Abstract
Eight members of the TRP-melastatin (TRPM) subfamily have been identified, whose physiological functions and distribution are poorly characterized. Although tissue expression and distribution patterns have been reported for individual TRPM channels, comparisons between individual studies are not possible because of variations in analysis techniques and tissue selection. We report here a comparative analysis of the expression patterns of all of the human TRPM channels in selected peripheral tissues and the central nervous system (CNS) using two distinct but complimentary approaches: TaqMan and SYBR Green real-time quantitative reverse transcription polymerase chain reaction (RT-PCR). These techniques generated comparative distribution profiles and demonstrated tissue-specific co-expression of TRPM mRNA species, indicating significant potential for the formation of heteromeric channels. TRPM channels 2, 4, 5, 6, and 7 in contrast to 1, 3, and 8 are widely distributed in the CNS and periphery. The tissues demonstrating highest expression for individual family members were brain (TRPM1), brain and bone marrow (TRPM2), brain and pituitary (TRPM3), intestine and prostate (TRPM4), intestine, pancreas, and prostate (TRPM5), intestine and brain (TRPM6), heart, pituitary, bone, and adipose tissue (TRPM7), and prostate and liver (TRPM8). The data reported here will guide the elucidation of TRPM channel physiological functions.
Key Words: :
INTRODUCTION
The mammalian transient receptor potential (TRP) channels form a superfamily consisting of diverse nonselective cation channels that both sense and respond to changes in the cellular environment [for reviews see (Citation1, Citation2, Citation3)]. These channels are structurally similar to the first nonmammalian TRP channels identified and characterized from Drosophila (Citation4, Citation5). Together with voltagegated Ca2+ channels, they likely provide the major route of Ca2+ entry in numerous cell types. Mammalian TRP channels possess six putative transmembrane domains that are thought to assemble in vivo as tetramers. They have a wide tissue distribution and most channels demonstrate the potential for alternative splicing. The existence of TRP heterodimers and splice variants increases significantly the potential for functional diversity. To date, the in vivo physiological functions of many TRP channels remain poorly characterized.
The TRPM subfamily was named after the first member identified (Melastatin) and is also referred to as the long TRP channel family due to the large N- and C-termini regions that these channels contain. This family is composed of eight channels that possess distinct biophysical and physiological properties [for review see (Citation6, Citation7)]. A number of these channels (including TRPM2) are permeable to extracellular calcium on activation, and others (including TRPM5) are modulated by the intracellular free calcium ion concentration [Ca2+]i. Three TRPM family members are highly unusual because they contain both ion channel and enzymatic activity in the same protein. TRPM2 contains an ADP-ribose pyrophosphatase domain (Citation8) and both TRPM6 and TRPM7 contain α-kinase domains in their C-termini (Citation9). The physiological functions of TRPM are the least well characterized of all of the TRP families and remain the subject of considerable interest. For example, the basic biophysical properties of TRPM1 have yet to be characterized electrophysiologically (Citation6). The functions of the enzymatic domains contained within TRPM2, TRPM6 and TRPM7 are also poorly understood. In addition, the potential for heterodimerization of these channels bringing together unique biophysical and enzymatic properties may reveal novel physiological potentials.
To understand the potential physiological roles of TRPM channels, it is necessary to know their spatial and temporal expression profiles in vivo. However, to date, limited data are available concerning TRPM expression in human tissues and the data that are available have been obtained by using non-quantitative techniques such as dot-blot, Northern blotting, and reverse transcription polymerase chain reaction (RT-PCR). Here, we report a detailed quantitative comparison of the expression pattern of the human TRPM channel family both in peripheral tissues and the CNS. We have used the genomic structure of the TRPM genes to design primers in the same equivalent exonic region as near to the functional ion channel domains as possible. TaqMan and SYBR Green RT-PCR techniques were used to generate quantitative data to enable a relevant comparison of the distribution profiles of TRPM channels in a way similar to that described for the TRPC channel family and the two pore domain potassium channels (Citation10, Citation11).
METHODS
RNA Tissue Samples and cDNA Synthesis
Poly A+ RNA from 20 separate tissues obtained from four individuals, two males and two females (except placenta and prostate, respectively), was prepared and reverse transcribed. Briefly, 1 μg of poly A+ RNA was reverse transcribed by using random priming and the cDNA produced was used to make replicate plates with each well containing the cDNA from 0.4 ng poly A+ RNA in 2 μL. Genomic DNA standards (Promega, Madison, WI, USA) were prepared by passing the DNA through a 25-gauge needle and were added to the plates as a 10-fold dilution series beginning at 40,000 copies per 2 μL.
