Abstract
Context
Rehabilitation after spinal cord injury (SCI) relies on the use of exercise training, which has limited functional gains. There is a need to develop more efficient approaches to facilitate recovery after SCI.
Methods
This review focuses on a neuromodulation method where transcranial magnetic stimulation (TMS) over the primary motor cortex is paired with transcutaneous electrical stimulation over a peripheral nerve to induce plasticity at corticospinal-motoneuronal synapses. These two stimuli are applied at precise inter-stimulus intervals to reinforce corticospinal synaptic transmission using principles of spike-timing-dependent plasticity applied alone or in combination with exercise training.
Results
Transmission in residual corticospinal axons, assessed using TMS and maximal voluntary motor output, increased after stimulation combined with exercise training in persons with SCI. There were also significant improvements in functional outcomes, including walking speed and grasping function, which persisted after 6–9 months post stimulation. Moreover, the data suggested that the effects of the stimulation protocol can be augmented with a higher number of sessions and with multiple stimulation sites in the spinal cord.
Conclusions
Voluntary movement is enhanced in people with SCI through the strengthening of corticospinal-motoneuronal synapses using paired stimulation. This neuromodulation technique represents a novel powerful strategy to facilitate functional recovery after SCI.
Introduction
Spinal cord injury (SCI) can lead to devastating consequences predominantly resulting from impairment in motor function. To date, there is no medical treatment that improves recovery of motor function after SCI. Considering that SCI rarely results in a complete spinal cord transection,Citation1 promoting neuroplasticity to strengthen residual connections is key to restoration of motor function. The corticospinal tract is a major descending pathway in the spinal cord that contributes to the control of voluntary movement.Citation2 Therefore, interventions that successfully engage residual corticospinal neurons and strengthen the connections between corticospinal neurons and spinal motoneurons are crucial to increase corticospinal transmission to facilitate functional recovery. The conventional approach to promote plasticity following SCI is rehabilitative exercise training.Citation3,Citation4 While exercise training aims to drive neural networks in an activity-dependent manner to facilitate functional recovery,Citation3,Citation4 the functional gains remain limited.
Invasive and noninvasive neurostimulation strategies have been combined with traditional rehabilitative exercise training in an attempt to restore function more effectively following SCI.Citation5–13 Combined with exercise training, several approaches have demonstrated to more efficiently recover function in people with complete or incomplete SCI, surpassing the effects of exercise training alone. Specifically, epiduralCitation5,Citation9,Citation13 or transcutaneousCitation8,Citation10 stimulation of the spinal cord and paired associative stimulation targeting the spinal cordCitation11 have demonstrated comparable effects in recent studies. Paired associative stimulation targeting the spinal cord, also referred to as paired corticospinal-motoneuronal stimulation (PCMS), is based on the well-known principles of spike-timing-dependent plasticityCitation14,Citation15 and it is a relatively new method compared to epidural and transcutaneous stimulation approaches. This review will focus on the application of PCMS in humans with SCI to discuss its potential as a clinical therapeutic intervention.
Short-term effects of PCMS
PCMS is an efficient physiological strategy to enhance connectivity at corticospinal-motoneuronal synapses, thereby exploiting the naturally occurring adaptation in neuronal connections based on principles of spike-timing-dependent plasticity – ‘neurons that fire together, wire together’.Citation15 This mechanism relies on precisely timed stimulation of presynaptic neurons just prior (within a few milliseconds) to stimulation of their postsynaptic neurons to engage endogenous plasticity, which enhances synaptic strength. In humans, PCMS targeting corticospinal-motoneuronal synapses can induce temporary increases in corticospinal excitability and motor output in people with incomplete chronic SCICitation6,Citation7,Citation11,Citation12 and in people without SCI.Citation16,Citation17 Specifically, PCMS-like plasticity is elicited at the corticospinal-motoneuronal synapse via the combination of peripheral nerve stimulation and transcranial magnetic stimulation (TMS). Descending volleys elicited by TMS over the primary motor cortex can be precisely timed to reach the corticospinal-motoneuronal synapses in the spinal cord a few milliseconds prior to antidromic volleys elicited by electrical stimulation of the peripheral nerve ().
