Abstract
Apolipoprotein M (apoM), an apolipoprotein predominantly associated with high-density lipoprotein (HDL), is considered a mediator of the numerous roles of HDL, including reverse cholesterol transport, anti-atherosclerotic, anti-inflammatory and anti-oxidant, and mediates pre-β-HDL formation. ApoM expression is known to be regulated by a variety of in vivo and in vitro factors. The transcription factors farnesoid X receptor, small heterodimer partner, liver receptor homolog-1, and liver X receptor comprise the signaling cascade network that regulates the expression and secretion of apoM. Moreover, hepatocyte nuclear factor-1α and c-Jun/JunB have been demonstrated to exert opposing regulatory effects on apoM through competitive binding to the same sites in the proximal region of the apoM gene. Furthermore, as a carrier and modulator of sphingosine 1-phosphate (S1P), apoM binds to S1P within its hydrophobic-binding pocket. The apoM/S1P axis has been discovered to play a crucial role in the apoM signaling pathway through its ability to regulate glucose and lipid metabolism, vascular barrier homeostasis, inflammatory response and other pathological and physiological processes. Using the findings of previous studies, the present review aimed to summarize the regulation of apoM expression by various factors and its role in different physiological and pathological conditions, and provide a new perspective for the further treatment of these diseases.
Introduction
Apolipoprotein M (apoM) is a member of the lipocalin superfamily, which was initially discovered to be present in triglyceride-rich lipoproteins by Xu et al. [Citation1] in 1999. ApoM is predominantly present in high-density lipoprotein (HDL), with a small proportion present in low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL). The human apoM gene is located on chromosome 6 in the major histocompatibility complex class III region, which is rich in genes involved in immunity and inflammation, and encodes a protein with a molecular weight of ∼26 kDa [Citation1]. Owing to the lack of a signal peptidase cleavage site, the mature apoM protein retains its hydrophobic signal peptide, which is anchored to the phospholipid monomolecular layer of HDL and prevents apoM from being filtered through the glomerulus. ApoM secreted by tubular epithelial cells is reabsorbed in a megalin-dependent manner; therefore, it is not detected in urine under physiological conditions [Citation2–4].
Sphingosine 1-phosphate (S1P) is a bioactive sphingolipid that acts as a secondary messenger through five G protein-coupled receptors [S1P receptor (S1PR1–5)]. S1P can bind to the hydrophobic-binding pocket of the apoM molecule, and together, apoM, S1P, and HDL regulate multiple biological functions [Citation5,Citation6]. For example, apoM delivers S1P to S1P receptors in endothelial cells, which activates the phosphorylated AKT signaling, and subsequently protecting endothelial cells (ECs) and maintaining the integrity of the vascular endothelial barrier [Citation7,Citation8].
Given the location of the apoM gene, it is unsurprising that single nucleotide polymorphisms in the apoM gene are associated with numerous diseases, including type 2 diabetes mellitus (T2DM) [Citation9–12], chronic obstructive pulmonary disease [Citation13], and systemic lupus erythematosus [Citation14]. For instance, in a previous study, the blood glucose levels in apoM−/− mice were decreased compared with those in wild-type mice, and apoM was suggested to promote insulin secretion and sensitivity [Citation15–17]. ApoM has also been shown to play a role in lipid metabolism; in particular, it was found to be essential for the formation of pre-β-HDL and reverse cholesterol transport [Citation18,Citation19]. ApoA1, the most abundant apolipoprotein in HDL, has been demonstrated to interact with ATP-binding cassette transporter A1 (ABCA1) to play a key role in reverse cholesterol transport. Faber et al. [Citation20] reported that apoM levels in apoA1-deficient mice were decreased compared with those in wild-type mice, which indicated a potential link between apoM and the biological effects of HDL, suggesting that apoM might affect the pathogenesis of several cardiometabolic diseases.
To improve current understanding of the association between apoM and numerous types of disease, and provide evidence to support apoM as a possible therapeutic target, it is vital to study the regulatory mechanisms of the signaling pathways between apoM and different molecules.
Regulation of apoM expression levels
ApoM expression is highly tissue-specific and is primarily localized into the liver and kidney, particularly in hepatocytes and renal tubular epithelial cells, in humans [Citation21]. Plasma apoM is mainly derived from hepatocytes. Recently, several studies have reported the function of apoM expression in and secretion by other types of cells, including adipocytes [Citation22] and porcine brain capillary ECs [Citation23]. As a component of HDL, apoM is essential for the formation and particle size of pre-β-HDL through its regulation of ABCA1 [Citation19] and for the reverse cholesterol transport of excess cholesterol from the peripheral tissues to the liver for secretion, which is considered an important mechanism for the anti-atherosclerotic effect of HDL. In recent years, the regulation of apoM expression and secretion has been extensively studied. Hepatocyte nuclear factor (HNF)-1α, HNF-4α, liver receptor homolog-1 (LRH-1), ABCA1 and apoA1 upregulate apoM expression levels, whereas liver X receptor (LXR), retinoid X receptor (RXR), farnesoid X receptor (FXR) and small heterodimer partner (SHP) downregulate expression levels of apoM. These regulatory factors are presented in .
