?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context
Brain–Computer Interface (BCI) is an emerging neurorehabilitation therapy for people with spinal cord injury (SCI).
Objective
The study aimed to test whether priming the sensorimotor system using BCI-controlled functional electrical stimulation (FES) before physical practice is more beneficial than physical practice alone.
Methods
Ten people with subacute SCI participated in a randomized control trial where the experimental (N = 5) group underwent BCI-FES priming (∼15 min) before physical practice (30 min), while the control (N = 5) group performed physical practice (40 min) of the dominant hand. The primary outcome measures were BCI accuracy, adherence, and perceived workload. The secondary outcome measures were manual muscle test, grip strength, the range of motion, and Electroencephalography (EEG) measured brain activity.
Results
The average BCI accuracy was 85%. The experimental group found BCI-FES priming mentally demanding but not frustrating. Two participants in the experimental group did not complete all sessions due to early discharge. There were no significant differences in physical outcomes between the groups. The ratio between eyes closed to eyes opened EEG activity increased more in the experimental group (theta Pθ = 0.008, low beta Plβ = 0.009, and high beta Phβ = 1.48e-04) indicating better neurological outcomes. There were no measurable immediate effects of BCI-FES priming.
Conclusion
Priming the brain before physical therapy is feasible but may require more than 15 min. This warrants further investigation with an increased sample size.
Introduction
Priming is a type of implicit learning where exposure to one stimulus causes a behavioral change in the form of an altered response to another stimulus (Citation1,Citation2). When used successfully in conjunction with a therapeutic intervention, priming results in a behavioral change coinciding with changes in neural processes (Citation2). Motor priming can be achieved through mental training via motor imagery and action observation, repetitive unilateral/bilateral movements, aerobic exercises, and stimulation of the brain and peripheral nerves (Citation1,Citation2).
In the literature, priming is often performed with repetitive transcranial magnetic stimulation, transcranial direct current stimulation, or Functional Electrical Stimulation (FES) (Citation1,Citation3). While the former two methods activate the motor cortex directly and the sensory cortex indirectly, FES activates the sensory cortex directly (orthodromically) as well as the motor cortex indirectly (Citation3). FES is currently the most widely used form of neuromodulation for SCI and multiple studies have validated the use of FES in helping to restore upper extremity function following SCI (Citation4,Citation5).
The indirect activation of the motor cortex presents a basis for neurorehabilitation with FES. During conventional rehabilitation, the neuromuscular electrical stimulation is set to repeatedly and automatically move the patient’s hands, i.e. without any voluntary input from the patient, and often without a functional goal. The main purpose of this stimulation is to strengthen the muscles. However, for FES to work effectively, voluntary effort must coincide with the FES to enable beneficial plasticity (Citation6). This is where Brain–Computer Interface (BCI) can help by decoding the voluntary effort, even in the absence of muscle contraction, relying on input from Electroencephalograph (EEG) (Citation7).
In people with spinal cord injury (SCI), motor priming has already been performed with transcutaneous spinal cord stimulation prior to robotic gait training (Citation8). Upper limbs are under the direct control of the cortico-spinal tract and might additionally benefit from the motor priming from the cortex rather than from the spinal cord. This motor priming can be achieved through BCI, where BCI can be used as a priming intervention to facilitate the excitability of the sensorimotor cortical networks which can maximize the effects of subsequent physical therapy (Citation9,Citation10). Movement-related BCI systems are typically based on the modulation of sensorimotor oscillations recorded through EEG (Citation11–14).about:blank It has been shown that corticomotor excitability is significantly higher when the power of sensorimotor rhythms in the alpha band (8–12 Hz, also called the “mu”-rhythm), or beta band (12–30 Hz) are low, or when the primary motor cortex M1 is stimulated during the trough of the oscillatory cycle of these rhythms (Citation15,Citation16). A phenomenon popularly called event-related desynchronization (ERD), which corresponds to the reduction of the power, is also an index of corticospinal tract or corticomotor excitability (Citation17,Citation18).about:blank.
BCI is often used in conjunction with an external device such as FES (Citation11,Citation19–21). In a BCI-triggered FES system or BCI-FES, first the motor cortex is primed using movement-related action such as motor imagery/attempt with neurofeedback to increase the effect of FES. This is followed by the combined modulating effect of BCI-FES. The facilitating effect of BCI-FES was demonstrated in a study on healthy participants by Suzuki et al. where BCI-FES intervention elicited muscle-specific short-term corticospinal excitability of the intervention targeted muscle only, whereas randomly applied FES was ineffective in eliciting any changes (Citation22).
BCI-FES has typically been used as a standalone intervention in people with SCI (Citation11,Citation19–21) and stroke (Citation12,Citation13,Citation23,Citation24). The duration of these interventions can be about an hour with the number of BCI-FES trials ranging from 40 to 120 repetitions. However, the same actions are repeated in each trial like attempt/imagination of hand extension or grasping. In a rehabilitation setting, the patients undergo a range of hand exercises that cater to improving their specific functional deficits, so the hand therapy is not the same for all patients. This calls for using BCI-FES as a priming intervention, followed by a patient-specific hand therapy session, formally referred to as physical practice. BCI-FES can facilitate priming on two levels: firstly, motor priming while attempting to perform movement, before FES, in order to increase the effect of stimulation, and secondly, sensorimotor cortex activation by BCI-triggered FES.
In a previous study by our group, we found the therapeutic effects of BCI-FES to be better than FES alone for the rehabilitation of the hand (Citation19). However, in this study, we were interested in testing the priming effects of BCI-FES, which goes beyond targeting specific functional improvements to the general improvement of the sensorimotor system and functional recovery. The objective of this study is (i) to test whether motor priming using BCI-FES before physical practice is feasible in clinical settings and (ii) whether it leads to better physical and neurological outcomes than physical practice alone.
