Abstract
Exosomes from human umbilical cord mesenchymal stem cells (HUCMSCs) containing microRNAs (miRNAs) have been underscored as possible therapeutic options for cancers. Hence, our goal here was to investigate the relevance of miR-320a-containing exosomes from HUCMSCs to lung cancer. First, H1299 and H460 cells were co-cultured with the exosomes overexpressing miR-320a from HUCMSCs. The data displayed that HUCMSCs-secreted exosomes expressing miR-320a exerted anti-tumor effects in vitro and in vivo. Online analysis available at TargetScan database revealed that miR-320a bound to sex-determining region Y-box 4 (SOX4), and the luciferase reporter gene assay clarified this targeting relationship. Next, a β-catenin-specific agonist WAY-262611 was delivered into the H1299 and H460 cells to assess the effects of the Wnt/β-catenin pathway on lung cancer cellular processes. The results demonstrated that WAY-262611 potentiated lung cancer cell viability, invasion, and migration, but inhibited cell apoptosis. Altogether, exosomes carrying miR-320a from HUCMSCs might suppress lung cancer cell growth via the SOX4/Wnt/β-catenin axis, which highpoints the potency of exosomes expressing miR-320a as a possible therapeutic option for lung cancer treatment.
Introduction
Lung cancer characterizes as the most commonly diagnosed cancer (11.6%) and the leading cause of cancer-related death (18.4%) in the worldwide in 2018 [Citation1]. The main driving forces for lung cancer involve genetic mutations and environmental factors, such as smoking history, radiation exposure, as well as toxic substances [Citation2]. The relatively short survival of lung cancer patients results from metastasis and recurrence, with bone, brain and liver ranking the most common positions for metastases [Citation3]. As a consequence, a clear explication of the mechanism of lung cancer metastasis may be beneficial to develop more effective treatment measures, which is of great significance to the improvement of the prognosis of patients with lung cancer [Citation4].
Exosomes, nanovesicles that are linked to various physiological and pathological conditions, may be applied as possible diagnostic tools for cancers, particularly lung cancer [Citation5]. Exosome-modulated transfer of microRNAs (miRNAs) has been validated to impact all kinds of cancer progression, including cancer cell invasion and proliferation, along with drug resistance through cell–cell communications [Citation6]. Extracellular vesicles secreted by human umbilical cord-derived mesenchymal stem cells (HUCMSCs), for example, have been established as an attractive therapeutic measure for the clinical application that might reduce undesirable side effects [Citation7]. HUCMSCs, a subtype of adult stem cell, hold the capacity of self-renewal and multi-potency [Citation8]. It has been reported that exosomes secreted from HUCMSCs overexpressing miR-148b-3p restrained the development of breast cancer [Citation9]. Interestingly, a significant decline in the miR-320a expression was found in cancer-associated fibroblasts-derived exosomes, and miR-320a performed as a tumor suppressor by interacting with its direct target PBX3 to impede the cell proliferation, migration and metastasis in hepatocellular carcinoma [Citation10]. Moreover, the tumor suppressor role of miR-320a has been reported in colorectal cancer [Citation11], prostate cancer [Citation12], and nasopharyngeal carcinoma [Citation13]. In addition, sex determining region Y (SRY)-box 4 (SOX4) was enhanced in different cancers and has been observed to be correlated with cellular transformation, cell survival, and metastasis [Citation14,Citation15]. Besides, SOX4 was extensively reported in the regulation of lung cancer through its interaction with different miRNAs and long non-coding RNAs [Citation16–18]. Furthermore, the connection between SOX family and the canonical Wnt/β-catenin pathway has been long established [Citation19]. In the present study, the potential relevance of HUCMSCs-derived exosomes to lung cancer was investigated. The effects of exosomes containing miR-320a from HUCMSCs on cell growth and metastasis were tested in vitro. Furthermore, the underlying mechanism of HUCMSCs-derived exosomes in lung cancer cells was explored, which might offer new insights into the understanding of lung cancer.
Material and methods
Antibodies, primers and reagents
Antibodies to fluorescein isothiocyanate (FITC)-labeled HUCMSCs surface markers CD105 (ab11414), CD29 (ab24693), CD44 (ab51037) and CD34 (ab81289), FITC-labeled exosome-specific markers TSG101 (ab125011), CD63 (ab59479) and CD81 (ab79559), and horseradish peroxidase (HRP)-labeled goat anti-mouse antibodies to SOX4 (ab80261), Wnt1 (ab15251), β-catenin (ab32572), GAPDH (ab8245) and immunoglobulin G (ab6728) were from Abcam Inc. (Cambridge, UK). The primer sequences used for the qPCR were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China), and the primer sequences are exhibited in . A β-catenin specific agonist WAY-262611 was purchased from MedChemExpress Inc. (HY-11035, Monmouth Junction, NJ, USA).
Table 1. Primer sequence for RT-qPCR.