Table 1. Regulation of apoM expression and function. ↑ indicates an increased apoM expression level, and ↓ indicates a decreased apoM expression level.
HNF-1α and HNF-4α
HNF-1α is an important transcription factor for gene expression in the intestine, liver, and kidney and pancreatic β-cells. Additionally, HNF-1α is indispensable for apoM expression. Compared with HNF-1α+/+ mice, HNF-1α−/− mice had markedly downregulated apoM mRNA and protein levels [Citation42]. Mosialou et al. [Citation43] demonstrated that the phorbol-induced transcriptional repression of apoM during inflammation was the result of competitive binding of Jun and HNF-1 to the same regulatory element in the proximal apoM promoter region. Moreover, Ma et al. [Citation24] reported that propofol, a common clinical intravenous anesthetic, compensated for the suppression of apoM expression in LPS-induced inflammation by upregulating HNF-1α levels, which has an anti-inflammatory role in vitro. Simvastatin, a lipid-lowering agent, was found to upregulate apoM and HNF-1α expression levels and downregulate LXRα expression in HepG2 cells. Furthermore, the increase in apoM expression was suppressed in the presence of the HNF-1α inhibitor (UCDA) or LXRα agonist (TO901317) suggesting that apoM may be involved in the lipid-lowering action of simvastatin by regulating the HNF-1α and LXRα pathways [Citation25]. Mutations in the HNF-1α gene are responsible for development of maturity-onset diabetes in the young (MODY3). However, the results of a previous case-control study concluded that there were no significant differences in the plasma apoM concentration between MODY3 and type 2 diabetes patients; thus, apoM cannot be used as a specific biomarker for MODY3 [Citation44]. It has recently been reported that apoM expression levels are downregulated in cases of hepatitis C virus (HCV) infection in vitro and in vivo and that apoM is indispensable for HCV replication [Citation26]. Therefore, based on previous studies that suggested HCV infection might downregulate the expression levels of HNF-1α and forkhead box A2 (FOXA2), both of which could regulate apoM transcription in the liver [Citation30], we hypothesized that apoM expression might be suppressed through the HNF-1α/FOXA2 signaling pathway in HCV infection, which might provide a novel perspective for research into antiviral therapies.
HNF-4α is an upstream regulator of HNF-1α. Mutations in the human HNF-4α gene cause an autosomal dominant disease, MODY1. HNF-4α binds to the −33/-21 region of the hormone response element in the proximal apoM promoter and positively regulates the expression of apoM [Citation45]. Ding et al. [Citation27] demonstrated that, compared with the liver tissue of young mice, the expression level of regulatory factor HNF-4α in liver tissue of old mice was significantly inhibited and the synthesis of apoM was reduced, resulting in attenuated S1P signaling. Furthermore, they demonstrated that the suppression of HNF-4α expression was associated with reduced sirtuin-1 level in the livers of aged mice. In summary, activation of the sirtuin-1/HNF-4α/apoM/S1PR1 pathway promoted regeneration and anti-fibrosis in injured lungs and kidneys. Targeting the apoM signaling pathway in the liver to promote regeneration of distant organs is therefore a potential therapy for age-related fibrosis.
LXR
LXR is a member of the nuclear receptor superfamily, and it is implicated in lipid metabolism and inflammation. Activated LXR forms heterodimers with RXR to regulate lipid homeostasis. Zhang et al. [Citation46] revealed that hepatic apoM expression levels were significantly downregulated by the synthetic LXR agonist, TO901317, in HepG2 cells. Moreover, synergistic effect was achieved with the combination of 9-cis retinoic acid and TO901317 via the LXR/RXR signaling pathway. ABCA1 is critical for the binding of apoA1 to free cholesterol to form pre-β-HDL. TO901317 has been found to activate ABCA1 to promote reverse cholesterol transport to HDL. In addition, dihydrocapsaicin, a capsaicinoid associated with cardiovascular disease, could suppress FOXA2 and promote LXRα expression to downregulate apoM expression in HepG2 cells [Citation47]. Interestingly, Di et al. [Citation37] demonstrated that ABCA1 upregulated apoM expression via the RXR/LXR signaling pathway in HepG2 cells, whereas LRH-1 was not involved. Calayir et al. [Citation28] also reported that the LXR agonist could upregulate apoM expression levels in the small intestine, and not in the liver, which was further supported by the findings of another study [Citation29]. Zhu et al. [Citation29] revealed that TO901317 markedly upregulated apoM expression in Caco-2 cells, whereas the FXR antagonist guggulsterone eliminated this positive regulatory effect, indicating that TO901317 may upregulate apoM expression via the LXR/FXR signaling pathway in Caco-2 cells. Although the underlying mechanism remains to be clarified, a possible reason for the dual effect on the regulation of apoM levels in the liver and intestine exhibited by TO901317 may be that TO901317 is more potent in activating FXR than its natural ligand and LRH-1 is present in low concentration in the intestine. Niacin, an important nutrient for humans, has been shown to effectively increase HDL-C levels. In vivo and in vitro experimental studies have revealed that niacin upregulated apoM mRNA and protein expression levels in a dose-dependent manner, which was subsequently inhibited by an LXRα inhibitor or short hairpin RNA targeting LXRα [Citation48].