Materials and methods
Study design
The study was a randomized controlled feasibility trial comprising of initial and final assessments, as well as intervention (20 sessions, min 3 times per week). The intervention was performed on the dominant hand or, on the weaker hand in the case of mixed-handedness (individual uses one hand for some tasks and the other hand for other tasks), assessed using the Edinburgh handedness inventory (Citation25). The participants were assigned experimental (BCI-FES priming followed by physical practice) or control (Physical Practice only) groups in a semi-randomized way using minimization (Citation26) to match the age (sub-groups 16–30, 31–45, 46–60, 61–75, and 76+) and the level of injury (sub-groups C1–C4 and C5–C8) between groups (Citation27).about:blank The study was approved by NHS Greater Glasgow & Clyde (ClinicalTrials.gov registration no. NCT04367623).
Participants
Ten people (M ± SD age 52 ± 13, 3 F) participated in the study following the inclusion criteria: age between 18 and 80, time since injury less than 6 months, and incomplete injury level C2–C8 or complete level C3–C8 with an area of partially preserved innervation. Participants with neurological disorders, peripheral nerve injury, brachial plexus injury, brain injury, and conditions contraindicative of FES usage were excluded. The participants’ details are shown in . All participants provided a written informed consent and the study was conducted according to the Declaration of Helsinki.
Table 1 Participant details. Lev and Com correspond to American Spinal Cord Injury Association (ASIA) impairment scale level and completeness of injury respectively. The hand column mentions the hand on which the therapy was performed. L and R refer to left-hand and right-hand, respectively. Ex and Co refer to experimental and control groups, respectively.
Intervention
The intervention flowchart is shown in A and the intervention setup for the experimental group is shown in B. Items 1–5 and 7 were used by experimental group only, while multiple items marked 6, were used for physical practice, were used by both groups. The experimental group underwent BCI-FES priming (∼10–15 min) before physical practice (30 min) while the control group performed 40 min of physical practice. The use of hand therapy devices during physical practice is shown in . FES was not part of physical practice in either group.
Figure 1 (a) Experimental protocol (b) Experimental setup for the intervention of the experimental group. BCI calibration which preceded the intervention involved a follow along video (item 7). During BCI-FES figure, items 1–5 were used, comprising of EEG cap with electrodes (1), FES electrodes (2), FES device (3), movement cue (4), neurofeedback gauge (5). Note that the green ball being squeezed was part of both the calibration and BCI-FES session for participants whose flexor muscles were targeted to make the session more realistic and provide sensory feedback. Item 6 shows devices used for hand therapy immediately after BCI-FES figure. The able-bodied volunteer provided informed consent for the photo to be taken
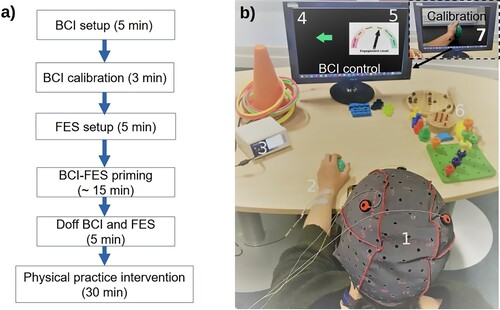
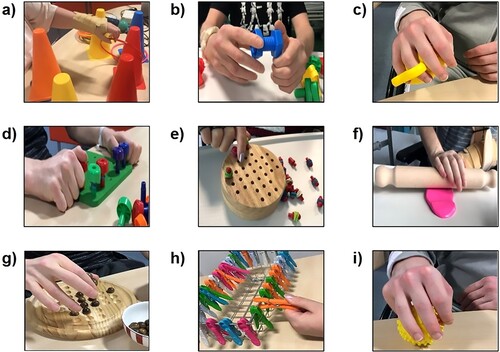
Figure 2 Examples of exercises used in physical practice as part of both experimental and control intervention: (a) reach and grasp, (b) hand manipulation (rotation), (c) increasing grip strength and finger flexion, (d) lateral tripod grasp, (e) fine motor skills, (f) hand strength and wrist extension, (g) pincer grasp, (h) lateral pinch, and (i) power grip
BCI-FES priming hardware and software setup
EEG was recorded from the electrode locations C3 and C4 (Citation28).about:blank More details are provided in Appendix . The details of BCI software such as real-time processing, visual feedback, online BCI control, and offline EEG processing have been explained in a previous publication by our group (Citation29) and additional details are provided in Appendix . The BCI control was based on a threshold switch where the alpha sensorimotor power (8–12 Hz) of the contralateral side had to be maintained below a predefined threshold (power threshold) for a predefined time (threshold time) to activate FES (Citation11,Citation12,Citation30).about:blank Essentially, the BCI control was based on modulation of the sensorimotor rhythm, with ERD i.e. decrease in power, being encouraged to activate FES. Stronger ERD corresponds to the stronger activation of the cortico-spinal pathways (Citation6). The power threshold was defined as a percentage of the calibration power (derived from a calibration session) (Citation29), set in a range from 80% to 100% in steps of 5%, and was fixed for a run. The threshold time was set between 1 and 1.6 s in steps of 0.2 s and was did not change during a single run.
To deliver FES stimulation (using Hasomed RehaStim, Hasomed, Germany), bipolar FES electrodes were placed over the forearm extensor (extensor digitorum superficialis) or flexor (flexor digitorum superficialis) muscles depending on the task. The pulses were biphasic, delivered at 33 Hz stimulation frequency. The pulse intensity (16–28 mA) and pulse width (200–260 µs) were adjusted to trigger wrist extension or flexion of fingers without discomfort. These muscles were chosen for simplicity of electrode placement to reduce donning i.e. setup type. While FES is capable of producing complex movements, participants mainly practiced a simple task, a grasp and release of an object, because the purpose of BCI-FES priming was a task nonspecific activation of the sensorimotor cortex. The purpose of this was to facilitate a subsequent task-specific practice during physical therapy. It must be noted that this is different from neuromuscular electrical stimulation (NMES), which primarily focuses on muscle contractions in order to increase muscle mass, and does not necessarily involve active patient engagement. As a result of active, BCI-driven engagement in functional task, FES produces a stronger activation of the sensory-motor cortex than NMES (Citation31).