Cell culture and treatment of lung cancer cells
Human lung cancer cell lines National Cancer Institute (NCI)-H1299 (RRID: CVCL_0060), 95 C (RRID: CVCL_7109), NCI-H460 (RRID: CVCL_0459), HCC827 (RRID: CVCL_2063) and immortalized human bronchial epithelial cells BEAS-2B (RRID: CVCL0168) were from ATCC (Manassas, VA, USA). All cell lines were maintained at 37 °C with 5% CO2 in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Subsequently, H1299 and H460 cells in good growth conditions were seeded at 5 × 104 cells/well in a six-well plate, followed by overnight incubation. Subsequently, RPMI-1640 medium containing HUCMSCs-derived exosomes at a final concentration of 20 μM was added. After 12 h, the medium was removed, and the cells were washed twice with phosphate buffered saline (PBS) and collected for subsequent experiments. For exosome uptake, nuclei staining was conducted with 4′,6-diamidino-2-phenylindole for 5 min. A fluorescence microscope (Olympus Optical Co., Ltd., Tokyo, Japan) was utilized to observe the uptake of exosomes in lung cancer cells. For cell transfection, we transfected SOX4-containing overexpression vectors into H1299 and H460 cells using Lipofectamine 2000 transfection reagent and subsequently verified the transfection efficiency using RT-qPCR.
Culture and identification of HUCMSCs
HUCMSCs were from the Cell Bank of Shanghai Institute of Cells, Chinese Academy of Science (Shanghai, China). HUCMSCs at passage three were obtained and loaded onto a flow cytometer (Beckman Coulter, Inc., Chaska, MN, USA). The expression of the cell surface markers CD105, CD29, CD44, and CD34 was detected. Adipocyte induction medium and osteoblast induction medium (Gibco, Grand Island, NY, USA) were used to induce differentiation of HUCMSCs into adipocytes and osteoblasts, respectively.
Exosome extraction and identification
The HUCMSCs were exposed to Dulbecco’s modified Eagle’s medium containing 10% exosome-free serum (A2720801, Gibco, Carlsbad, CA, USA) for a period of 48 h. The supernatant was harvested for exosome purification using the ExoQuick ULTRA EV isolation kit (SBI, Palo Alto, CA, USA). The characteristics of exosomes were substantiated by transmission electron microscopy (TEM) and particle size by Nanosight analysis (Shanghai Dajiang Biotechnology Co., Ltd., Shanghai, China) as previously described [Citation20]. The expression of exosome-specific markers TSG101, CD63 and CD81 was identified by flow cytometry. miRNA was extracted from exosomes using Trizol reagent (Invitrogen Inc., Carlsbad, CA, USA) for RT-qPCR analysis or sequencing in Sangon Biotech (Shanghai, China). To identify exosome transport, exosomes were labeled with PKH26 (Sigma-Aldrich Chemical Company, St Louis, MO, USA).
RNA isolation and quantification
Total RNA was obtained from HUCMSCs, exosomes and lung cancer cells using Trizol (Invitrogen) to determine their concentration, and the RNA was purified and then reversely transcribed into cDNA. Using the SYBRPCR MasterMix kit (Applied Biosystems, Inc., Carlsbad, CA, USA), miR-320a and mRNA expression of SOX4 was quantified with U6 and β-actin serving as their respective endogenous controls. The primers used in the experiment were designed by the Primer 3Plus website and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Cell Titer-Glo assay
H460 and H1299 cells were plated into the 96-well plate at 1 × 104 cells each well. According to the instructions, the proliferation analysis was performed using CellTiter-Glo reagent (Promega). After the removal of the supernatant, cells were supplemented with 100 μL SmGM-2 Bullet Kit medium containing 20 μL 3-(4,5-dimethylthiazole)-5-(3-carboxyl methoxyphenyl)-2-(4-sulfonyl phenyl). Afterwards, the cells were subjected to another 4 h-incubation with the mixture of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny l)-2H-tetrazolium, inner salt (MTS)/phenazine methyl sulfate. The optical density (OD) values were read on a microplate scanner (BioTek, Winooski, VT, USA) at 490 nm.
5-Ethynyl-2’-deoxyuridine (EdU)
The EdU labeling was performed by a BeyoClick™ EdU cell proliferation kit (C0085L, Beyotime, Shanghai, China) with Alexa Fluor594. The treated or untreated H1299 or H460 cells were rinsed with PBS (Sigma-Aldrich). After the addition of fresh medium, the cells were incubated for 2 h with EdU. Subsequently, cells were washed with PBS to remove the medium and the free EdU probe, fixed for 30 min with paraformaldehyde (Sigma-Aldrich) and stained with direct fluorescent antibody (Sigma-Aldrich). After being washed with PBS, the cells were observed by an inverted microscope (Olympus, Tokyo, Japan).
Colony formation assay
A total of 1 × 103 H1299 and H460 cells were plated into a 6 cm plate. After 2 weeks of culture at 37 °C under 5% CO2, the colonies formed were fixed with methanol for 20 min, treated with 0.1% crystal violet at room temperature (Sigma), counted and imaged.
Caspase-3 activity detection
Caspase-3 activity was measured using a Caspase-3 activity kit (BC3830, Solarbio, Beijing, China) to assess apoptosis. The total protein was extracted from H1299 and H460 cells and seeded into a 96-well petri dish with a 90 μL reaction buffer and a substrate of Caspase-3. After a 4-h incubation at 37 °C, the activity of Caspase-3 was determined by a microplate reader (Tecan, Switzerland) at 405 nm.