Primer Design
Primers were designed close to the functional ion channel pore region, in the same equivalent exonic region for each family member (exon 21, overlapping with exon 20 for TRPM5) according to the exon numbering for TRPM2 (Citation12). The Primer3 program (Citation13) was used for primer design. The selective primers and probes used in this study together with a generic diagram showing their relative TRPM localisation are shown in .
Table 1 Primers and probes used for the quantification of expression of each of the TRPM channels.![]() Localization of TRPM primers around the TRP domain region for each channel (Citation5)
Localization of TRPM primers around the TRP domain region for each channel (Citation5) ![]() Kinase or nudix hydrolase domains, in TRPMs 6/7 and 2, respectively
Kinase or nudix hydrolase domains, in TRPMs 6/7 and 2, respectively
TaqMan Quantitative PCR Analysis
SYBR Green and TaqMan RT-PCR are fluorescence-based techniques used for RNA quantification and genetic analysis (Citation14). SYBR Green analysis is based on the property of the fluorescent dye SYBR Green I (PE Applied Biosystems, Warrington, UK) to bind to the PCR product. TaqMan chemistry consists of the hybridization of a dual-labeled probe to the PCR product and the development of the signal by loss of fluorescence quenching as PCR degrades the probe. Both techniques are sensitive and easy to use. SYBR Green chemistry has the advantage of being inexpensive, but the disadvantage is that without a sequence-specific probe, nonspecific amplification can occur and increase the signal to noise apparent from specific amplification. The production of multiple fragments during the SYBR Green PCR can be identified by the use of a dissociation curve run immediately after the PCR.
We used TaqMan quantitative PCR to assess the level of each gene relative to genomic DNA standards as previously described (Citation11). Briefly, PCR primers and TaqMan probe, designed as described above, were added to TaqMan universal 2× mastermix (Applied Biosystems, CA, USA) together with water to give a final concentration of 400 nM primers and 100 nM probe. Eight microliters of this mastermix was then added to 2 μL of complementary DNA (cDNA) preplated into a 384-well PCR plate. The plate was PCR-cycled by using TaqMan default conditions (50°C for 2 min, 95°C for 10 min, and 45 cycles consisting of 95°C for 15 sec and 60°C for 1 min) using an ABI7900HT Sequence Detector (Applied Biosystems).
SYBR Green Quantitative PCR
SYBR Green quantitative PCR (Applied Biosystems) was performed in a manner similar to the TaqMan quantitative PCR described above, with substitution of the TaqMan probe for the same volume of water and the exchange of TaqMan PCR mastermix for SYBR Green 2× mastermix (Applied Biosystems). In addition, the ABI7900HT Sequence Detector was set to use the SYBR Green factory default detector range. The factory default melt curve heating stage was undertaken at the end of the 35-cycle PCR stage.
Data Analysis
The data are presented as the means of mRNA copies detected for each gene/ng poly A+ RNA from four individuals ±SEM (n = 4). These values were calculated from a standard curve generated on the ABI7900HT Sequence Detector by using the sheared genomic standards present on the plate and SDS2.0 software (Applied Biosystems).
RESULTS
Comparison of SYBR Green and TaqMan Sensitivities
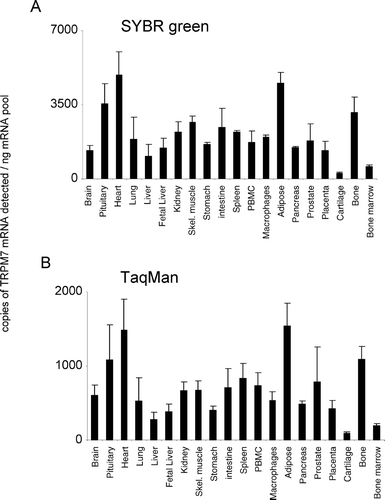
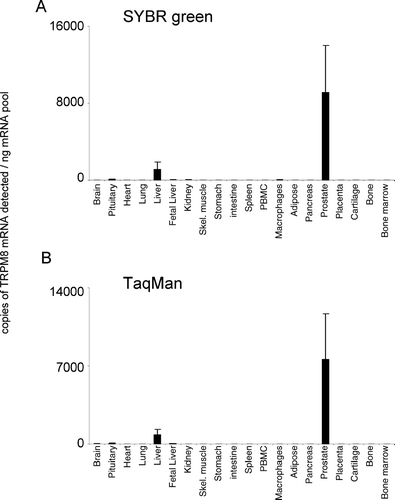
In the present study, both SYBR Green and TaqMan techniques delivered comparable patterns of tissue mRNA distribution for all TRPM channels (, , , , , , , ). For TRPM1, the TaqMan and SYBR Green mRNA patterns are consistent because the levels of TRPM1 mRNA are extremely low. The 45 PCR cycles that were used for TaqMan would detect more copies of TRPM1 mRNA than the 35 cycles conducted with SYBR Green analysis. The latter would be insufficient to allow quantification, indicating that we are reaching the limits of detection for both technologies with this gene. Comparable mRNA expression levels were obtained from both analysis techniques for TRPMs 1, 3, 4, 5, and 8 (, , , , ). In addition, although SYBR Green detected more copies of TRPM2 and TRPM7 mRNA and less copies of TRPM6 mRNA than TaqMan analysis, the relative levels of expression of these channels between tissues were the same. In general, both technical approaches were equally sensitive, although individual SYBR Green assays could be limited in their sensitivity by primer dimer formation.