BCI-FES priming protocol
The BCI-FES priming was performed in two stages: BCI calibration and BCI-FES online-control. The calibration involved a follow-along video to facilitate 10 repetitions of attempted movements accompanied by action observation. The observed actions were presented on a computer screen. The participants attempted to perform either wrist extension or finger flexion, whichever function was more impaired. These movements are top in the hierarchy of tetraplegia hand management (Citation32). The same movement was used for all sessions.
There were at least 3 runs in the BCI-FES online-control stage, each comprising of 10 trials. Within one trial, the user was cued to attempt movements (motor attempt, MA) such that they move the pointer of the gauge (, 5th part) on a computer screen, towards the green side. The pointer was controlled through movement-related EEG, indicative of ERD. The participants had a minimum of 1 s and a maximum of 10 s time in order to activate the FES, which was active for 5 s. This was followed by a resting period of 15 s. The total time of 30 trials (3 runs × 10 trials) of BCI-FES online-control including the resting period was the range of 10.5–15 min. Breaks between runs were provided as needed. Every 4 sessions, a 2-minute resting-state EEG activity was recorded in the eyes open and eyes closed state pre and post BCI-FES priming.
Physical practice
Various devices were used to provide hand therapy such as putty, hand ergometer, stress balls, pegboards, wooden solitaires, cones and rings, rotating knobs, nuts and bolts, clothespins, keyboards, coins, decorative pebbles, and books These devices facilitated the practice of grip, grasp, and range of motion as well as activities of daily living. shows examples of exercises used in physical practice as part of both experimental and control interventions. The first author of this study administered these activities in both groups. As the participants had different levels of functional impairment, the same activities were not performed for every participant, rather a range of exercises from different exercise domains (grip, grasp, range of motion, fine motor skills) were chosen which needed practice. Fine motor skills or exercises with small objects were often practised during later sessions of the intervention due to the participants’ inability to perform at the start when functional deficits were larger.
Feasibility and usability
These were primary outcome measures. Feasibility was assessed through the FES Activation Rate (AR) and usability through a NASA task load index questionnaire.
Activation rate
The AR was calculated as shown in Equation (1), where Ns and NT refer to the number of successful trials and total trials, respectively. There were no false positives as attempted movement could be observed in each trial. For each session, the mean activation rate was calculated by averaging activation rates over all participants. To quantify the trend of activation rate, a regression line was fitted to the mean activation rates of all sessions. A statistically significant (P < 0.05) positive slope indicates that the rates increased over sessions while a negative slope indicates a decrease over time.
(1)
(1)
NASA task load index
The NASA task load index (Citation33) was used to assess the workload at the end of the 1st, 5th, 10th, and 15th BCI-FES priming sessions. It is a 21-point scale wherein participants rated their perceived workload on six aspects: mental demand, physical demand, performance, effort, and frustration. Lower scores imply less task load and therefore good performance.
Attrition rate
This measure shows the number of participants that dropped out from the study as well as looking at the reasons for dropping out.
Functional assessments
Assessments
The Manual Muscle Test (MMT) was conducted by a physiotherapist blinded to the group assignment of the participant (Citation34). The hand and arm muscles such as Latissimus dorsi, Pectoralis major, Serratus anterior, Deltoid, Triceps, Biceps, Brachioradialis, Supinator, Pronator, Extensor digitorum communis, Extensor carpi radialis, Extensor pollicis longus, Flexor carpi radialis, Flexor digitorum profundus, and intrinsic hand muscles were graded according to the criteria shown in appendix , based on isometric “Break test”, which aims to evaluate the muscle’s ability to resist a gradually increasing pressure (Citation35).
The Catz-Itzkovich Spinal Cord Independence Measure (SCIM) was used to assess the participant’s ability to complete activities of daily living (Citation36). The 5th (Respiration), 6th (Sphincter Management-Bladder) and 7th (Sphincter Management-Bowel) functions were skipped as they were less relevant for upper extremities. The maximum grip strength of each hand was measured using a grip force transducer (MLT004/ST, AD Instruments, USA; resolution 25 mN). The active Range of Motion (ROM) of elbow flexion-extension, wrist flexion-extension, wrist abduction–adduction, thumb flexion-extension, and thumb abduction–adduction of each hand was measured using twin axis goniometers (SG series, Biometrics Ltd, USA; ±150°, resolution 0.1°, accuracy of ±2°).
Analysis
The score change (final -initial) for each assessment was compared between the experimental and control group, separately for the targeted and non-treated hands using Wilcoxon rank-sum test. For the ROM assessment, the changes were pooled for movement of all joints while for the MMT assessment, the changes were pooled separately for the upper arm (9 muscles) and forearm (6 muscles). The significance level was set to P = 0.05 for this and all subsequent statistical analysis in this study.
Neurological assessment
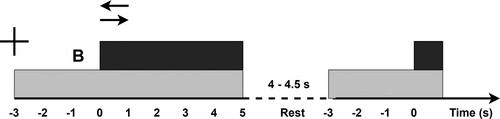
The neurological assessment involved 64 channel EEG recording (for setup details refer Appendix ) in 3 conditions: 2 min rest with eyes closed, 2 min rest with eyes open, and cue-based left and right-hand MA, the paradigm shown in .