Flow cytometry
H460 and H1299 cells were seeded into a 6-well plate. After 48 h of culture, the cells were resuspended in 100 ml buffer and ice-bathed with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) for 15 min. Finally, the cells were loaded onto a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The apoptosis rate was then analyzed using the CytExpert software (Beckman-Caulter, Chaska, MN, USA).
Transwell assays
After 48 h of culture, the cells were trypsinized, dispersed in serum‐free RPMI-1640 medium with a density of 3 × 105 cells per mL and plated in the apical chamber with a volume of 200 μL. Then, a total of 600 μL medium containing 20% serum was added into the basolateral chambers. All of these chambers were placed in the incubator for 24 h. With the removal of cells on the upper surface of the membrane by cotton swabs, cells that passed through the membrane were fixed with 4% paraformaldehyde for 10 min and stained by crystal violet for 8 min, followed by the observation under a microscope (Olympus Optical Co., Ltd., Tokyo, Japan).
Luciferase reporter gene assay
TargetScan Human 7.0 (http://www.targetscan.org/) miRNA database was used to predict target genes of miR-320a. The wild-type (WT) (MT) SOX4 3’untranslated region (3’UTR) sequences and mutant binding sites of miR-320a (MT) were amplified by polymerase chain reaction and cloned into pGL3 basic vector (Promega), respectively. The reporter vectors and miR-320a mimic were then co-transfected into 293 cells. The luciferase activity was assessed with a dual-luciferase reporter gene assay system (Promega).
Immunoblotting
The total protein was isolated using radioimmunoprecipitation assay reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA). Protein concentrations were quantified with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). After isolation by polyacrylamide gel electrophoresis, the extracted protein was transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). At room temperature, the membrane was sealed for 1 h with 5% skim milk powder in Tris-buffered saline containing 20 mM Tris, 150 mM NaCl and 0.1% Tween 20 (pH = 7.6) and probed overnight with the primary antibodies at 4 °C overnight. The membranes were subjected to a 1-h incubation with HRP-labeled secondary antibody at room temperature and visualized with an enhanced chemiluminescence system (Pierce). The ImageJ software (National Institutes of Health, Bethesda, MD, USA) was lastly used for protein quantification on the basis of the gray value ratio of target protein to GAPDH.
Tumorigenicity assays in nude mice
Ten 4-week-old athymic male BALB/c nude mice were purchased from the Animal Facility of Model Animal Research Institute of Nanjing University (Nanjing, Jiangsu, China). For xenograft models, the mice were randomly assigned to 2 groups (n = 5). The mice were inoculated subcutaneously with 1 × 107 H1299 cells in the right flank and treated with exosomes daily. The size of tumor was measured every 7 days. After 28 days of inoculation, mice were euthanized, and tumors were isolated and weighed. Tumor volume was calculated as the following formula: (length × width2)/2. Tumors were dissected out for tissue slice or proteins analysis. Immunohistochemistry was performed under the manufacturer’s instructions. Briefly, the slides were incubated with primary antibodies overnight at 4 °C and then incubated with secondary antibody at room temperature for 2 h. The expression was evaluated using a score obtained by multiplying the values of staining intensities.
Statistics
All calculations were carried out using GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). All data are displayed as the form of mean ± standard error of the mean (SEM). The differences among groups were compared by two-way analysis of variance (ANOVA) along with Tukey’s post hoc test or a student t-test, as appropriate. p < 0.05 was defined as indicating statistical significance.
Results
HUCMSCs and their exosomes are successfully isolated
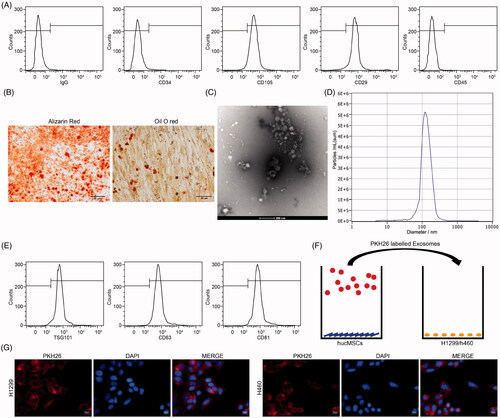
As shown in , the surface marker antigens of purified cells were determined by flow cytometric analysis. The results illustrated that the cells harbored typical MSCs markers: positive for CD29 and CD105, whereas negative for CD34 or CD45, indicating that the cells used were HUCMSCs. Then, we investigated the properties of HUCMSCs by inducing osteogenic and adipogenic differentiation. We observed the presence of calcium deposits with alizarin-red staining and intracytoplasmic lipid droplets with Oil-red O staining (), indicating the potentials for adipogenic and osteogenic differentiation of the acquired HUCMSCs. TEM revealed that the exosomes obtained from HUCMSCs were oval-shaped membrane vesicles (). Nanosight analysis demonstrated a size distribution between 0 and 200 nm in diameter (). Furthermore, these harvested particles were identified by flow cytometry, which showed positive results for the exosome surface marker proteins TSG101, CD81 and CD63 (). The above analysis identified these collected particles as exosomes derived from HUCMSCs. Subsequently, we treated H1299 cells and H460 cells with exosomes (Exo). Fluorescence microscopy further confirmed that PKH26-labeled exosomes had been internalized and transferred to H1299 cells and H460 cells (), and was mainly distributed in the cytoplasm ().
