeller A, Buck VS, Friedenberg DA, Bouton CE, et al. A high definition noninvasive neuromuscular electrical stimulation system for cortical control of combinatorial rotary hand movements in a human with tetraplegia. IEEE Trans Biomed Eng. 2019;66(4):910–9.
- Tigra W, Navarro B, Cherubini A, Gorron X, Gelis A, Fattal C, et al. A novel EMG interface for individuals with tetraplegia to pilot robot hand grasping. IEEE Trans Neural Syst Rehabil Eng. 2018 Feb;26(2):291–8.
- Skomrock ND, Schwemmer MA, Ting JE, Trivedi HR, Sharma G, Bockbrader MA, et al. A characterization of brain-computer interface performance trade-offs using support vector machines and deep neural networks to decode movement intent. Front Neurosci. 2018;12:763.
- Colachis SC, Bockbrader MA, Zhang M, Friedenberg DA, Annetta NV, Schwemmer MA, et al. Dexterous control of seven functional hand movements using cortically-controlled transcutaneous muscle stimulation in a person with tetraplegia. Front Neurosci. 2018;12:208.
- Andreasen Struijk LNS, Egsgaard LL, Lontis R, Gaihede M, Bentsen B. Wireless intraoral tongue control of an assistive robotic arm for individuals with tetraplegia. J Neuroeng Rehabil. 2017 Nov 6;14(1):110.
- Libedinsky C, So R, Xu Z, Kyar TK, Ho D, Lim C, et al. Independent mobility achieved through a wireless brain-machine interface. PLos One 2016 Nov 1;11(11):e0165773.
- Wang W, Collinger JL, Degenhart AD, Tyler-Kabara EC, Schwartz AB, Moran DW, et al. An electrocorticographic brain interface in an individual with tetraplegia. PLoS ONE 2013;8(2):e55344.
- Ortner R, Allison BZ, Korisek G, Gaggl H, Pfurtscheller G. An SSVEP BCI to control a hand orthosis for persons with tetraplegia. IEEE Trans Neural Syst Rehabil Eng. 2011 Feb;19(1):1–5.
- Chadwick EK, Blana D, Simeral JD, Lambrecht J, Kim SP, Cornwell AS, et al. Continuous neuronal ensemble control of simulated arm reaching by a human with tetraplegia. J Neural Eng. 2011 Jun;8(3):034003.
- Rakos M, Freudenschuss B, Girsch W, Hofer C, Kaus J, Meiners T, et al. Electromyogram-controlled functional electrical stimulation for treatment of the paralyzed upper extremity. Artif Organs 1999 May;23(5):466–9.
- Popovic DB, Popovic MB, Sinkjaer T. Neurorehabilitation of upper extremities in humans with sensory-motor impairment. Neuromodulation 2002 Jan;5(1):54–66.
- Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord 2006 Mar;44(3):143–51.
- Kowalczewski J, Chong SL, Galea M, Prochazka A. In-home tele-rehabilitation improves tetraplegic hand function. Neurorehabil Neural Repair. 2011 Jun;25(5):412–22.
- Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair 2011 Jun;25(5):433–42.
- Rudhe C, Albisser U, Starkey ML, Curt A, Bolliger M. Reliability of movement workspace measurements in a passive arm orthosis used in spinal cord injury rehabilitation. J Neuroeng Rehabil. 2012 Jun 9;9(1):37.
- Cortes M, Elder J, Rykman A, Murray L, Avedissian M, Stampas A, et al. Improved motor performance in chronic spinal cord injury following upper-limb robotic training. NeuroRehabil. 2013;33(1):57–65.
- Hortal E, Planelles D, Resquin F, Climent JM, Azorín JM, Pons JL. Using a brain-machine interface to control a hybrid upper limb exoskeleton during rehabilitation of patients with neurological conditions. J Neuroeng Rehabil. 2015 Oct 17;12(1):92.
- Francisco GE, Yozbatiran N, Berliner J, O'Malley MK, Pehlivan AU, Kadivar Z, et al. Robot-assisted training of arm and hand movement shows functional improvements for incomplete cervical spinal cord injury. Am J Phys Med Rehabil. 2017 Oct;96(10 Suppl 1):S171–7.
- Iwahashi K, Hayashi T, Watanabe R, Nishimura A, Ueta T, Maeda T, et al. Effects of orthotic therapeutic electrical stimulation in the treatment of patients with paresis associated with acute cervical spinal cord injury: a randomized control trial. Spinal Cord 2017;55(12):1066–70.