Figure 3 The sequence of events for motor attempt trials. The cross represents the readiness cue at t = −3 s. At t = 0 s the execution cue appears on top of the readiness cue in the form of an arrow. The gray rectangle represents the time for which the readiness cue is present on the screen while the black rectangle represents the time the execution cue is present on the screen. B stands for the beep
Neurological outcomes
Event-related desynchronization
Event-related desynchronization (ERD) was assessed during the initial and final assessment of both groups. The EEG recorded during the initial and final assessment was analyzed offline using the EEGLAB toolbox in MATLAB (Citation37) after pre-processing (for details refer appendix ). The noise-free data were used to obtain baseline-normalized event-related spectral perturbations (ERSP) or changes in power corresponding to MA. ERSP is a way of presenting ERD in different frequency ranges at the same time in the form of a spectrogram. The baseline period was t = −4.5 s–t = −3.5 s (a time immediately preceding the cue was not chosen as it would have had components of auditory evoked potential induced by the beep) and the MA period was t = 0.4 s–t = 1.4 s with respect to the MA cue at t = 0. The ERSP was averaged over the theta (4–8 Hz), alpha (8–12), lower beta (12–20 Hz), and higher beta (Citation19–29) bands. The post and pre-intervention ERSPs were compared in EEGLAB STUDY (Citation38), using the non-parametric permutation test (10,000 randomizations). The False Discovery Rate (FDR) method was used to correct for multiple comparisons (Citation39). While the ERSP includes both synchronization (ERS) and desynchronization (ERD), the latter is predominantly induced by movement-related tasks. Previous studies have shown high temporal stability of the right-hand motor imagery ERD in the alpha and upper beta bands as well as high intra-individual reliability of the movement-related beta oscillations (Citation40,Citation41).
EC/EO reactivity
In healthy people, eyes closed to eyes open power ratio or EC/EO reactivity indicates that compared to EC, EO is associated with reduced EEG amplitude in each band in a topographically distinctive manner: global reduction in alpha, posterior theta, and decreased posterior as well as increased frontal beta (Citation42,Citation43). Boord et al. (Citation44) found that EC/EO reactivity is reduced in SCI compared to able-bodied people, and the presence of central neuropathic pain (CNP) further accentuates this effect. Therefore, an increase in EC/EO reactivity would indicate a good EEG-based neurological outcome. Even though the test-retest reliability hasn’t been directly tested, EEG-based measures of theta, alpha, and beta powers were found to be highly repeatable in healthy participants. 46EC measurements were found to be more reproducible than EO measurements.
After cleaning the data, 3 participants per group (S1, S7, and S10 in experimental; S2, S6, and S9 in control) were available. The EC/EO reactivity was calculated for all 64 electrodes in four frequency bands. Changes in EC/EO reactivity were pooled over occipital electrodes (PO3, POz, PO4, O1, Oz, and O2) and compared between the experimental and control group using unpaired t-test. Only occipital electrodes were selected for comparison as EC/EO reactivity is expected to be highest in the occipital area.
Effects of BCI-FES priming
In order to assess the immediate effects of BCI-FES priming on the EEG in the experimental group, EC/EO reactivity was derived from the pre and post session resting state power spectral density (Welch’s method, window size = 2 s, with 50% overlap) of the contralateral side. The reactivity was pooled over sessions and participants and compared using a 2-way repeated measures ANOVA with time (before vs after) and frequency band (theta, alpha, lower beta, and higher beta) as the within-subjects factors.
Results
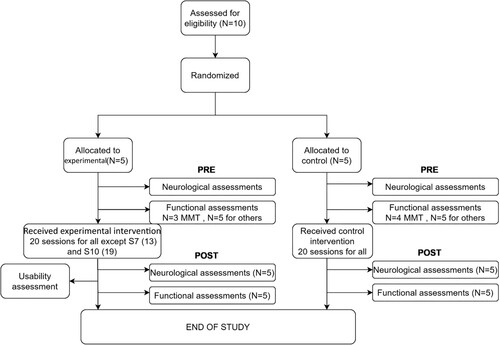
The flow diagram for the study is shown in .
Figure 4 Flow diagram for the clinical study protocol and implementation. MMT stands for manual muscle test. Most participants completed all sessions two in the experimental group. Nevertheless, they completed the post-assessments before leaving.
Feasibility and usability
The mean activation rates increased over sessions with the slope of the regression line equal to 0.479 (P = 0.032), and had minimum and maximum values of 84.7% and 98.7%, respectively.
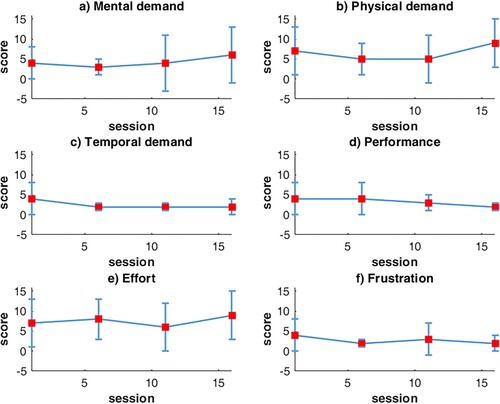
The average NASA questionnaire values are shown in . Overall, the perceived mental demand, physical demand, and effort increased over sessions, but the variability amongst participants was high. The perceived frustration, temporal demand, and performance decreased over time. Note that lower scores for performance imply high performance according to the NASA task load index.
Figure 5 Changes in the NASA Task Load Index scores over 15 sessions, averaged over all participants of the experimental group
Two participants in the experimental intervention group did not complete all 20 sessions (one completed 13 and the other 19 sessions) because of early discharge from the hospital, but they completed the assessment sessions. The reason for early termination was the inability to organize transport from home.