Figure 1. Identification of HUCMSCs and their derived Exo. (A) HUCMSC surface markers CD34, CD45, CD29 and CD105 detected by flow cytometry; (B) osteogenic and adipogenic differentiation of HUCMSCs by alizarin red staining and Oil red O staining; (C) the morphology of HUCMSCs-derived Exo observed under TEM; (D) particle size distribution of HUCMSCs-Exo determined by Nanosight analysis; (E) exosomal surface markers TSG101, CD63 and CD81 detected by flow cytometry; (F) H1299 cells and H460 cells co-cultured with extracted exosomes; (G) fluorescence staining of internalization of PKH26-labeled exosomes by H1299 and H460 cells. Each assessment was done in triplicate with 3-time repetition to ensure minimum deviation.
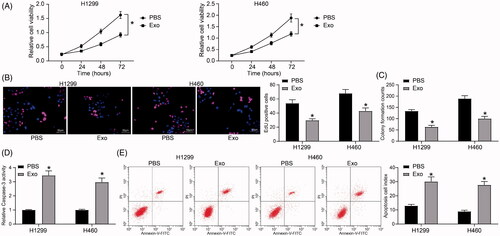
HUCMSCs-derived exosomes inhibit proliferation of lung cancer cells
Firstly, the changes in cell viability of H1299 and H460 cells after exosome treatment were examined using the Cell Titer-Glo kit. The results showed that exosome treatment significantly inhibited the viability of lung cancer cells (). We subsequently demonstrated through EdU staining and colony formation assays that exosomes also inhibited proliferation of lung cancer cells (). A Caspase-3 activity kit was then used to detect apoptosis levels in lung cancer cells. We found that after exosome treatment, the activity of Caspase-3 increased significantly in lung cancer cells (), and the identical results were also monitored after flow cytometry ().
Figure 2. HUCMSCs-derived exosomes inhibit proliferation of lung cancer cells. (A) H1299 and H460 cell viability evaluated by a Cell Titer-Glo assay; (B) cell proliferation evaluated by EdU assays; (C) colony formation of H1299 and H460 cell proliferation; (D) Caspase-3 activity in cells examined by a Caspase-3 kit; (E) detection of apoptosis levels examined by flow cytometry. All experiments were repeated three times. All data are displayed as the mean ± SEM. The differences among groups were compared by a two-way ANOVA followed by Tukey’s post hoc test. *p < 0.05.
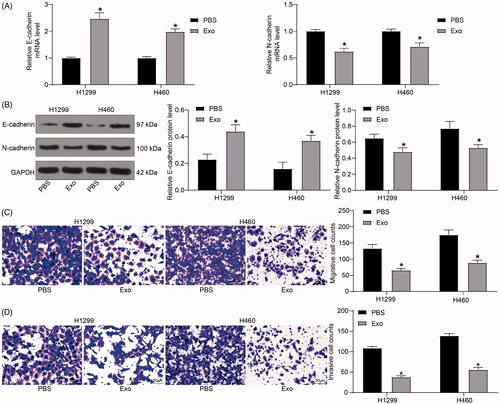
Lung cancer cell aggressiveness is inhibited by exosomes derived from HUCMSCs
Subsequently, the expression of E-cadherin and N-cadherin was tested by RT-qPCR and immunoblotting. After exosome treatment, the expression of E-cadherin in H1299 and H460 cells was notably increased, while N-cadherin expression was significantly reduced (). Moreover, we demonstrated by Transwell assays that the number of migrated and invaded H1299 cells and H460 cells was diminished after exosome treatment ().
Figure 3. HUCMSCs-derived exosomes inhibit lung cancer cell aggressiveness. (A) the mRNA expression of E-cadherin and N-cadherin in H1299 and H460 cells assayed by RT-qPCR; (B) the protein expression of E-cadherin and N-cadherin in H1299 and H460 cells determined by immunoblotting; (C) the migration of H1299 and H460 cells evaluated by Transwell assays; (D) the invasion of H1299 and H460 cells evaluated by Transwell assays. Each assessment was done in triplicate with 3-time repetition to ensure minimum deviation. All data are displayed as the mean ± SEM. The differences among groups were analyzed by a two-way ANOVA followed by Tukey’s post hoc test. *p < 0.05.
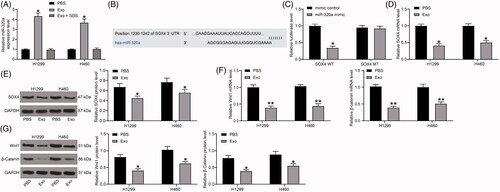
HUCMSC-derived exosomes increase miR-320a and inhibit SOX4 expression in lung cancer cells
We then assayed the miR-320a expression in H1299 and H460 cells after exosome co-culturing, which demonstrated that miR-320a was significantly promoted in lung cancer cells under the co-culture system. Furthermore, to further determine that lung cancer cells can take up miR-320a through exosomes, we treated exosomes with sodium dodecyl sulfate (SDS), thereby disrupting the membrane structure of exosomes, and subsequently co-cultured them with H1299 and H460 cells. We found that the expression of miR-320a in lung cancer cells co-cultured with exosomes treated with SDS did not change significantly, which can indicate that miR-320a can be delivered to lung cancer cells through HUCMSCs-derived exosomes (). After that, we used the TargetScan human 7.0 (http://www.targetscan.org/) miRNA database to predict target genes for miR-320a, and we predicted that miR-320a targeted the 3’UTR of SOX4 (). In addition, we demonstrated by dual-luciferase assays that the relative luciferase activity of 293 cells transfected with miR-320a and SOX4-WT sequences was significantly reduced, confirming that miR-320a could target SOX4 (). Therefore, we hypothesized that exosomes inhibited SOX4 expression by carrying miR-320a. Thus, we examined expression of SOX4 in H1299 and H460 cells by RT-qPCR analysis and immunoblotting, and found that exosome treatment significantly inhibited SOX4 expression (). To further determine the downstream molecular mechanism, we detected the Wnt1 and β-catenin expression in H1299 cells and H460 cells by RT-qPCR and immunoblotting. After exosome treatment, Wnt1 and β-catenin in cells were significantly inhibited ().