- Lu Z, Tong K-Y, Shin H, Stampas A, Zhou P. Robotic hand-assisted training for spinal cord injury driven by myoelectric pattern recognition: a case report. Am J Phys Med Rehabil. 2017 Oct;96(10 Suppl 1):S146–9.
- Freyvert Y, Yong NA, Morikawa E, Zdunowski S, Sarino ME, Gerasimenko Y, et al. Engaging cervical spinal circuitry with non-invasive spinal stimulation and buspirone to restore hand function in chronic motor complete patients. Sci Rep. 2018;8(1):15546.
- Collinger JL, Boninger ML, Bruns TM, Curley K, Wang W, Weber DJ. Functional priorities, assistive technology, and brain-computer interfaces after spinal cord injury. J Rehabil Res Dev 2013 Apr;50(2):145–60.
- Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014 Sep 23;6:309–31.
- Aung TS, el Masry WS. Audit of a British centre for spinal injury. Spinal Cord 1997 Mar;35(3):147–50.
- McCaughey EJ, Purcell M, McLean AN, Fraser MH, Bewick A, Borotkanics RJ, et al. Changing demographics of spinal cord injury over a 20-year period: a longitudinal population-based study in Scotland. Spinal Cord 2016 Apr;54(4):270–6.
- DeVivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 2012 May;50(5):365–72.
- Miyakoshi N, Suda K, Kudo D, Sakai H, Nakagawa Y, Mikami Y, et al. A nationwide survey on the incidence and characteristics of traumatic spinal cord injury in Japan in 2018. Spinal Cord 2020 Aug 11. doi:10.1038/s41393-020-00533-0.
- Nas K, Yazmalar L, Şah V, Aydın A, Öneş K. Rehabilitation of spinal cord injuries. World J Orthop. 2015 Jan 18;6(1):8–16.
- Diong J, Harvey LA, Kwah LK, Eyles J, Ling MJ, Ben M, et al. Incidence and predictors of contracture after spinal cord injury–a prospective cohort study. Spinal Cord 2012 Aug;50(8):579–84.
- Scivoletto G, Galli G, Torre M, Molinari M, Pazzaglia M. The overlooked outcome measure for spinal cord injury: use of assistive devices. Front Neurol. 2019 Mar 22;10:1–9.
- Anderson K, Aito S, Atkins M, Biering-Sørensen F, Charlifue S, Curt A, et al. Functional recovery measures for spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med. 2008;31(2):133–44.
- Kalsi-Ryan S, Beaton D, Curt A, Duff S, Popovic MR, Rudhe C, et al. The graded redefined assessment of strength sensibility and prehension: reliability and validity. J Neurotrauma 2012 Mar 20;29(5):905–14.
- Wuolle KS, Van Doren CL, Thrope GB, Keith MW, Peckham PH. Development of a quantitative hand grasp and release test for patients with tetraplegia using a hand neuroprosthesis. J Hand Surg Am. 1994 Mar;19(2):209–18.
- Sainburg RL, Ghilardi MF, Poizner H, Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol. 1995 Feb;73(2):820–35.
- Patil S, Raza WA, Jamil F, Caley R, O’Connor RJ. Functional electrical stimulation for the upper limb in tetraplegic spinal cord injury: a systematic review. J Med Eng Technol. 2015 Oct 3;39(7):419–23.
- Popovic MR, Popovic DB, Keller T. Neuroprostheses for grasping. Neurol Res. 2002 Jul 1;24(5):443–52.
- Keith MW, Peckham PH, Thrope GB, Stroh KC, Smith B, Buckett JR, et al. Implantable functional neuromuscular stimulation in the tetraplegic hand. J Hand Surg [Am]. 1989 May 1;14(3):524–30.
- Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–60.
- Dunn JA, Sinnott KA, Rothwell AG, Mohammed KD, Simcock JW. Tendon transfer surgery for people with tetraplegia: an overview. Arch Phys Med Rehabil. 2016 Jun 1;97(6):S75–S80.
- Cain SA, Gohritz A, Fridén J, van Zyl N. Review of upper extremity nerve transfer in cervical spinal cord injury. J Brachial Plex Peripher Nerve Inj. 2015 Aug 6;10(1):e34–42.