Functional outcomes
The values for functional outcomes are shown in . The MMT changes were not significantly different between the experimental and control groups for the forearm muscles of both targeted and non-treated hand. For the upper arm, changes were still not significantly different for the targeted hand, however, they were significantly greater for the non-treated hand. The change in secondary motor outcomes was not different between groups.
Table 2 Changes (mean ± std, median [min, max]) in scores of functional outcomes such as manual muscle test (MMT), spinal cord independence measure (SCIM), grip strength (GS), and range of motion (ROM) along with P-values of comparison between the experimental and control groups using Wilcoxon rank-sum test. F and U refer to forearm and upper-arm, respectively.
Neurological outcomes
Event related desynchronization
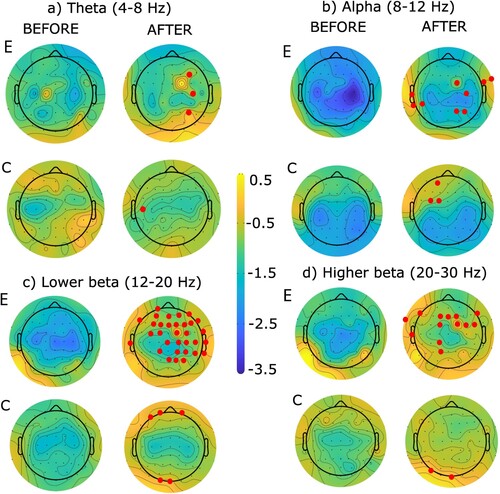
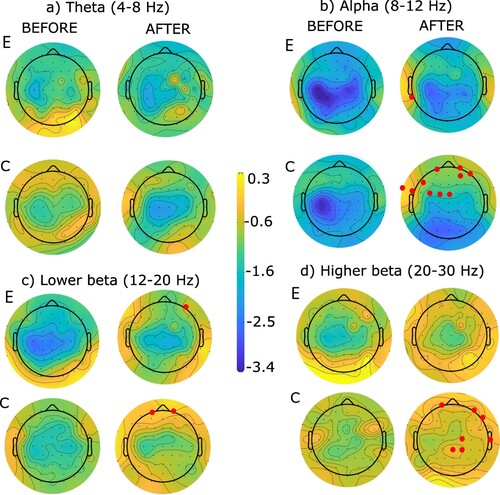
The ERSP topographies are shown in for left-hand MA and for right-hand MA. The blue color represents ERD (desynchronization or motor-related activity) while the yellow color represents ERS (synchronization, or less motor-related activity). In all conditions, ERS P-values became less negative i.e. ERD decreased. The electrodes presenting a significant difference are marked in red in all figures. The experimental group showed more changes for the left hand while the control group showed more changes for the right hand.
EC/EO reactivity
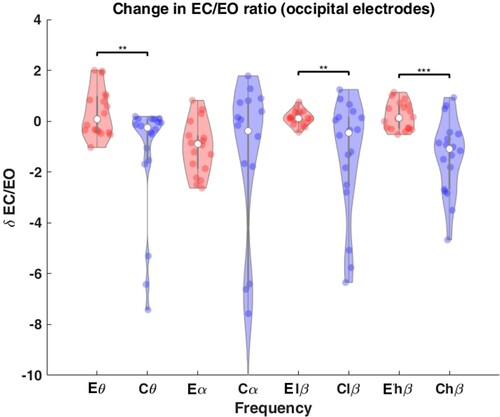
The change in EC/EO reactivity for occipital electrodes, δ EC/EO is shown in . In the control group, the reactivity decreased significantly compared to the experimental group in the theta (P = 0.008), lower beta (P = 0.009) and higher beta (P = 1.48e-04) bands.
Effects of BCI-FES priming
The EC/EO reactivity did not change immediately after BCI-FES priming session (F(1,27) = 3.980, P = 0.056, time x frequency interaction P = 0.676). The mean EC/EO reactivity was 1.98 (CI [1.53 2.2]) before therapy and 1.52 (CI [1.27 1.77]) after therapy.
Discussion
This study showed that it is feasible to deliver BCI-FES priming intervention prior to physical practice in a clinical setting. The adherence to the intervention was high, and none of the hospitalized participants withdrew from the study. The mean true positive FES activation rate (a measure of BCI-FES performance) was above 85% for all sessions and increased over time. This compares to activation rates reported in previous studies by our research group (Citation11,Citation30) and exceeds those reported by Jovanovic et al. (Citation21). Studies based on BCI-FES with stroke participants also achieved high true positive activation rate (Citation20,Citation23,Citation26). The heuristic settings of parameters in each run enabled participants to minimize true negative and false positive rate, however, an increase in performance over sessions also reflects a learning effect.
The low NASA scores for frustration and temporal demands indirectly indicate that participants were relaxed. Previous studies have attributed high motivation and low frustration to high accuracies (Citation20,Citation45). The increase in mental demand, physical demand, and effort might be attributed to increasing the difficulty level over sessions by increasing the value of threshold parameters. The difficulty was increased in order to avoid false positives and to increase the therapeutic effect. However, the perceived performance also increased over time, echoing a real increase in performance as measured by the FES activation rate. Biasiucci et al. (Citation13) also adjusted BCI confidence intervals for each session in each individual participant to make the FES acquisition hard but feasible. They hypothesized that the adjustment facilitated effective engagement i.e. active participation and attention to the motor task, which play an important role in neural plasticity.
The results from primary and secondary functional outcomes indicate that both groups had the same amount of motor recovery for the forearms of the targeted hand, where the therapy was administered. The upper arms of the non-treated hand improved better for the control group in terms of MMT, likely due to better natural recovery. However, the small number of MMT sampling points and lack of differences in secondary outcomes between groups require further investigation in this regard.