Figure 4. HUCMSCs-derived exosomes increase miR-320a expression in lung cancer cells, while inhibit SOX4 expression. (A) miR-320a expression in H1299 and H460 cells examined by RT-qPCR; (B) the binding sites between miR-320a and SOX4 predicted by the online website Target Scan Human 7.0 (http://www.targetscan.org/) miRNA database; (C) the luciferase activity of SOX4-WT and SOX4-MUT after transfection with mimic control and miR-320a mimic evaluated by dual-luciferase reporter gene assay; (D) the mRNA expression of SOX4 in H1299 and H460 cells examined by RT-qPCR; (E) the protein expression of SOX4 in H1299 and H460 cells examined by immunoblotting; (F) the mRNA expression of Wnt1 and β-catenin in H1299 cells and H460 cells detected by RT-qPCR; (G) the protein expression of Wnt1 and β-catenin in H1299 cells and H460 cells detected by immunoblotting. All experiments were repeated three times. All data are displayed as the mean ± SEM. The differences among groups were analyzed by a two-way ANOVA followed by Tukey’s post hoc test. *p < 0.05, **p < 0.01.
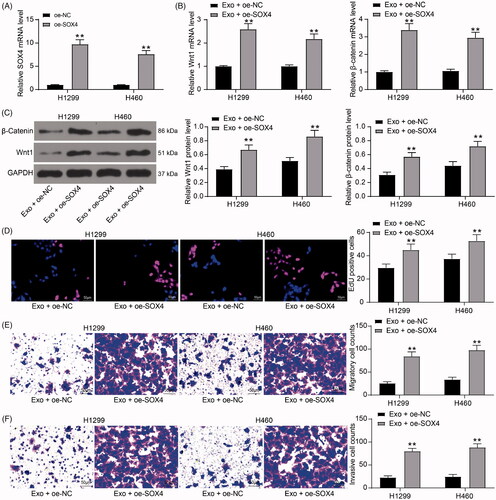
Inhibition of lung cancer cell activity by exosomes is diminished after overexpression of SOX4
To verify the effect of SOX4 on the growth of lung cancer cells, SOX4 overexpression plasmids () were transfected into H1299 and H460 cells, which were subsequently treated with HUCMSCs. We found that the activation level of Wnt/β-catenin signaling pathway was significantly increased in lung cancer cells after overexpression of SOX4 (). The results of EdU assays showed that overexpression of SOX4 significantly attenuated the inhibitory effect of HUCMSCs-exosome on lung cancer cell proliferation (). In the results of Transwell assay, we observed that the increased expression of SOX4 in cells could significantly restore the migration and invasion ability of lung cancer cells in vitro ().
Figure 5. Inhibition of lung cancer cell activity by exosomes is diminished after overexpression of SOX4. (A) SOX4 mRNA expression in H1299 and H460 cells transfected with an overexpression plasmid of SOX4 by RT-qPCR; (B) the mRNA expression of Wnt1 and β-catenin in H1299 cells and H460 cells detected by RT-qPCR; (C) the protein expression of Wnt1 and β-catenin in H1299 cells and H460 cells detected by immunoblotting; (D) cell proliferation evaluated by EdU assays; (E) the migration of H1299 and H460 cells evaluated by Transwell assays; (F) the invasion of H1299 and H460 cells evaluated by Transwell assays. Each assessment was done in triplicate with 3-time repetition to ensure minimum deviation. All data are displayed as the mean ± SEM. The differences among groups were analyzed by a two-way ANOVA followed by Tukey’s post hoc test. *p < 0.01.
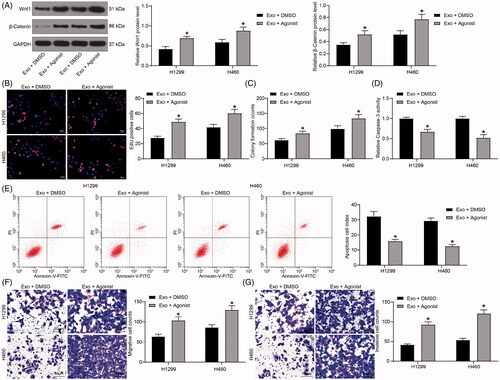
The β-catenin-specific agonist weakens the effects of exosomes on lung cancer cells
Finally, H1299 and H460 cells under the co-culture system were treated with the β-catenin-specific agonist WAY-262611. We then assessed the expression of Wnt1 and β-catenin using immunoblotting and observed that their expression enhanced significantly after the WAY-262611 treatment (). The results of EdU and colony formation assays showed that the proliferation of cells was increased significantly after WAY-262611 treatment (). Flow cytometry and Caspase-3 kit determination showed that apoptosis levels and Caspase-3 activity were decreased significantly () following WAY-262611 delivery. Finally, the Transwell assays revealed that the number of cell migration and invasion increased significantly after activation of β-catenin ().