LRH-1/SHP
LRH-1 is an orphan nuclear receptor mainly expressed in tissues of endodermal origin [Citation49], and it is involved in a diverse array of biological effects, including lipid and bile acid metabolism [Citation50,Citation51], inflammation [Citation52], and cell differentiation and proliferation [Citation53,Citation54]. Bile acids are produced by cholesterol in the liver and are regulated via negative feedback. Bile acids first bind to FXR to initiate the transcription of SHP, thereby leading to the suppression of LRH-1 transcription and cholesterol 7a-hydroxylase gene expression, which is a rate-limiting enzyme in bile acid synthesis [Citation50,Citation55]. Venteclef et al. [Citation32] demonstrated that the expression levels of apoM were significantly downregulated (−60%) in HepG2 cells following LRH-1 knockdown. In contrast, the expression levels of apoM were upregulated four-fold in HepG2 cells following the overexpression of LRH-1, which occurred in a dose-dependent manner. LRH-1 is bound to the LRH-1 response element, which is located in the proximal promoter region of apoM, where it can directly promote apoM transcription. This binding was reported to be suppressed by SHP, as both LRH-1and SHP have been observed to bind to a similar regulatory region, which suggests a competitive inhibitory relationship between SHP and LRH-1. Therefore, bile acids are suggested to exert an inhibitory effect on apoM expression [Citation32].
Peroxisome proliferator-activated receptors (PPARs)
PPARs are ligand-activated transcription factors comprising three subtypes: PPARα, PPARγ, and PPARβ/δ. Luo et al. [Citation39] demonstrated that in HepG2 cells, palmitic acid inhibited apoM expression levels, which could not be counteracted by either the PI3K inhibitor (LY294002) or protein kinase C inhibitor (GFX), but reversed by PPARβ/δ antagonist (GSK3787), suggesting that palmitic acid-mediated apoM downregulation occurs via the PPARβ/δ pathway. Furthermore, they showed that rosiglitazone, a PPARγ activator, mediated the increased expression of hepatic apoM and downregulation of ABCA1 in rats [Citation34]. This result is apparently at odds with the notion that ABCA1 upregulates apoM expression levels. Presumably, rosiglitazone upregulated apoM by directly binding to the gene promoter region of apoM rather than via the ABCA1 pathway. By overexpressing and knocking down the expression of PPARγ in HepG2 cells, Kurano et al. [Citation35] reported that PPARγ-regulated apoM expression levels demonstrated a bell-shaped curve, that is, both overexpression and knockdown of PPARγ downregulated apoM expression levels and only in the presence of mild inhibition increased the expression levels of apoM. The different types of models used (in vivo or in vitro) may account for the controversial conclusions regarding the regulation of apoM by PPARγ in these studies.
Long non-coding RNAs
Long non-coding RNAs (lncRNAs) are a class of transcripts which can exceed 200 nt in length and lack the ability to encode proteins. LncRNAs have been associated with many diseases, including cancer and atherosclerosis. Xue et al. [Citation36] observed that both lncRNA HLA complex group 11 (HCG11) and apoM levels were downregulated in laryngeal carcinoma tissues and cells. Further cell experiments verified that HCG11 suppressed laryngeal carcinoma cell proliferation and promoted cell apoptosis by sponging miR-4469 and upregulating apoM expression. Utilizing animal and cell models with lncRNA taurine up-regulated gene 1 (TUG1) gain- and loss-of-function, Yang et al. [Citation40] demonstrated a promoting effect of TUG1 on the progression of atherosclerosis. Subsequently, this mechanism was clarified, indicating that TUG1 could competitively inhibit the binding of FXR1 and miR-92a, thereby promoting FXR1 to further downregulate apoM expression, together with elevated inflammatory cytokines and impeded cholesterol efflux; this resulted in deterioration of atherosclerosis. The mechanism of apoM regulation by lncRNAs in disease requires further in-depth research.