Figure 1 Illustration of paired corticospinal-motoneuronal stimulation (PMCS). (a). Corticospinal neurons are activated via transcranial magnetic stimulation (TMS) over each motor cortex (green lines). Spinal motor neurons are activated antidromically via peripheral nerve stimulation (purple line). (b). The interstimulus interval (ISI) between paired pulses allows descending volleys, elicited by TMS, to arrive at the presynaptic terminal of corticospinal neurons (1st, green spike) 1–2 ms before antidromic volleys, elicited by peripheral nerve stimulation, arrive at the dendrites of the corresponding spinal motor neurons (2nd, purple spike). The precise timing is measured for each muscle targeted using central and peripheral conduction times.
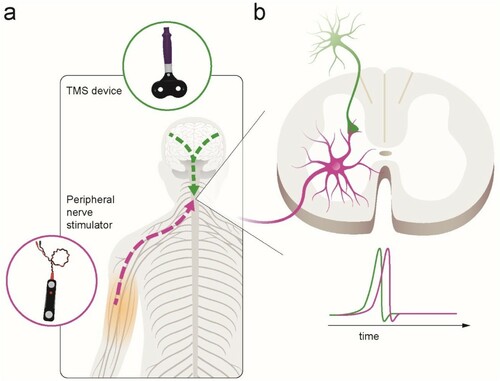
Bunday and PerezCitation6 applied PCMS for the first time in humans with incomplete cervical SCI and demonstrated that plasticity at residual corticospinal-motoneuronal synapses can be achieved. These authors showed that the arrival of presynaptic volleys prior to motoneuronal volleys in the spinal cord enhanced corticospinal excitability in the first dorsal interosseous (FDI) muscle, and in hand motor function as measured by time to perform the Nine-Hole-Peg-Test. Importantly, the changes in corticospinal transmission positively correlated with enhancements in voluntary motor output, suggesting an association between motor output and strength of the induced plasticity. In a subsequent study, PCMS was used to target spinal synapses of lower-limb motoneurons in humans with chronic incomplete SCI.Citation12 During PCMS, presynaptic volleys elicited by TMS of the leg motor cortex arrived at the spinal cord a few milliseconds prior to antidromic volleys elicited by electrical stimulation of the common peroneal nerve. The results showed that the size of motor evoked potentials (MEPs) in the tibialis anterior muscle increased after PCMS but not sham-stimulation. In addition, physiological changes present after the stimulation were accompanied by increases in electromyographic activity in the tibialis anterior and increases in isometric dorsiflexion force. Overall, the effect of PCMS on physiological and behavioral outcomes in upper and lower limb muscles occurred after 100–200 pairs of stimuli at 0.1 Hz and lasted for up to 30–120 min.
Additionally, the effect of PCMS on corticospinal excitability when pairs of stimuli were delivered during short-lasting low-intensity isometric voluntary contractions was explored.Citation7 The results showed that MEPs elicited by TMS and electrical stimulation at the cervicomedullary junction increased to a larger extent when PCMS was applied during voluntary activity compared with rest in SCI participants. Here, SCI participants who did not respond to PCMS at rest responded to voluntary activity and those participants who responded to both protocols showed larger increments in corticospinal transmission when PCMS was applied during voluntary activity. Possibly, this is because spinal lesions are associated with reduced corticospinal inputs to motoneurons and increasing the number and size of descending volleys by voluntary contraction could boost spinal plasticity after SCI.