Figure 1. Expression of hTRPM1 mRNA. mRNA profiles in human CNS and peripheral tissues. Data are expressed as copies of TRPM1 mRNA detected normalized to ng mRNA pool to correct for RNA quantity and integrity and are means ± SEM for triplicate reverse transcription reactions from each RNA pool.
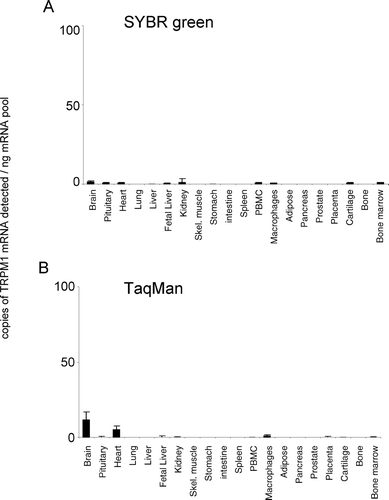
Figure 2. Expression of hTRPM2 mRNA. mRNA profiles in human CNS and peripheral tissues. Data are expressed as copies of TRPM2 mRNA detected normalized to ng mRNA pool to correct for RNA quantity and integrity and are means ± SEM for triplicate reverse transcription reactions from each RNA pool.
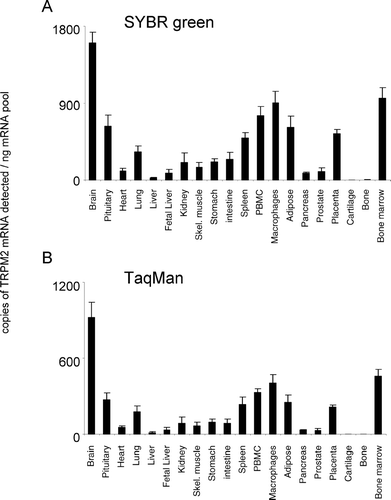
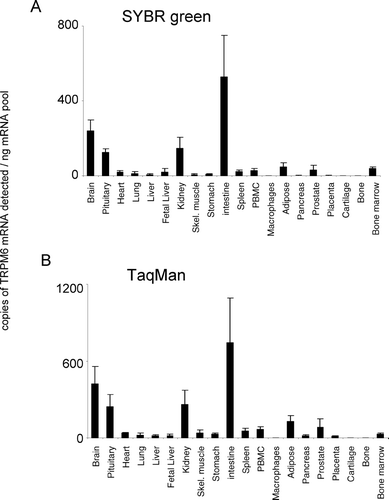
Figure 3. Expression of hTRPM3 mRNA. mRNA profiles in human CNS and peripheral tissues. Data are expressed as copies of TRPM3 mRNA detected normalized to ng mRNA pool to correct for RNA quantity and integrity and are means ± SEM for triplicate reverse transcription reactions from each RNA pool.
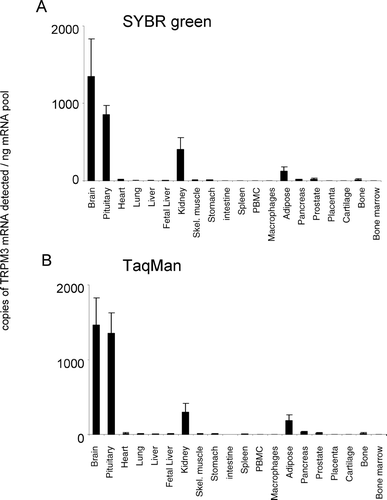
Figure 4. Expression of hTRPM4 mRNA. mRNA profiles in human CNS and peripheral tissues. Data are expressed as copies of TRPM4 mRNA detected normalized to ng mRNA pool to correct for RNA quantity and integrity and are means ± SEM for triplicate reverse transcription reactions from each RNA pool.