Other regulatory factors
Sramkova et al. [Citation22] were the first to identify apoM expression in adipocytes. Their results revealed that the expression levels of apoM, a novel adipokine, were downregulated in obese individuals compared with those in lean individuals, and calorie restriction in obese individuals subsequently upregulated the expression levels of apoM in adipose tissue. The participants were then provided a high-carbohydrate diet instead of a diet high in monounsaturated fats, and analysis identified that apoM was removed from HDL in individuals with a high-carbohydrate diet. Additionally, the hypercatabolic state of the HDL proteins was suggested to be a potential indicator of cardioprotective properties of HDL [Citation56]. Although apoM is present to a less extent in LDL and VLDL, it is associated with the catabolism of LDL and VLDL. In mice lacking LDL receptors or LDL receptor-related protein 1, apoM expression levels were found to be upregulated, whereas the clearance of apoM-enriched VLDL and LDL from the plasma was decreased, which was inconsistent with the reported anti-atherogenic effects of apoM [Citation57]. Estrogen is an important factor in the regulation of lipid metabolism. Wei et al. [Citation38] determined that estrogen receptor-α could bind to the −1580 to 1575 bp promoter region (-GGTCA-) of the human apoM gene to promote transcriptional activity, resulting in upregulation of apoM expression by estrogen. Furthermore, Ren et al. [Citation41] revealed that TGF-β downregulated apoM expression levels via the transforming growth factor-β-activated kinase 1/JNK/c-Jun signaling pathway in HepG2 cells.
ApoM-mediated signal transduction
Recently, apoM/S1P signaling is an axis of the apoM signaling pathway that has attracted significant attention. Studies have demonstrated that S1P is involved in the regulation of various biological functions. For example, S1P could promote lymphocyte trafficking in the presence of S1PR1 [Citation58], serving an important role in maintenance of vascular integrity and regulation of endothelial barrier function, and its role has been implicates in the process of angiogenesis [Citation59–62]. The primary source of S1P is erythrocytes, followed by platelets and ECs [Citation63]. Notably, apoM has been found to affect the S1P export from erythrocytes [Citation64]. S1P in the plasma is mainly associated with apoM-containing HDL, of which ∼30% binds to albumin [Citation5,Citation65]. One previous study reported that S1P levels were downregulated in apoM-deficient mice compared to those in control mice [Citation5]. As a carrier of S1P, apoM is known to mediate the interaction between S1P and HDL. Therefore, S1P may be a crucial molecule for future apoM-HDL research.
Kobayashi et al. [Citation66] demonstrated that in high-glucose conditions, apoM polymerizes and demonstrates a weakened ability to bind to S1P, resulting in a decrease in HDL particles containing S1P and an attenuation in numerous HDL functions. ApoM-containing HDL suppressed lymphopoiesis and lymphocyte proliferation by activating the S1P/S1PR1 signaling in bone marrow lymphocyte progenitors [Citation67]. Endothelial dysfunction, as a manifestation of vascular disease, is associated with vascular permeability. The suppression of circulating apoM in aged mice was found to reduce S1P signaling associated with S1PR1, which is the most abundantly expressed S1PR in ECs, resulting in enhanced vascular leakage and further promoting fibrosis in injured lungs and kidneys [Citation27]. These findings are consistent with the concept that age causes a gradual decrease in the regenerative capacity of tissues and organs. Endothelial permeability was increased in the lungs of apoM-/- mice compared with wild-type mice, which was reversed following the injection of apoM/S1P-enriched plasma or the activation of S1P/S1PR1 signaling [Citation68]. In addition, apoM-deficient mice exhibited increased blood-brain barrier permeability to small molecules and upregulated transcellular transport, which were eliminated by stimulating the S1PR1 signaling pathway [Citation69].
The apoM/S1P/S1PR1 axis plays an important role in protecting the vascular barrier and regulating inflammation. Immune complex (IC) deposition is associated with vascular permeability. In mouse models of IC-induced vascular injury, an S1PR1 agonist (CYM-5542) was reported to increase the resistance to inflammation in response to ICs, which was subsequently mitigated by S1P carried by apoM [Citation60]. A recombinant soluble apoM therapeutic, apoM-Fc, was devised to stabilize apoM-bound S1P to achieve sustained activation of S1PRs by protecting apoM-Fc from degradation, thereby restoring the homeostasis of the endothelium from a pathological state [Citation70]. In addition, an apoM-Fc fusion protein was found to prevent fibrosis, promote regeneration of the kidney following injury, increase EC barrier function and attenuate the inflammatory response [Citation27,Citation60].