Figure 6. The β-catenin-specific agonist weakens the effects of exosomes on lung cancer cells. (A) the Wnt1 and β-catenin protein levels in H1299 and H460 cells immunoblotting; (B) cell proliferation evaluated by EdU assays; (C) colony formation of H1299 and H460 cell proliferation; (D) Caspase-3 activity in cells examined by a Caspase-3 kit; (E) detection of apoptosis levels examined by flow cytometry; (F) the migration of H1299 and H460 cells evaluated by Transwell assays; (G) the invasion of H1299 and H460 cells evaluated by Transwell assays. All experiments were repeated three times. All data are expressed as the mean ± SEM. The differences among groups were analyzed by a two-way ANOVA followed by Tukey’s post hoc test. *p < 0.05.
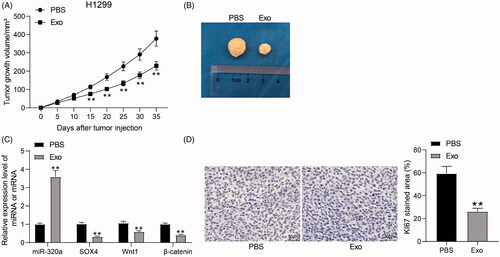
HUCMSCs-derived exosomes inhibit the growth of H1299 cells in vivo
To further investigate the effect of HUCMSCs on lung cancer cell growth in in vivo experiments, we used H1299 cells to generate a tumor-bearing animal model in nude mice and treated mice with HUCMSCs-derived exosomes. After treatment with exosomes, the growth rate of tumors formed by H1299 in mice was significantly reduced (). RT-qPCR exhibited that the expression of miR-320a was elevated, while the mRNA expression of SOX4, Wnt1, and β-catenin was significantly reduced in the tumors (). Moreover, we found that the staining intensity of KI67 in tumors was also significantly reduced after HUCMSCs-exosome treatment (). The above results suggest that HUCMSCs-exosome can inhibit the growth of lung cancer cells in vivo.
Figure 7. HUCMSCs-derived exosomes inhibit the growth of H1299 cells in vivo. (A) growth rate of tumors formed by H1299 cells in vivo; (B) representative images of tumors; (C) miR-320a expression and mRNA expression of SOX4, Wnt1 and β-catenin in xenograft tumors by RT-qPCR; (D) the staining intensity of KI67 in xenograft tumors examined by immunohistochemical staining. All data are expressed as the mean ± SEM. The differences among groups were analyzed by a two-way ANOVA followed by Tukey’s post hoc test or unpaired t test (n = 5). **p < 0.01.
Discussion
Despite that exosomes have been believed as possible candidates in modulating the cell-cell communication during cancer metastasis and the progression of other diseases, its role has not been thoroughly investigated in the lung [Citation21]. Therefore, identification of the molecular biomarkers transmitted by exosomes during metastasis remains a huge challenge for lung cancer treatment. In this investigation, the data disclosed that HUCMSCs-derived exosomes could lower cell viability, migration and invasion, yet accelerate apoptosis in lung cancer. In addition, HUCMSCs could deliver miR-320a to lung cancer cells via exosomes. Moreover, SOX4 was a putative target of miR-320a. HUCMSCs-derived exosomes containing miR-320a revert the malignant phenotype in lung cancer cells through the Wnt/β-catenin pathway by targeting SOX4, indicating that the miR-320a/SOX4/Wnt/β-catenin axis involves in the lung cancer progression and metastasis.
HUCMSCs have been observed to exert anti-tumor functions in various primary malignancies and metastases [Citation22]. Moreover, HUCMSCs-secreted extracellular matrix prohibits the proliferation capacities of metastatic breast cancer cells [Citation23]. Cells are capable of secreting three types of extracellular vesicles: exosomes (30-150 nm), microvesicles and apoptotic bodies in accordance with their release mechanisms and sizes [Citation24]. Similar to cells, exosomes comprise a plethora of molecules, involving lipids, proteins as well as nucleic acids (mRNA and non-coding RNA) which could be transmitted to recipient cells [Citation25]. Importantly, Kahlert et al. [Citation26] highlighted the significance of exosomes in cancers through their participations in tumor microenvironment, angiogenesis as well as metastasis. Metastasis is an intricate process of tumor cell invasion, survival in blood vessels and colonization of the host organs, and exosomes function as regulatory players in cell-cell communication, manipulating various steps of the metastatic process [Citation27]. Exosomes derived from MSCs at 100 μg/mL significantly promoted the expression of E-cadherin versus the control group in the repair of injured endometrium [Citation28]. Of note, Manterola et al. [Citation29] observed the downregulation of miR-320a in exosomes of 25 patients with glioblastoma multiforme relative to healthy controls. Besides, the tumor-suppressor role of miR-320a has been underscored in chronic myeloid leukemia [Citation30], nasopharyngeal carcinoma [Citation31], colorectal cancer [Citation32], along with hepatocellular carcinoma [Citation33]. More importantly, miR‑320a was also reduced in non‑small cell lung cancer [Citation34] and connected to p100, a metastasis activator in lung cancer [Citation35]. Consequently, we consider that exosomes containing miR-320a isolated from HUCMSCs may play a carcinostatic role in lung cancer by curtailing cancer cell growth and metastasis.