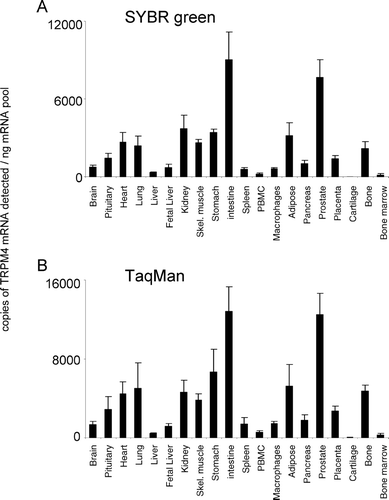
Figure 5. Expression of hTRPM5 mRNA. mRNA profiles in human CNS and peripheral tissues. Data are expressed as copies of TRPM5 mRNA detected normalized to ng mRNA pool to correct for RNA quantity and integrity and are means ± SEM for triplicate reverse transcription reactions from each RNA pool.
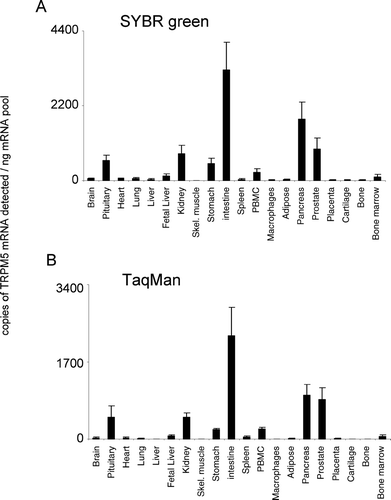
Figure 6. Expression of hTRPM6 mRNA. mRNA profiles in human CNS and peripheral tissues. Data are expressed as copies of TRPM6 mRNA detected normalized to ng mRNA pool to correct for RNA quantity and integrity and are means ± SEM for triplicate reverse transcription reactions from each RNA pool.
Expression Profiles
TRPM1 showed negligible expression (up to 28 mRNA copies detected/ng polyA+ RNA), in all tissues tested (). Brain and heart were the only regions to exhibit significant mRNA expression levels that were conserved between both analysis techniques used (). In addition to these tissues, isolated cells of the macrophage lineage also possessed significant and conserved detectable levels of TRPM1. No other tissues revealed significant expression of TRPM1. In contrast to TRPM1, TRPM2 was widely expressed, being detectable in all peripheral tissue regions analyzed except for cartilage and bone (). Highest relative tissue expression levels were apparent in the brain. In addition to brain, bone marrow and macrophage cells also possessed high relative expression levels. The relative pattern of expression was conserved between both analysis techniques used.
TRPM3 was detected at highest levels in the brain and pituitary with low but significant levels of expression detected in kidney, adipose tissue, pancreas, prostate, and bone (). TRPM3 was barely detectable or absent from all other tissues tested (). The relative pattern of TRPM3 expression was conserved between both analysis techniques used. TRPM4 was the most highly expressed of the TRPM channels. TRPM4 displayed a widespread expression pattern, being expressed at highest levels in intestine and prostate (). TRPM4 mRNA was not present at significant levels in cartilage tissue. The relative pattern of expression was conserved for TRPM4 between both analysis techniques used.
TRPM5 displayed a discrete tissue distribution, with highest expression in intestine, pancreas, prostate, kidney, and pituitary (). Significant expression was also observed in stomach, peripheral blood mononuclear cell (PBMC), and bone marrow. For other tissue regions, the levels of expression were not significantly above background. The relative pattern of expression was conserved for TRPM5 between both analysis techniques used. TRPM6 was highly expressed in intestine (). Lower levels of expression were detected in brain, pituitary, adipose, prostate, bone marrow, and kidney. TRPM6 mRNA was virtually undetectable in all other tissues analyzed. The relative pattern of expression was conserved for TRPM6 between both analysis techniques used.