Following binding to the ligand-binding pocket of apoM, S1P interacts with S1PRs by lifting the N-terminal S1PR1 cap to activate downstream signaling pathways involved in inflammation, cardiovascular diseases, and glucose and lipid metabolism [Citation71]. The apoM-S1P complex could induce AKT and MAPK phosphorylation via S1PR1 to protect the endothelial barrier [Citation5]. Tumor necrosis factor-α (TNF-α), a pro-inflammatory cytokine, is involved in systemic inflammation and is secreted by a variety of cells, including activated monocyte-macrophages, fat cells, T cells and B cells. S1P signaling through S1PRs activates the serine/threonine kinase AKT and endothelial nitric oxide synthase phosphorylation [Citation72]. Liu et al. [Citation73] demonstrated that apoM delivered S1P to S1PR2, thereby activating the PI3K/AKT signaling pathway and resulting in alleviation of TNF-α-induced injury and inflammation in human umbilical vein endothelial cells (HUVECs). Consistently, the HDL/apoM/S1P complex could prevent apoptosis of HUVES through S1PR1 and/or S1PR3, which depended on the activation of AKT and ERK1/2 [Citation7]. Zheng et al. [Citation8] induced an inflammatory model of HUVECs through stimulation with oxidized LDL (ox-LDL) and demonstrated that S1PR2 expression levels were significantly upregulated in the presence of ox-LDL. By activating the S1PR2/PI3K/AKT signaling pathway and inhibiting nuclear translocation of NF-κB, apoM-S1P inhibited the expression levels of several pro-inflammatory factors, thus alleviating inflammation. Gao et al. [Citation74] discovered that TNF-α could induce the phosphorylation of inhibitor of NF-κB-α and the activation of NF-κB signaling to increase intracellular adhesion molecule-1 and vascular cell adhesion molecule-1 (VCAM-1) expression in HepG2 cells, the effect of which was significantly suppressed by apoM overexpression or markedly enhanced by small interfering RNA targeting apoM. Additionally, similar to HDL-apoM in adults, Gaudio et al. [Citation75] reported that neonatal HDL-apoM delivered S1P to S1PR1 to suppress the phosphorylation of NF-κB and alleviate inflammation induced by TNF-α stimulation.
Frej et al. [Citation76] demonstrated that apoM/S1P complexes move from dense HDL particles to light HDL particles, which are unable to activate AKT signaling and inhibit TNF-α-induced VCAM-1 expression in women with type 1 diabetes mellitus. Yao et al. [Citation16] reported that apoM-deficient mice had increased insulin resistance, reduced levels of AKT phosphorylation, and developed a glucose metabolism disorder, which eventually resulted in diabetes. Consistent with these findings, Kurano et al. [Citation77] demonstrated that apoM/S1P protected against insulin resistance by activating the AKT and AMPK signaling via S1PR1 and/or S1PR3. However, a recent prospective study of a Danish population showed that although plasma apoM concentration was inversely correlated with the risk of T2DM, the causal relationship between apoM levels and T2DM remains unclear [Citation12]. The role of apoM in diabetes and glucose metabolism warrants further investigation.
In addition to inflammation and diabetes, several studies were conducted on the mechanism of apoM in various diseases. Zhu et al. [Citation78] reported that, compared with normal lung tissues, apoM expression levels in non-small cell lung cancer (NSCLC) tissues were significantly higher. By overexpressing apoM in vitro and in vivo, it was demonstrated that apoM/S1P/S1PR1 promoted the proliferation and invasion of NSCLC by activating the PI3K/AKT and ERK1/2 pathways. In contrast, apoM inhibited proliferation in the hepatocellular carcinoma cell line SMMC7721 cells via the vitamin D receptor [Citation79]. ApoM appears to exert different functions by acting specifically in cancer cells. Moreover, apoM could improve the survival rate of LPS-treated mice and have a protective effect on organ injury, which might imply the role of apoM in the treatment of sepsis [Citation80]. In summary, currently, research on apoM-mediated signal transduction has mainly focused on inflammation, diabetes, and cancer. In most cases, apoM relies on the apoM/S1P axis to perform its biological function. With respect to cancer, the role of apoM is highly controversial and the underlying mechanism needs to be further explored.
Conclusion
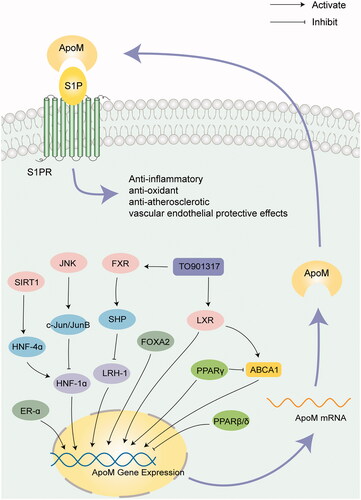
Recently, numerous studies have reported changes in apoM levels in various types of disease and have further investigated the underlying mechanisms; however, to date, the knowledge of apoM regulation and associated signaling pathways remains limited. Certain transcription factors, including HNF-1α, c-Jun/JunB, LRH-1 and SHP, directly bind to the regulatory elements located in the apoM proximal promoter region to regulate apoM expression at the transcriptional level. Structurally, apoM binds to S1P and apoM-S1P complexes subsequently associates with S1PR1–5 and regulates the phosphorylation levels of downstream molecules, which form an amplified cascade leading to the homeostasis of the vascular barrier, and glucose and lipid metabolism. For example, the apoM/S1P/S1PR2/PI3K/AKT signaling pathway was found to play a role in mitigating inflammation and protecting the vascular endothelium. ApoM was reported to improve insulin sensitivity via AKT signaling and protect against insulin resistance, whereas in diabetic patients, the ability to form apoM-S1P-HDL complexes is impaired because of apoM glycosylation and decreased levels of apoM. shows a schematic diagram of the regulation of apoM expression levels and the role of apoM/S1P.