Long-term effects of PCMS
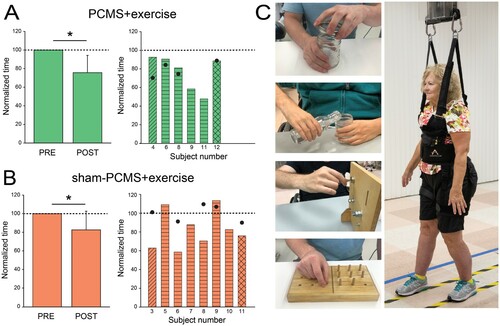
After proof-of-principle studies showing that PCMS can elicit short-term functional improvements in both upper and lower extremities after SCI, efforts have been made to potentiate the aftereffects of this type of stimulation. For the first time, the clinical potential of PCMS was tested when this stimulation was combined with exercise training for repeated sessions. Jo and PerezCitation11 studied individuals with different levels of chronic incomplete SCI who underwent 10 sessions of PCMS with or without exercise training. Upper limb exercises involved gross and fine grasping and hand cycling, and lower limb exercises involved over-ground and treadmill walking, and stair climbing. Note that in this study, we were able to involve people with different levels of SCI and therefore during PMCS peripheral nerve stimulation was delivered to different nerves to target different muscles (ulnar nerve at the wrist to target the first dorsal interosseous; median nerve at the wrist to target the abductor pollicis brevis; brachial plexus at the Erb’s point to target the deltoid and biceps brachii; common peroneal nerve under the head of the fibula to target the tibialis anterior). The results showed that corticospinal excitability and maximal voluntary contraction in targeted muscles increased ∼50% after PCMS with or without exercise but not after sham-stimulation with exercise, suggesting that the exercise program tested alone was not sufficient to elicit physiological changes. Although previous studies showed that a single session of PCMS facilitated small levels of voluntary output after SCI,Citation6,Citation12 it did not increase maximal voluntary contraction.Citation18 However, the results of this trial suggested that repeated stimulation sessions potentiated the effect on maximal voluntary contractions enhancing the therapeutic potential of this approach. Another important result was that behavioral effects obtained after 10 sessions were preserved for up to 6 months in the group receiving PCMS combined with exercise but not sham-stimulation, suggesting that this stimulation protocol can be used to maximize the effect of exercise rehabilitation ().
One of the promising features of PCMS is its functional effects after repeated sessions. In a follow-up study, we applied PCMS combined with exercise training for 40 sessions and targeted multiple spinal levels in parallel.Citation19 Although its application has been restricted to one specific muscle at a time in previous studies, PCMS in theory could target multiple muscles if each antidromic volley from different peripheral nerves is precisely timed with the corresponding descending volleys. With this principle, our precisely timed stimulation protocol targeted corticospinal-motoneuronal synapses of multiple upper and lower limb muscles simultaneously in eight different muscle groups. We found that after 40 sessions, corticospinal responses increased by 328% and maximal voluntary motor output by 193% in all target muscles. Parallel to changes in electrophysiological parameters reflecting synaptic potentiation, participants improved significantly across several functional metrics. The time to perform a grasping task and 10-m walk decreased ∼50% (compared to 20% after 10 sessions) and persisted 9-month post-intervention. Also, both motor and sensory function evaluated by the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) exam significantly improved after 40 sessions. The functional gains in this study were comparable to those reported with epidural stimulationCitation13 but, notably, with significantly less sessions. Although further investigations are needed to adequately determine superiority in the efficiency of different types of neuromodulation techniques, our results strongly support the view that targeting spinal synapses using principles of spike-timing-dependent plasticity represents a novel effective strategy to facilitate exercise-mediated recovery in humans with different levels of SCI.
Figure 2 Functional improvements after paired corticospinal-motoneuronal stimulation (PCMS). Graphs show group (left) and individual (right) data for PCMS + exercise (A; n = 6), and sham-PCMS + exercise (B; n = 8)groups. The x-axes show the time of measurements (PRE = pre-assessment; POST = post-as-sessment) and the y-axes show the time to perform tasks as percentage of the time at pre-assessment. The x-axes of the right graphs show indi-vidual subjects and filled circles indicate the 6-month follow-up results. (C) Tests involved subcomponents of the GRASSP and the 10-m walk tests. Data of participants included in the shoulder (transverse lines), hand (horizontal lines), and leg (crossed lines) block are shown for each intervention. Filled circles show individual functional outcomes in a subset of subjects at the 6-month follow-up after PCMS + exercise and sham-PCMS + exercise. Error bars indicate SDs, *P< 5 0.05.