The ERD for MA decreased post-therapy for both groups in all frequency bands. This is in contrast to studies with stroke patients studies, where ERD increased post-intervention for active groups compared to control groups (Citation13,Citation46).about:blank This might reflect the differences in the nature of the injury (SCI vs stroke) and related neurological changes in the subacute phase. Lopez et al. (Citation47) followed people with complete SCI over several months in the subacute phase. They found that SCI participants with persisting paralysis had a decrease in MA-ERD (in α and β) while those who had functional and neurological improvement had an increase in α MA-ERD and a small decrease in β ERD.
Most participants in the current study had incomplete injuries and were measured within 2–3 months post-injury, therefore the decrease in MA ERD found in this study does not imply a lack of improvement. In our previous study on people with subacute SCI, we found that participants had initial widespread ERD, rather than ERD over the sensory-motor cortex. This “overactivation” was reduced in BCI FES intervention group as compared to passive FES control group, also accompanied by increased muscle strength in the former (Citation19). It is interesting to note that in the current study, most ERD changes in the experimental group were found for the left-hand MA, although the left hand was treated in only two participants. Also, larger ERD changes were found in the beta band rather than the alpha band which was modulated during BCI-FES priming. This might reflect the dynamic changes already happening as part of regular rehabilitation and natural recovery.
The EC/EO reactivity decreased post-intervention in the control group while it increased in the experimental group. The largest difference between the groups was at the occipital sites. Published literature shows that the EC/EO reactivity is reduced post-SCI compared to able-bodied controls, an effect that is further exacerbated by the presence of CNP (Citation44). It has been proposed that thalamocortical dysrhythmia is a mechanism responsible for lowering the reactivity in both SCI and SCI-CNP (Citation44). Therefore, the experimental intervention in this study might have a positive effect on the brain thalamocortical function.
The same long-term EEG-based neurological outcome was also used to evaluate short-term priming. The immediate short-term effect of BCI FES can be reasonably expected if there is an effect of priming. The EC/EO reactivity, only measured at central electrodes, was not immediately affected by BCI-FES priming. As reactivity changes in the long-term, it is possible that a single 15 min session of BCI-triggered FES might not be long enough to cause measurable changes in reactivity. Another plausible explanation is that central cortex might not be an appropriate location to measure changes in reactivity, because the largest long-term changes were measured over the occipital area, something to consider in future studies.
Single-session BCI-based priming has only been tested in several studies. In a study on healthy participants, Suzuki et al. (Citation22) demonstrated an increase in corticospinal excitability (assessed through motor evoked potential) immediately and post 30 min of BCI-triggered FES session compared to a random FES session where no changes were observed. The session lasted 25 min, comprised 76 trials, and used the same control strategy i.e. modulating sensorimotor rhythm. Kersting et al. (Citation48) tested the effects of BCI-controlled peroneal nerve stimulators on people with stroke and found increased excitability in the BCI associative stimulation intervention compared to the BCI non-associative intervention where no changes were observed. The session involved 30–50 attempted trials, effectively 15 min of BCI intervention. In that study, however, the stimulation was timed with the maxima of motor cortex activation seen through movement-related cortical potential, promoting Hebbian plasticity more precisely. Therefore, we believe that the type of BCI control strategy may govern the time needed to prime the brain. In addition to this, since the neurological deficit in people with SCI is not in the brain but in the spinal cord, the effect of priming might be different.
There are several limitations of the study. The first involves the type and repetitions of physical therapy exercises. These were not standardized across participants and may have affected the dose, subsequently confounding the differences in recovery between the experimental and control group. It is however a common practice to have individual exercise according to patient’s needs. Another limitation is that there was no hand function outcome measure even though the therapeutic activities focused on hand therapy interventions. However, due to very low initial manual muscle scores and the experience from our previous study (Citation19) we did not expect to see large functional improvements, hence we chose general measures of muscle strength, range of motion, and grip strength. Still, in this patient group, even a minimal improvement in muscle strength of fingers or wrist may enable them to use assistive devices including keyboards and switches.
There are some points to explore in future studies. The most important is increased sample size. Secondly, the priming time could be increased by increasing the number of trials with multiple baseline recordings to see if there is dose–response relationship in the short-term. Further, short-term priming could be assessed by other measures typically used to check excitability such as motor evoked potentials.
Conclusions
Priming the brain using BCI-FES priming before physical practice may not have better functional and sensory outcomes than physical practice alone, however, it may have a positive effect on the EEG-based neurological outcomes. There was no evidence of short-term priming in EC/EO reactivity and individual alpha peak frequency.
Disclaimer statements
Contributors None.
Funding This study has been funded by the Commonwealth PhD Scholarship [grant number INCS-2018-244]; Commonwealth Foundation.
Conflicts of interest Authors have no conflict of interest.
AppendixFigures.docx
Download MS Word (476.1 KB)Appendix.docx
Download MS Word (18.3 KB)Acknowledgements
We would like to thank all participants for taking part in the study.
References
- Stoykov ME, Madhavan S. Motor priming in neurorehabilitation. J Neurol Phys Ther 2015;39(1):33–42. doi:10.1097/NPT.0000000000000065.
- Stoykov ME, Corcos DM, Madhavan S. Movement-based priming: Clinical applications and neural mechanisms. J Mot Behav 2017;49(1):88–97. doi:10.1080/00222895.2016.1250716.
- Popović DB. Advances in functional electrical stimulation (FES). J Electromyogr Kinesiol 2014;24(6):795–802. doi:10.1016/j.jelekin.2014.09.008.
- Zhang H, Liu Y, Zhou K, Wei W, Liu Y. Restoring sensorimotor function through neuromodulation after spinal cord injury: Progress and remaining challenges. Front Neurosci [Internet] 2021;15. [accessed 2023 Aug 27]. doi:10.3389/fnins.2021.749465.