Figure 1. Regulation of the apoM signaling pathway. The regulatory factors regulate the expression levels of apoM directly or indirectly. ApoM binds to S1P and delivers it to S1PRs to serve an anti-inflammatory, anti-atherosclerotic, and vascular endothelial protective role. ApoM: apolipoprotein M; S1P: sphingosine 1-phosphate; JNK: c-Jun N-terminal kinase; HNF: hepatocyte nuclear factor; LRH-1: liver receptor homolog-1; FXR: farnesoid X receptor; SHP: small heterodimer partner; LXR: liver X receptor; ABCA1: ATP-binding cassette transporter A1; PPAR: peroxisome proliferator-activated receptor; ER-α, estrogen receptor-α.
However, despite the controversy surrounding the anti-atherosclerotic effect of apoM, it remains a potential therapeutic target for atherosclerosis and diabetes mellitus. To the best of our knowledge, the roles of the apoM signaling pathway in numerous diseases and under normal physiology have not been completely elucidated. Further studies are required to thoroughly investigate the mechanism of apoM in the pathogenesis of these diseases and determine its potential as a novel therapeutic target.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Xu N, Dahlback B. A novel human apolipoprotein (apoM). J Biol Chem. 1999;274(44):31286–31290.
- Christoffersen C, Ahnstrom J, Axler O, et al. The signal peptide anchors apolipoprotein M in plasma lipoproteins and prevents rapid clearance of apolipoprotein M from plasma. J Biol Chem. 2008;283(27):18765–18772.
- Axler O, Ahnstrom J, Dahlback B. Apolipoprotein M associates to lipoproteins through its retained signal peptide. FEBS Lett. 2008;582(5):826–828.
- Faber K, Hvidberg V, Moestrup SK, et al. Megalin is a receptor for apolipoprotein M, and kidney-specific megalin-deficiency confers urinary excretion of apolipoprotein M. Mol Endocrinol. 2006;20(1):212–218.
- Christoffersen C, Obinata H, Kumaraswamy SB, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A. 2011;108(23):9613–9618.
- Sevvana M, Ahnstrom J, Egerer-Sieber C, et al. Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. J Mol Biol. 2009;393(4):920–936.
- Ruiz M, Okada H, Dahlbäck B. HDL-associated ApoM is anti-apoptotic by delivering sphingosine 1-phosphate to S1P1 & S1P3 receptors on vascular endothelium. Lipids Health Dis. 2017;16(1):36.
- Zheng Z, Zeng Y, Zhu X, et al. ApoM-S1P modulates Ox-LDL-induced inflammation through the PI3K/Akt signaling pathway in HUVECs. Inflammation. 2019;42(2):606–617.
- Liu D, Pan JM, Pei X, et al. Interaction between apolipoprotein M gene single-nucleotide polymorphisms and obesity and its effect on type 2. Sci Rep. 2020;10(1):7859.
- Zhang PH, Gao JL, Pu C, et al. A single-nucleotide polymorphism C-724/del in the proter region of the apolipoprotein M gene is associated with type 2 diabetes mellitus. Lipids Health Dis. 2016;15(1):142.
- Liu M, Frej C, Langefeld CD, et al. Plasma apoM and S1P levels are inversely associated with mortality in African Americans with type 2 diabetes mellitus. J Lipid Res. 2019;60(8):1425–1431.
- Hajny S, Christoffersen M, Dalila N, et al. Apolipoprotein M and risk of type 2 diabetes. J Clin Endocrinol Metab. 2020;105(9):dgaa433.
- Yu Y, Zhang J, Qiao Y, et al. Apolipoprotein M gene single nucleotide polymorphisms discovery in patients with chronic obstructive pulmonary disease and determined by the base-quenched probe technique. Gene. 2017;637:9–13.
- Du W, Shen T, Li H, et al. Low apolipoprotein M serum levels correlate with Systemic lupus erythematosus disease activity and apolipoprotein M gene polymorphisms with Lupus. Lipids Health Dis. 2017;16(1):88.
- Kurano M, Hara M, Tsuneyama K, et al. Induction of insulin secretion by apolipoprotein M, a carrier for sphingosine 1-phosphate. Biochim Biophys Acta. 2014;1841(9):1217–1226.
- Yao S, Zhang J, Zhan Y, et al. Insulin resistance in apolipoprotein M knockout mice is mediated by the protein kinase Akt signaling pathway. Endocr Metab Immune Disord Drug Targets. 2020;20(5):771–780.
- Yu Y, Zhang J, Yao S, et al. Apolipoprotein M overexpression through adeno-associated virus gene transfer improves insulin secretion and insulin sensitivity in Goto-Kakizaki rats. J Diabetes Investig. 2020;11(5):1150–1158.
- Wolfrum C, Poy MN, Stoffel M. Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat Med. 2005;11(4):418–422.
- Mulya A, Seo J, Brown AL, et al. Apolipoprotein M expression increases the size of nascent pre beta HDL formed by ATP binding cassette transporter A1. J Lipid Res. 2010;51(3):514–524.
- Faber K, Axler O, Dahlback B, et al. Characterization of apoM in normal and genetically modified mice. J Lipid Res. 2004;45(7):1272–1278.