TRPM7 was ubiquitously expressed across all tissues analyzed, showing similar levels of expression across the majority of tissues tested (). The relative pattern of expression was conserved for TRPM7 between both analysis techniques used. In the present study, TRPM8 displayed the most discrete expression pattern; highest levels of mRNA were detected in the prostate (). TRPM8 mRNA was also shown to be present in human liver. TRPM8 mRNA was virtually undetectable in all other tissues analyzed. The relative pattern of expression was conserved for TRPM8 between both analysis techniques used.
DISCUSSION
This study provides for the first time, a comparative mRNA distribution profile for the human TRPM channel family. Those tissues demonstrating highest mRNA expression for TRPM channels were brain (TRPM1), brain macrophage and bone marrow (TRPM2), brain and pituitary (TRPM3), intestine and prostate (TRPM4), intestine, pancreas, and prostate (TRPM5), intestine and brain (TRPM6), heart, pituitary, adipose tissue, and bone (TRPM7) and prostate and liver (TRPM8). TRPM1 is a putative tumor suppressor cloned from murine cells of the melanocyte lineage [for review see (Citation15, Citation16)], which is thought to act as a Ca2+ permeable ion channel. However, to date, its electrophysiological properties are unknown. In the present study, human TRPM1 mRNA expression was detected at extremely low levels at the limit of our detection techniques, with brain the only tissue possessing significant expression (). A splice variant has been reported, TRPM1-S, terminating at exon 14, which inhibits TRPM1 function by interfering in its trafficking to the plasma membrane (Citation15). Fang and Setaluri (Citation16) observed that PCR products of human TRPM1 cDNA spanning exons from 4 to 16 or 17 were very weak compared with products spanning exons from 4 to 15. Because we designed our primers close to the functional ion channel domain (exon 21), we would have underestimated the abundance of such variants of TRPM1.
TRPM2 is a calcium permeant channel, highly expressed in brain, pancreas, and immune cells, activated by intracellular ADP ribose, Ca2+ and by oxidative stress [for review see (Citation17, Citation18, Citation19)]. Prolonged activation of TRPM2 causes a sustained elevation in intracellular free calcium levels and subsequent cell death. Three human naturally occurring splice variants are reported in the literature and have been characterized following heterologous expression in HEK293 cells. Wehage et al. (Citation20) reported a splice variant with a deletion in the C-terminus that responds to reactive oxidant species but not to ADP-ribose. Recently, Uemura et al. (Citation21) reported a splice variant with an N-terminal deletion that responds to oxidants and which is selectively expressed in human striatum. Zhang et al. (Citation22) reported a different splice variant that could not be detected by our primer set. This variant acts as a dominant negative of full length TRPM2 by direct interaction of the two forms at the membrane level. In the present study, TRPM2 displayed a widespread expression pattern, being present in brain and all peripheral regions analyzed except cartilage and bone, which could imply a significant role for TRPM2 in human physiology.
To date, two studies report TRPM3 molecular and functional characterization each using a different variant of human TRPM3 (Citation23, Citation24). Human TRPM3 was cloned from kidney in both studies and was heterologously expressed in HEK293 cells. TRPM3 is permeable to calcium and a recent study showed that different splice variants differ in their relative permeability to divalent cations (Citation25). In the study of Lee et al. quantitative RT-PCR revealed a higher expression in kidney than in brain, spinal cord, and testis, and TRPM3 was virtually absent in the remaining tissues studied (Citation23). Similarly, Grimm et al. (Citation24) demonstrated higher expression in the kidney than in other tissues. The primers used by Lee et al. and Grimm et al., in contrast to the primers used in this study, were designed to the terminal exon 23 and exons 1–11, respectively [according to the equivalent exon numbering for TRPM2 (Citation12)]. Therefore, it is possible that they were able to detect additional channel variants to those detected in this study, expressed at greater levels in kidney. Although TRPM3 has been reported to be activated by a small molecule, the sphingolipid D-erythro-sphingosine (Citation26), no physiological function has been reported for native TRPM3. Because of its expression in kidney and its proximity to TRPM6 (9q21.13), it has been suggested that TRPM3 may play a contributory role in the renal insufficiency phenotype of hypomagnesemia with secondary hypocalcemia (Citation23). Our data reveal significant brain distribution of pore-forming channels and, therefore, may indicate a significant role for TRPM3 in brain function.