The decline of miR-320a in exosomes occurred concomitant with the promotion of SOX4 in the present study. Bioinformatics analysis displayed that the SOX4 3’UTR harbors potential binding sites for miR-320a, and luciferase reporter assays validated that SOX4 is a possible target of miR-320a. SOX4 is found to be necessary for the process of epithelial-mesenchymal transition, migration and metastatic spread [Citation15]. In addition, miR-129-5p repressed cell proliferation, migration and induced apoptosis by targeting SOX4 and modulating the Wnt/β-catenin pathway in chondrosarcoma [Citation36]. Meanwhile, miR-320a downregulation prohibited liver cancer cell from proliferation via the Wnt/β‑catenin pathway [Citation37]. Glioma cells co-cultured with exosomes carrying miR-133b mimic demonstrated reduced protein expression of Wnt1 and β-catenin, while the opposite trends were observed in cells treated with exosomes containing miR-133b inhibitor [Citation38], which was consistent with the obtained data from our research. Accordingly, lung cancer cells co-cultured with exosomes were further introduced with the β-catenin-specific agonist WAY-262611, and the repressive role of exosomes on lung cancer progression was reversed. In line with our observations, the Wnt/β-catenin pathway inhibitor IWP-2 was found to halt cell proliferation and potentiate cell apoptosis, and could counteract the stimulative role of SOX9 in lung cancer cell proliferation [Citation39], implying the possible interaction between SOX4 and the Wnt/β-catenin pathway in lung cancer.
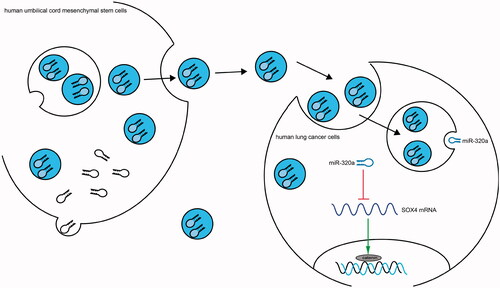
The findings of this study illustrated that treatment with HUCMSCs-derived exosomes hindered the progression of lung cancer by transferring miR-320a to inhibit migration, invasion and viability, and to stimulate apoptosis of lung cancer cells. We also found that SOX4 was a candidate target of miR-320a. Furthermore, the Wnt/β‑catenin pathway was engaged in the development and metastasis of lung cancer cells (). Previously, Besse et al. [Citation40] performed a phase II clinical trial evaluating the clinical benefit of exosomes derived from dendritic cells as maintenance immunotherapy in non-small cell lung cancer and found that the median progression-free survival was 2.2 months and median overall survival was 15 months, with 32% of patients had stable disease beyond 4 months. Therefore, we believe that exosomes may be an alternative regimen for lung cancer therapy.
Figure 8. Schematic diagram summarizes the miR‐320a-containing exosomes/SOX4/Wnt/β-catenin axis and its functions in lung cancer. HUCMSCs-derived exosomes block the Wnt/β-catenin pathway and therefore inhibit cell growth and aggressiveness by transferring miR-320a to inhibit SOX4 expression in lung cancer.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
All the data generated or analyzed during this study are included in this published article.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Lai XN, Li J, Tang LB, et al. MiRNAs and LncRNAs: dual roles in TGF-beta signaling-regulated metastasis in lung cancer. Int J Mol Sci. 2020;21:1193.
- Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84.
- Lin H, Shangguan Z, Zhu M, et al. lncRNA FLVCR1-AS1 silencing inhibits lung cancer cell proliferation, migration, and invasion by inhibiting the activity of the Wnt/beta-catenin signaling pathway. J Cell Biochem. 2019;120:10625–10632.
- Amiri A, Pourhanifeh MH, Mirzaei HR, et al. Exosomes and lung cancer: roles in pathophysiology, diagnosis and therapeutic applications. Curr Med Chem. 2021;28:308–328.
- Xia Y, Wei K, Hu LQ, et al. Exosome-mediated transfer of miR-1260b promotes cell invasion through Wnt/beta-catenin signaling pathway in lung adenocarcinoma. J Cell Physiol. 2020;235:6843–6853.
- Dong L, Pu Y, Zhang L, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018;9:218.
- Yuan Y, Zhou C, Chen X, et al. Suppression of tumor cell proliferation and migration by human umbilical cord mesenchymal stem cells: a possible role for apoptosis and Wnt signaling. Oncol Lett. 2018;15:8536–8544.
- Yuan L, Liu Y, Qu Y, et al. Exosomes derived from microRNA-148b-3p-overexpressing human umbilical cord mesenchymal stem cells restrain breast cancer progression. Front Oncol. 2019;9:1076.