- Zhang XY, Dong X, Zheng L, et al. Specific tissue expression and cellular localization of human apolipoprotein M as determined by in situ hybridization. Acta Histochem. 2003;105(1):67–72.
- Sramkova V, Berend S, Siklova M, et al. Apolipoprotein M: a novel adipokine decreasing with obesity and upregulated by calorie restriction. Am J Clin Nutr. 2019;109(6):1499–1510.
- Kober AC, Manavalan APC, Tam-Amersdorfer C, et al. Implications of cerebrovascular ATP-binding cassette transporter G1 (ABCG1) and apolipoprotein M in cholesterol transport at the blood-brain barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(6):573–588.
- Ma X, Hu YW, Zhao ZL, et al. Anti-inflammatory effects of propofol are mediated by apolipoprotein M in a hepatocyte nuclear factor-1alpha-dependent manner. Arch Biochem Biophys. 2013;533(1–2):1–10.
- Yang L, Zhao S. Effect of simvastatin on the expression and regulation mechanism of apolipoprotein M. Int J Mol Med. 2012;29(3):510–514.
- Cai H, Yao W, Huang J, et al. Apolipoprotein M, identified as a novel hepatitis C virus (HCV) particle associated protein, contributes to HCV assembly and interacts with E2 protein. Antiviral Res. 2020;177:104756.
- Ding BS, Yang D, Swendeman SL, et al. Aging suppresses sphingosine-1-phosphate chaperone ApoM in circulation resulting in maladaptive organ repair. Dev Cell. 2020;53(6):677–690 e4.
- Calayir E, Becker TM, Kratzer A, et al. LXR-agonists regulate ApoM expression differentially in liver and intestine. Curr Pharm Biotechnol. 2008;9(6):516–521.
- Zhu C, Di D, Zhang X, et al. TO901317 regulating apolipoprotein M expression mediates via the farnesoid X receptor pathway in Caco-2 cells. Lipids Health Dis. 2011;10(1):199.
- Wolfrum C, Howell JJ, Ndungo E, et al. Foxa2 activity increases plasma high density lipoprotein levels by regulating apolipoprotein M. J Biol Chem. 2008;283(24):16940–16949.
- Ma X, Zhao J, Zhao Z, et al. Propofol attenuates lipopolysaccharide-induced monocyte chemoattractant protein-1 production through enhancing apoM and foxa2 expression in HepG2 cells. Inflammation. 2015;38(3):1329–1336.
- Venteclef N, Haroniti A, Tousaint JJ, et al. Regulation of anti-atherogenic apolipoprotein M gene expression by the orphan nuclear receptor LRH-1. J Biol Chem. 2008;283(7):3694–3701.
- Pan Y, Zhou HG, Zhou H, et al. Apolipoprotein M regulates the orphan nuclear receptor LRH-1 gene expression through binding to its promoter region in HepG2 cells. Drug Des Devel Ther. 2015;9:2375–2382.
- Luo G, Feng Y, Zhang J, et al. Rosiglitazone enhances apolipoprotein M (Apom) expression in rat's liver. Int J Med Sci. 2014;11(10):1015–1021.
- Kurano M, Ikeda H, Iso ON, et al. Regulation of the metabolism of apolipoprotein M and sphingosine 1-phosphate by hepatic PPARgamma activity. Biochem J. 2018;475(12):2009–2024.
- Xue HX, Li HF, Wang T, et al. LncRNA HCG11 suppresses laryngeal carcinoma cells progression via sponging miR-4469/APOM axis. Eur Rev Med Pharmacol Sci. 2020;24(6):3174–3182.
- Di D, Wang Z, Liu Y, et al. ABCA1 upregulating apolipoproein M expression mediates via the RXR/LXR pathway in HepG2 cells. Biochem Biophys Res Commun. 2012;421(1):152–156.
- Wei J, Yu Y, Luo GH, et al. 17beta-estradiol regulates the expression of apolipoprotein M through estrogen receptor alpha-specific binding motif in its promoter. Lipids Health Dis. 2017;16(1):66.
- Luo G, Shi Y, Zhang J, et al. Palmitic acid suppresses apolipoprotein M gene expression via the pathway of PPARbeta/delta in HepG2 cells. Biochem Biophys Res Commun. 2014;445(1):203–207.
- Yang L, Li T. LncRNA TUG1 regulates ApoM to promote atherosclerosis progression through miR-92a/FXR1 axis. J Cell Mol Med. 2020;24(15):8836–8848.
- Ren K, Mo ZC, Liu X, et al. TGF-beta down-regulates apolipoprotein M expression through the TAK-1-JNK-c-Jun pathway in HepG2 cells. Lipids. 2017;52(2):109–117.
- Richter S, Shih DQ, Pearson ER, et al. Regulation of apolipoprotein M gene expression by MODY3 gene hepatocyte nuclear factor-1alpha: haploinsufficiency is associated with reduced serum apolipoprotein M levels. Diabetes. 2003;52(12):2989–2995.