Two variants of human TRPM4 have been characterized that differ in the length of the N-terminus: TRPM4a and TRPM4b [for review see (Citation17)]. The shorter human TRPM4a channel is reported to conduct Ca2+ (Citation15), whereas TRPM4b is a Ca2+-activated Ca2+ impermeable cation channel (Citation27). Northern blot analysis of TRPM4b revealed expression in kidney, heart, prostate, colon, intestine, placenta, skeletal muscle, pancreas, at lower levels in thymus and spleen, and being absent in lung, brain, liver, and leukocytes (Citation15, Citation27, Citation28). Recently, TRPM4 has been proposed as a regulator of myogenic constriction in rat cerebral arteries (Citation29) and as a mediator of cytokine production in T lymphocytes (Citation30). In the present study, which will have detected both TRPM4a and b, TRPM4 displayed a widespread expression pattern: highly expressed in intestine and prostate and absent only in cartilage ().
Like TRPM4, TRPM5 is a Ca2+-activated Ca2+ impermeable monovalent cation channel (Citation31). TRPM5 is thought to mediate taste signaling, but native human and murine TRPM5 transcripts are also present in other tissues and cell lines (Citation32). In the present study, TRPM5 displayed a discrete tissue distribution, with highest expression in intestine, followed by pancreas, prostate, kidney, and pituitary (). This tissue distribution profile is consistent with high TRPM5 expression previously reported in the mouse intestine (Citation33).
TRPM6 and TRPM7 are closely related, being the only known ion channels with an intrinsic kinase domain, and share electrophysiological properties [for review see (Citation17)]. It is thought that both TRPM6 and TRPM7 regulate cellular Mg2+ homeostasis. Cloning of human TRPM6 has been described by two groups, with mRNA being detected in intestine and kidney (Citation34, Citation35). Several mutations in the TRPM6 gene have been identified in patients suffering with hypomagnesemia with secondary hypocalcemia (Citation34). Two such mutants resulted in truncated proteins, and a further miss-sense mutation has been shown to affect the ability of TRPM6 to interact with TRPM7 (Citation36, Citation37). In the present study, TRPM6 mRNA was highly expressed in intestine () with low levels of expression detected in brain, pituitary and kidney, being virtually absent in the rest of the tissues analyzed. However, to date, TRPM6 expression in the brain has not been reported. Recently, a TRPM6/7-like channel was reported in pig and rat cardiac tissue; thus, these channels may not be limited to a physiological role in renal function (Citation38).
TRPM7 is an ubiquitously expressed channel (Citation39), and it may be responsible for cellular entry of trace metal ions (Citation40) besides its function as a cellular Mg2+ homeostasis regulator (Citation6); see above. TRPM7 has been reported to be involved in cell growth in human retinoblastoma cells, anoxic neuronal cell death, and also a TRPM7-like current has been reported in rat microglia [for review see (Citation19)]. Recently, Annexin I has been identified as a substrate of the TRPM7 kinase (Citation41). Annexin I is a Ca2+-binding protein that has been implicated in processes such as inflammation, proliferation, and apoptosis. In the present study, TRPM7 was the most ubiquitously expressed channel, showing similar levels of expression across nearly all tissues tested (). These data are in agreement with previous literature findings (Citation6) and with the hypothesis that one critical function of TRPM7 is to act as a Mg2+ homeostasis regulator across the whole organism (Citation17).
Human TRPM8 expression was originally shown to be restricted mostly to the prostate, with increased expression occurring in prostate cancer, as well as a number of other primary neoplasms (Citation42). Human TRPM8 is also expressed in the bladder (Citation43), whereas rat and mouse TRPM8 expression seems to be restricted to trigeminal and dorsal root ganglion (Citation43, Citation44, Citation45). Several human TRPM8 transcripts have been observed in normal prostate and the melanoma cell line G361 (Citation42), and rat TRPM8 transcripts of various sizes have been observed in neuronal tissue (Citation45), suggesting that alternative splicing of TRPM8 occurs. Both human and rodent TRPM8 are activated by cold temperatures and by cooling agents (Citation44, Citation45, Citation46) and, thus, are likely to be involved in noxious thermal nociception (Citation47). TRPM8 has been also implicated in the survival of prostate cancer cells (Citation48). In the present study, TRPM8 displayed the most discrete expression pattern, with highest levels in the prostate (). In addition, our data indicate that TRPM8 is also present in human liver, a localization not reported to date.