Conclusions
We propose that noninvasive stimulation targeting corticospinal-motoneuronal synapses using principles of spike-timing-dependent plasticity can represent a method to strengthen simultaneously multiple lower and/or upper limb muscles after SCI. Overall, approaches targeting corticospinal-motoneuronal synapses represent a new promising tool to promote meaningful functional recovery after SCI.
Disclaimer statements
Contributors None.
Funding This work was supported by U.S. Department of Veterans Affairs [RR&D Merit Review I01RX002474; RR&D Merit Review I01RX002848]; National Institute of Neurological Disorders and Stroke [grant numbers R35NS122336; R01 NS090622].
Conflicts of interest Authors have no conflict of interests to declare.
References
- Layer RT, Ulich TR, Coric D, et al. New clinical-pathological classification of intraspinal injury following traumatic acute complete thoracic spinal cord injury: postdurotomy/myelotomy observations from the INSPIRE trial. Neurosurgery 2017;64(CN_suppl_1):105–9.
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain 1968;91(1):15–36.
- Behrman AL, Ardolino EM, Harkema SJ. Activity-based therapy: from basic science to clinical application for recovery after spinal cord injury. J Neurol Phys Ther. 2017;41(Suppl 3):S39–45.
- Smith AC, Knikou M. A review on locomotor training after spinal cord injury: reorganization of spinal neuronal circuits and recovery of motor function. Neural Plast. 2016;2016:1216258.
- Angeli CA, Boakye M, Morton RA, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379(13):1244–50.
- Bunday KL, Perez MA. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol. 2012;22(24):2355–61.
- Bunday KL, Urbin MA, Perez MA. Potentiating paired corticospinal-motoneuronal plasticity after spinal cord injury. Brain Stimul. 2018;11(5):1083–92.
- Gad P, Lee S, Terrafranca N, et al. Non-Invasive activation of cervical spinal networks after severe paralysis. J Neurotrauma 2018;35(18):2145–58.
- Gill ML, Grahn PJ, Calvert JS, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24(11):1677–82.
- Inanici F, Samejima S, Gad P, Edgerton VR, Hofstetter CP, Moritz CT. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng. 2018;26(6):1272–8.
- Jo HJ, Perez MA. Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury. Brain 2020;143(5):1368–82.
- Urbin MA, Ozdemir RA, Tazoe T, Perez MA. Spike-timing-dependent plasticity in lower-limb motoneurons after human spinal cord injury. J Neurophysiol. 2017;118(4):2171–80.
- Wagner FB, Mignardot JB, Le Goff-Mignardot CG, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018;563(7729):65–71.
- Feldman DE. The spike-timing dependence of plasticity. Neuron 2012;75(4):556–71.
- Hebb DO. The organization of behavior; a neuropsychological theory. New York: Wiley; 1949.
- D’Amico JM, Donges SC, Taylor JL. Paired corticospinal-motoneuronal stimulation increases maximal voluntary activation of human adductor pollicis. J Neurophysiol. 2018;119(1):369–76.
- Taylor JL, Martin PG. Voluntary motor output is altered by spike-timing-dependent changes in the human corticospinal pathway. J Neurosci. 2009;29(37):11708–16.
- Donges SC, Boswell-Ruys CL, Butler JE, Taylor JL. The effect of paired corticospinal-motoneuronal stimulation on maximal voluntary elbow flexion in cervical spinal cord injury: an experimental study. Spinal Cord 2019;57(9):796–804.
- Jo HJ, Kizziar E, Sangari S, Chen D, Kessler A, Kim K, Anschel A, Heinemann AW, Mensh BD, Lieber RL, Oudega M, Perez MA. Inducing Hebbian Plasticity at Multiple Spinal Cord Levels Restores Grasping and Walking in Humans with Tetraplegia: A Prospective Study. 2021, Under revision.