- Zhang Z, Li X, Sun W, et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017; 397:33–42.
- Cui Z, Sun Q, Yan W, et al. The role of miR-320a and its target gene GMEB1 in epithelial-mesenchymal transition and invasion of colorectal cancer. J Gene Med. 2021:e3327.
- Ma X, Wang Z, Ren H, et al. Long non-coding RNA GAS5 suppresses tumor progression and enhances the radiosensitivity of prostate cancer through the miR-320a/RAB21 Axis. CMAR. 2020;12:8833–8845.
- Xing B, Qiao XF, Qiu YH, et al. TMPO-AS1 regulates the aggressiveness-associated traits of nasopharyngeal carcinoma cells through sponging miR-320a. Cancer Manag Res. 2021;13:415–425.
- Grimm D, Bauer J, Wise P, et al. The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol. 2020;67:122–153.
- Tiwari N, Tiwari VK, Waldmeier L, et al. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–783.
- Quan X, Li X, Yin Z, et al. p53/miR-30a-5p/SOX4 feedback loop mediates cellular proliferation, apoptosis, and migration of non-small-cell lung cancer. J Cell Physiol. 2019;234:22884–22895.
- Yang J, Lin X, Jiang W, et al. lncRNA LEF1-AS1 promotes malignancy in non-small-cell lung cancer by modulating the miR-489/SOX4 axis. DNA Cell Biol. 2019;38:1013–1021.
- Zhong M, Wang WL, Yu DJ. Long non-coding RNA OR3A4 is associated with poor prognosis of human non-small cell lung cancer and regulates cell proliferation via up-regulating SOX4. Eur Rev Med Pharmacol Sci. 2019;23:6524–6530.
- Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239:56–68.
- Ying W, Riopel M, Bandyopadhyay G, et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171:372–384.e12.
- Lee H, Zhang D, Zhu Z, et al. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep. 2016;6:35250.
- Li T, Zhang C, Ding Y, et al. Umbilical cord-derived mesenchymal stem cells promote proliferation and migration in MCF-7 and MDA-MB-231 breast cancer cells through activation of the ERK pathway. Oncol Rep. 2015;34:1469–1477.
- Sun B, Yu KR, Bhandari DR, et al. Human umbilical cord blood mesenchymal stem cell-derived extracellular matrix prohibits metastatic cancer cell MDA-MB-231 proliferation. Cancer Lett. 2010;296:178–185.
- Guiot J, Struman I, Louis E, et al. Exosomal miRNAs in lung diseases: from biologic function to therapeutic targets. J Clin Med. 2019;8:1345.
- Fortunato O, Gasparini P, Boeri M, et al. Exo-miRNAs as a new tool for liquid biopsy in lung cancer. Cancers. 2019;11:888.
- Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med. 2013;91:431–437.
- Chen R, Xu X, Qian Z, et al. The biological functions and clinical applications of exosomes in lung cancer. Cell Mol Life Sci. 2019;76:4613–4633.
- Yao Y, Chen R, Wang G, et al. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-beta1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res Ther. 2019;10:225.
- Manterola L, Guruceaga E, Gallego Perez-LarCya J, et al. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro Oncol. 2014;16:520–527.
- Xishan Z, Ziying L, Jing D, et al. MicroRNA-320a acts as a tumor suppressor by targeting BCR/ABL oncogene in chronic myeloid leukemia. Sci Rep. 2015;5:12460.
- Qi X, Li J, Zhou C, et al. MicroRNA-320a inhibits cell proliferation, migration and invasion by targeting BMI-1 in nasopharyngeal carcinoma. FEBS Lett. 2014;588:3732–3738.
- Zhang Y, He X, Liu Y, et al. microRNA-320a inhibits tumor invasion by targeting neuropilin 1 and is associated with liver metastasis in colorectal cancer. Oncol Rep. 2012;27:685–694.
- Xie F, Yuan Y, Xie L, et al. miRNA-320a inhibits tumor proliferation and invasion by targeting c-Myc in human hepatocellular carcinoma. OTT. 2017;10:885–894.
- Wang J, Shi C, Wang J, et al. MicroRNA-320a is downregulated in non-small cell lung cancer and suppresses tumor cell growth and invasion by directly targeting insulin-like growth factor 1 receptor. Oncol Lett. 2017;13:3247–3252.
- Xing A, Pan L, Gao J. p100 functions as a metastasis activator and is targeted by tumor suppressing microRNA-320a in lung cancer. Thorac Cancer. 2018;9:152–158.
- Zhang P, Li J, Song Y, et al. MiR-129-5p inhibits proliferation and invasion of chondrosarcoma cells by regulating SOX4/Wnt/beta-catenin signaling pathway. Cell Physiol Biochem. 2017;42:242–253.
- Lu C, Liao Z, Cai M, et al. MicroRNA-320a downregulation mediates human liver cancer cell proliferation through the Wnt/beta-catenin signaling pathway. Oncol Lett. 2017;13:573–578.
- Xu H, Zhao G, Zhang Y, et al. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/beta-catenin signaling pathway by targeting EZH2. Stem Cell Res Ther. 2019;10:381.
- Guo YZ, Xie XL, Fu J, et al. SOX9 regulated proliferation and apoptosis of human lung carcinoma cells by the Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:4898–4907.
- Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5:e1071008.