The TRPM mRNA expression patterns presented in this study are in general consistent with published expression [for review see (Citation6, Citation49, Citation50)]; however, we show new TRPM mRNA localizations not reported previously. We demonstrate TRPM6 mRNA expression in the brain for the first time. In addition, we show that human TRPM8 mRNA is expressed in the liver. Because we use primers designed to equivalent elements of the TRPM channels that are functionally necessary, our data allow for cross-comparison of expression to be made between individual members of this ion channel family. It is possible that co-expression of different family members in the same tissues might be of physiological significance. Hetero-oligomerization is a feature of several members of distinct TRP channel families; for example, TRPC1 can co-assemble with TRPC5 to form a heteromeric neuronal channel (Citation51). For the TRPM channel family, experiments appear to show that TRPM6 requires TRPM7 to reach the plasma membrane to form a functional ion channel (Citation36). A number of naturally occurring splice variants and mutants have been described for members of the TRPM family, and expression of some of these may have significant physiological consequences. For example, two short variant forms have been shown to have dominant negative effects on the full-length members via direct interaction, namely, TRPM1-S and TRPM2-S (Citation15, Citation22), and several nonfunctional transcripts of TRPM6 have been identified (Citation34, Citation36, Citation37). However, because we designed the primers to be close to the functional domain, we have confined our analyses to the detection of pore-forming channels.
We have detailed a comparative study of the expression profiles of an important family of calcium permeable ion channels. Although this study provides a valuable comparison of expression at the mRNA level, it is important to remember that mRNA levels will not always correlate with the relative abundance of these channels at the protein level or their functional state. Systematic studies using TRPM subtype-selective antisera are required to extend our findings to the protein level, which will further guide experiments to understand the functions of these channels.
ACKNOWLEDGMENTS
EF was the recipient of EU Framework V Postdoctoral Fellowship project MCFH-2001-00746. The authors thank Dr. R. Ravid, Netherlands Brain Bank, the Netherlands, for donation of brain tissue. They also acknowledge the expert technical assistance of Jennifer M Wilson.
REFERENCES
- Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol 2005; 68: 685–717, [CSA]
- Birnbaumer L, Yidirim E, Abramowitz J. A comparison of the genes coding for canonical TRP channels and their M, V and P relatives. Cell Calcium 2003; 33: 419–432, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Clapham D E. TRP channels as cellular sensors. Nature 2003; 426: 517–524, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Vriens J, Owsianik G, Voets T, Droogmans G, Nilius B. Invertebrate TRP proteins as functional models for mammalian channels. Pflugers Arch 2004; 449: 213–226, [INFOTRIEVE], [CSA]
- Montell C. The TRP superfamily of cation channels. Sci STKE 2005; 272, re3, [CSA]
- Fleig A, Penner R. The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol Sci 2004; 25: 633–639, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Kraft R, Harteneck C. The mammalian melastatin-related transient receptor potential cation channels: An overview. Pflugers Arch 2005; 451: 204–211, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Perraud A L, Fleig A, Dunn C A, Bagley L A, Launay P, Schmitz C, Stokes A J, Zhu Q, Bessman M J, Penner R, Kinet J P, Scharenberg A M. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001; 411: 595–599, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Montell C. Mg2+ homeostasis: The Mg2+ nificent TRPM chanzymes. Curr Biol 2003; 13: R799–R801, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Chapman C G, Meadows H J, Godden R J, Campbell D A, Duckworth M, Kelsell R E, Murdock P R, Randall A D, Rennie G I, Gloger I S. Cloning, localisation and functional expression of a novel human, cerebellum specific, two pore domain potassium channel. Brain Res Mol Brain Res 2000; 82: 74–83, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Riccio A, Medhurst A D, Mattei C, Kelsell R E, Calver A R, Randall A D, Benham C D, Pangalos M N. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res 2002; 109: 95–104, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, Shimizu N. Molecular cloning of a novel putative Ca2+ channel protein (Trpc7) Highly expressed in brain. Genomics 1998; 54: 124–131, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 2000; 132: 365–386, [INFOTRIEVE], [CSA]
- Ponchel F, Toomes C, Bransfield K, Leong F T, Douglas S H, Field S L, Bell S M, Combaret V, Puisieux A, Mighell A J, Robinson P A, Inglehearn C F, Isaacs J D, Markham A F. Real-time PCR based on SYBR-Green I fluorescence: An alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol 2003; 3: 18, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Xu X Z, Moebius F, Gill D L, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci U S A 2001; 98: 10692–10697, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Fang D, Setaluri V. Expression and Up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem Biophys Res Commun 2000; 279: 53–61, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Perraud A L, Knowles H M, Schmitz C. Novel aspects of signaling and ion-homeostasis regulation in immunocytes. The TRPM ion channels and their potential role in modulating the immune response. Mol Immunol 2004; 41: 657–673, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Perraud A L, Schmitz C, Scharenberg A M. TRPM2 Ca2+ permeable cation channels: From gene to biological function. Cell Calcium 2003; 33: 519–531, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- McNulty S, Fonfria E. The role of TRPM channels in cell death. Pflugers Arch 2005; 451: 235–242, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Wehage E, Eisfeld J, Heiner I, Jungling E, Zitt C, Luckhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 2002; 277: 23150–23156, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Uemura T, Kudoh J, Noda S, Kanba S, Shimizu N. Characterization of human and mouse TRPM2 genes: Identification of a novel N-terminal truncated protein specifically expressed in human striatum. Biochem Biophys Res Commun 2005; 328: 1232–1243, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Zhang W, Chu X, Tong Q, Cheung J Y, Conrad K, Masker K, Miller B A. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem 2003; 278: 16222–16229, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Lee N, Chen J, Sun L, Wu S, Gray K R, Rich A, Huang M, Lin J H, Feder J N, Janovitz E B, Levesque P C, Blanar M A. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J Biol Chem 2003; 278: 20890–20897, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 2003; 278: 21493–21501, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Oberwinkler J, Lis A, Giehl K M, Flockerzi V, Philipp S E. Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J Biol Chem 2005; 280: 22540–22548, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Grimm C, Kraft R, Schultz G, Harteneck C. Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine. Mol Pharmacol 2005; 67: 798–805, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Launay P, Fleig A, Perraud A L, Scharenberg A M, Penner R, Kinet J P. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 2002; 109: 397–407, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem 2003; 278: 30813–30820, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Earley S, Waldron B J, Brayden J E. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 2004; 95: 922–929, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet J P. TRPM4 regulates calcium oscillations after T cell activation. Science 2004; 306: 1374–1377, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol 2003; 13: 1153–1158, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Prawitt D, Monteilh-Zoller M K, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A 2003; 100: 15166–15171, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Perez C A, Margolskee R F, Kinnamon S C, Ogura T. Making sense with TRP channels: Store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium 2003; 33: 541–549, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Schlingmann K P, Weber S, Peters M, Niemann N L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth H W, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 2002; 31: 166–170, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Walder R Y, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger M B, Beck G E, Englehardt R K, Carmi R, Sheffield V C. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 2002; 31: 171–174, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Chubanov V, Waldegger S, Schnitzler M, Vitzthum H, Sassen M C, Seyberth H W, Konrad M, Gudermann T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci U S A 2004; 101: 2894–2899, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Voets T, Nilius B, Hoefs S, Van Der Kemp C M, Droogmans G, Bindels J M, Hoenderop G J. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 2004; 279: 19–25, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Gwanyanya A, Amuzescu B, Zakharov S I, Macianskiene R, Sipido K R, Bolotina V M, Vereecke J, Mubagwa K. Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: Permeation of divalent cations and pH-mediated regulation. J Physiol 2004; 559: 761–776, [INFOTRIEVE], [CSA]
- Runnels L W, Yue L X, Capham D E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001; 291: 1043–1047, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Monteilh-Zoller M K, Hermosura M C, Nadler M J, Scharenberg A M, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol 2003; 121: 49–60, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Dorovkov M V, Ryazanov A G. Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem 2004; 279: 50643–50646, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Tsavaler L, Shapero M H, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Research 2001; 61: 3760–3769, [INFOTRIEVE], [CSA]
- Stein R J, Santos S, Nagatomi J, Hayashi Y, Minnery B S, Xavier M, Patel A S, Nelson J B, Futrell W J, Yoshimura N, Chancellor M B, De Miguel F. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol 2004; 172: 1175–1178, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Peier A M, Moqrich A, Hergarden A C, Reeve A J, Andersson D A, Story G M, Earley T J, Dragoni I, Mcintyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108: 705–715, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- McKemy D D, Neuhausser W M, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416: 52–58, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004; 430: 748–754, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Tominaga M, Caterina M J. Thermosensation and pain. J Neurobiol 2004; 61: 3–12, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Zhang L, Barritt G J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res 2004; 64: 8365–8373, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Vennekens R, Voets T, Bindels R J, Droogmans G, Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium 2002; 31: 253–264, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Clapham D E, Runnels L W, Strubing C. The TRP ion channel family. Nat Rev Neurosci 2001; 2: 387–396, [INFOTRIEVE], [CSA], [CROSSREF], [CROSSREF]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham D E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 2001; 29: 645–655, [INFOTRIEVE], [CSA]