- Karamian BA, Siegel N, Nourie B, Serruya MD, Heary RF, Harrop JS, Vaccaro AR. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J Orthop Traumatol 2022;23(1):1–17. doi:10.1186/s10195-021-00623-6.
- Rushton DN. Functional electrical stimulation and rehabilitation – an hypothesis. Med Eng Phys 2003;25(1):75–78. doi:10.1016/S1350-4533(02)00040-1.
- Pfurtscheller G, Müller GR, Pfurtscheller J, Gerner HJ, Rupp R. “Thought” – control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett 2003;351(1):33–36. doi:10.1016/S0304-3940(03)00947-9.
- Skiadopoulos A, Famodimu GO, Solomon SK, Agarwal P, Harel NY, Knikou M. Priming locomotor training with transspinal stimulation in people with spinal cord injury: Study protocol of a randomized clinical trial. Trials 2023;24(1):145. doi:10.1186/s13063-023-07193-4.
- Ramos-Murguialday A, Broetz D, Rea M, Läer L, Yilmaz Ö, Brasil FL, Liberati G, Curado MR, Garcia-Cossio E, et al. Brain–machine interface in chronic stroke rehabilitation: A controlled study. Ann Neurol 2013;74(1):100–108. doi:10.1002/ana.23879.
- Milosevic M, Marquez-Chin C, Masani K, Hirata M, Nomura T, Popovic MR, Nakazawa K. Why brain-controlled neuroprosthetics matter: Mechanisms underlying electrical stimulation of muscles and nerves in rehabilitation. BioMed Eng OnLine 2020;19(1):81. doi:10.1186/s12938-020-00824-w.
- Vučković A, Wallace L, Allan DB. Hybrid brain-computer interface and functional electrical stimulation for sensorimotor training in participants with tetraplegia: A proof-of-concept study. J Neurol Phys Ther 2015;39(1):3–14. doi:10.1097/NPT.0000000000000063.
- Kim T, Kim S, Lee B. Effects of action observational training plus brain-computer interface-based functional electrical stimulation on paretic arm motor recovery in patient with stroke: A randomized controlled trial: effects of AOT plus BCI-FES on arm motor recovery. Occup Ther Int 2016;23(1):39–47. doi:10.1002/oti.1403.
- Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, Corbet T, Schnider A, Schmidlin T, Zhang H, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun 2018;9(1):2421. doi:10.1038/s41467-018-04673-z.
- Mukaino M, Ono T, Shindo K, Fujiwara T, Ota T, Kimura A, Liu M, et al. Efficacy of brain-computer interface-driven neuromuscular electrical stimulation for chronic paresis after stroke. J Rehabil Med 2014;46(4):378–382. doi:10.2340/16501977-1785.
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol 1999;110(11):1842–1857. doi:10.1016/S1388-2457(99)00141-8.
- Neuper C, Wörtz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res [Internet] 2006;159:211–222. [place unknown]: Elsevier; [accessed 2022 Dec 12]; doi:10.1016/S0079-6123(06)59014-4.
- Rau C, Plewnia C, Hummel F, Gerloff C. Event-related desynchronization and excitability of the ipsilateral motor cortex during simple self-paced finger movements. Clin Neurophysiol 2003;114(10):1819–1826. doi:10.1016/S1388-2457(03)00174-3.
- Daly I, Blanchard C, Holmes NP. Cortical excitability correlates with the event-related desynchronization during brain–computer interface control. J Neural Eng 2018;15(2):026022. doi:10.1088/1741-2552/aa9c8c.
- Osuagwu BCA, Wallace L, Fraser M, Vuckovic A. Rehabilitation of hand in subacute tetraplegic patients based on brain computer interface and functional electrical stimulation: A randomised pilot study. J Neural Eng 2016;13(6):065002, doi:10.1088/1741-2560/13/6/065002.
- Trincado-Alonso F, López-Larraz E, Resquín F, Ardanza A, Pérez-Nombela S, Pons JL, Montesano L, Gil-Agudo Á. A pilot study of brain-triggered electrical stimulation with visual feedback in patients with incomplete spinal cord injury. J Med Biol Eng 2018;38(5):790–803. doi:10.1007/s40846-017-0343-0.
- Jovanovic LI, Kapadia N, Zivanovic V, Rademeyer HJ, Alavinia M, McGillivray C, Kalsi-Ryan S, Popovic MR, Marquez-Chin C. Brain–computer interface-triggered functional electrical stimulation therapy for rehabilitation of reaching and grasping after spinal cord injury: A feasibility study. Spinal Cord Ser Cases 2021;7(1):24. doi:10.1038/s41394-020-00380-4.
- Suzuki Y, Jovanovic LI, Fadli RA, Yamanouchi Y, Marquez-Chin C, Popovic MR, Nomura T, Milosevic M. Evidence that brain-controlled functional electrical stimulation could elicit targeted corticospinal facilitation of hand muscles in healthy young adults. Neuromodulation: Technology at the Neural Interface.:S 2023;26:1612–1621; doi:10.1016/j.neurom.2021.12.007.
- Sebastián-Romagosa M, Cho W, Ortner R, Murovec N, Von Oertzen T, Kamada K, Allison BZ, Guger C. Brain computer interface treatment for motor rehabilitation of upper extremity of stroke patients – A feasibility study. Front Neurosci 2020;14:591435. doi:10.3389/fnins.2020.591435.
- Miao Y, Chen S, Zhang X, Jin J, Xu R, Daly I, Jia J, Wang X, Cichocki A, Jung T-P. BCI-Based Rehabilitation on the stroke in sequela stage. Neural Plast 2020;2020:1–10. doi:10.1155/2020/8882764.
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971;9(1):97–113. doi:10.1016/0028-3932(71)90067-4.
- Altman DG, Bland JM. Treatment allocation by minimisation. Br Med J 2005;330(7495):843. doi:10.1136/bmj.330.7495.843.
- DeVivo MJ, Biering-Sørensen F, New P, Chen Y. Standardization of data analysis and reporting of results from the international spinal cord injury core data set. Spinal Cord 2011;49(5):596–599. doi:10.1038/sc.2010.172.
- Nuwer MR. 10-10 electrode system for EEG recording. Clin Neurophysiol 2018;129(5):1103. doi:10.1016/j.clinph.2018.01.065.
- Kumari R, Janković MM, Costa A, Savić AM, Konstantinović L, Djordjević O, Vucković A. Short term priming effect of brain-actuated muscle stimulation using bimanual movements in stroke. Clin Neurophysiol 2022;138:108–121. doi:10.1016/j.clinph.2022.03.002.
- Zulauf-Czaja A, Al-Taleb MKH, Purcell M, Petric-Gray N, Cloughley J, Vuckovic A. On the way home: A BCI-FES hand therapy self-managed by sub-acute SCI participants and their caregivers: A usability study. J NeuroEng Rehabil 2021;18(1):44. doi:10.1186/s12984-021-00838-y.
- Schick T. Functional electrical stimulation in neurorehabilitation. Springer Cham; 2022. doi:10.1007/978-3-030-90123-3 [accessed 2023 Sep 3].
- Kozin SH. Chapter 15 – Tetraplegia: Nerve and tendon transfers. In: Abzug JM, Kozin SH, Neiduski R, (eds.) Pediatric hand therapy [Internet]. San Diego, CA: Elsevier; 2020. p. 201–220. doi:10.1016/B978-0-323-53091-0.00015-4 [accessed 2023 Feb 11].
- Hart SG. Nasa-Task Load Index (NASA-TLX); 20 Years Later. th ANNUAL MEETING.
- Cuthbert SC, Goodheart GJ. On the reliability and validity of manual muscle testing: A literature review. Chiropr Man Therap 2007;15(1):4. doi:10.1186/1746-1340-15-4.
- Conable KM, Rosner AL. A narrative review of manual muscle testing and implications for muscle testing research. J Chiropr Med 2011: S1556370711000903. doi:10.1016/j.jcm.2011.04.001.
- Catz A, Itzkovich M, Stein F. The Catz-Itzkovich SCIM: A revised version of the spinal cord independence measure. Disabil Rehabil 2001;23(6):263–268. doi:10.1080/096382801750110919.
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134(1):9–21. doi:10.1016/j.jneumeth.2003.10.009.
- Delorme A, Mullen T, Kothe C, Akalin Acar Z, Bigdely-Shamlo N, Vankov A, Makeig S. EEGLAB, SIFT, NFT, BCILAB, and ERICA: New tools for advanced EEG processing. Comput Intell Neurosci 2011;2011:1–12. doi:10.1155/2011/130714.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B: Stat Methodol 1995;57(1):289–300. doi:10.1111/j.2517-6161.1995.tb02031.x.
- Friedrich EVC, Scherer R, Neuper C. Stability of event-related (de-) synchronization during brain–computer interface-relevant mental tasks. Clin Neurophysiol 2013;124(1):61–69. doi:10.1016/j.clinph.2012.05.020.
- Espenhahn S, de Berker AO, van Wijk BCM, Rossiter HE, Ward NS. Movement-related beta oscillations show high intra-individual reliability. Neuroimage 2017;147:175–185. doi:10.1016/j.neuroimage.2016.12.025.
- Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol 2007;118(12):2765–2773. doi:10.1016/j.clinph.2007.07.028.
- Barry RJ, De Blasio FM. EEG differences between eyes-closed and eyes-open resting remain in healthy ageing. Biol Psychol 2017;129:293–304. doi:10.1016/j.biopsycho.2017.09.010.
- Boord P, Siddall PJ, Tran Y, Herbert D, Middleton J, Craig A. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord 2008;46(2):118–123. doi:10.1038/sj.sc.3102077.
- Irimia DC, Ortner R, Poboroniuc MS, Ignat BE, Guger C. High classification accuracy of a motor imagery based brain-computer interface for stroke rehabilitation training. Front Robot AI 2018;5:130. doi:10.3389/frobt.2018.00130.
- Remsik AB, Williams L, Gjini K, Dodd K, Thoma J, Jacobson T, Walczak M, McMillan M, Rajan S, Young BM, et al. Ipsilesional Mu rhythm desynchronization and changes in motor behavior following post stroke BCI intervention for motor rehabilitation. Front Neurosci 2019;13:53. doi:10.3389/fnins.2019.00053.
- López-Larraz E, Figueiredo TC, Insausti-Delgado A, Ziemann U, Birbaumer N, Ramos-Murguialday A. Event-related desynchronization during movement attempt and execution in severely paralyzed stroke patients: An artifact removal relevance analysis. NeuroImage: Clinical 2018;20:972–986. doi:10.1016/j.nicl.2018.09.035.
- Mrachacz-Kersting N, Jiang N, Stevenson AJT, Niazi IK, Kostic V, Pavlovic A, Radovanovic S, Djuric-Jovicic M, Agosta F, Dremstrup K, et al. Efficient neuroplasticity induction in chronic stroke patients by an associative brain-computer interface. J Neurophysiol 2016;115(3):1410–1421. doi:10.1152/jn.00918.2015.
Appendix
Table A1 EEG details for BCI-FES setup and neurological assessments, recorded using GTec gUSBAmp Bio Amplifier.
Table A2 Criteria for assessing the strength of muscles using manual muscle test.
Table A3 The slopes and P-values for ERD trends. The underlined values represent statistically significant (P < 0.05) trends. α, lβ, and hβ refer to alpha, lower beta and higher beta bands respectively.