- Mosialou I, Krasagakis K, Kardassis D. Opposite regulation of the human apolipoprotein M gene by hepatocyte nuclear factor 1 and Jun transcription factors. J Biol Chem. 2011;286(19):17259–17269.
- Cervin C, Axler O, Holmkvist J, et al. An investigation of serum concentration of apoM as a potential MODY3 marker using a novel ELISA. J Intern Med. 2010;267(3):316–321.
- Mosialou I, Zannis VI, Kardassis D. Regulation of human apolipoprotein m gene expression by orphan and ligand-dependent nuclear receptors. J Biol Chem. 2010;285(40):30719–30730.
- Zhang X, Zhu Z, Luo G, et al. Liver X receptor agonist downregulates hepatic apoM expression in vivo and in vitro. Biochem Biophys Res Commun. 2008;371(1):114–117.
- Zhao J, Hu Y, Li S, et al. Dihydrocapsaicin down-regulates apoM expression through inhibiting Foxa2 expression and enhancing LXRα expression in HepG2 cells. Lipids Health Dis. 2014;13(1):50.
- Yang L, Li T, Zhao S, et al. Niacin regulates apolipoprotein M expression via liver X receptor-α. Mol Med Rep. 2019 Oct;20(4):3285–3291.
- Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14(5):250–260.
- Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6(3):507–515.
- del Castillo-Olivares A, Campos JA, Pandak WM, et al. The role of alpha1-fetoprotein transcription factor/LRH-1 in bile acid biosynthesis: a known nuclear receptor activator that can act as a suppressor of bile acid biosynthesis. J Biol Chem. 2004;279(16):16813–16821.
- Schwaderer J, Phan TS, Glockner A, et al. Pharmacological LRH-1/Nr5a2 inhibition limits pro-inflammatory cytokine production in macrophages and associated experimental hepatitis. Cell Death Dis. 2020;11(2):154.
- Bianco S, Jangal M, Garneau D, et al. LRH-1 controls proliferation in breast tumor cells by regulating CDKN1A gene expression. Oncogene. 2015;34(34):4509–4518.
- Seitz C, Huang J, Geiselhoringer AL, et al. The orphan nuclear receptor LRH-1/NR5a2 critically regulates T cell functions. Sci Adv. 2019;5(7):eaav9732.
- Boulias K, Katrakili N, Bamberg K, et al. Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. Embo J. 2005;24(14):2624–2633.
- Andraski AB, Singh SA, Lee LH, et al. Effects of replacing dietary monounsaturated fat with carbohydrate on HDL (high-density lipoprotein) protein metabolism and proteome composition in humans. Arterioscler Thromb Vasc Biol. 2019;39(11):2411–2430.
- Christoffersen C, Pedersen TX, Gordts PL, et al. Opposing effects of apolipoprotein m on catabolism of apolipoprotein B-containing lipoproteins and atherosclerosis. Circ Res. 2010;106(10):1624–1634.
- Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316(5822):295–298.
- Galvani S, Sanson M, Blaho VA, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015;8(389):ra79.
- Burg N, Swendeman S, Worgall S, et al. Sphingosine 1-phosphate receptor 1 signaling maintains endothelial cell barrier function and protects against immune complex-induced vascular injury. Arthritis Rheumatol. 2018;70(11):1879–1889.
- Argraves KM, Gazzolo PJ, Groh EM, et al. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem. 2008;283(36):25074–25081.
- Terao R, Honjo M, Totsuka K, et al. The role of sphingosine 1-phosphate receptors on retinal pigment epithelial cells barrier function and angiogenic effects. Prostaglandins Other Lipid Mediat. 2019;145:106365.
- Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21(4):1202–1209.
- Christensen PM, Bosteen MH, Hajny S, et al. Apolipoprotein M mediates sphingosine-1-phosphate efflux from erythrocytes. Sci Rep. 2017;7(1):14983.
- Aoki S, Yatomi Y, Ohta M, et al. Sphingosine 1-phosphate-related metabolism in the blood vessel. J Biochem. 2005;138(1):47–55.
- Kobayashi T, Kurano M, Nanya M, et al. Glycation of HDL polymerizes apolipoprotein M and attenuates its capacity to bind to sphingosine 1-phosphate. J Atheroscler Thromb. 2020;27.
- Blaho VA, Galvani S, Engelbrecht E, et al. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature. 2015;523(7560):342–346.
- Christensen PM, Liu CH, Swendeman SL, et al. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 2016;30(6):2351–2359.
- Mathiesen Janiurek M, Soylu-Kucharz R, Christoffersen C, et al. Apolipoprotein M-bound sphingosine-1-phosphate regulates blood-brain barrier paracellular permeability and transcytosis. Elife. 2019;8:e49405.
- Swendeman SL, Xiong Y, Cantalupo A, et al. An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci Signal. 2017;10(492):eaal2722.
- Jiang Z, Zhang H. Molecular mechanism of S1P binding and activation of the S1P1 receptor. J Chem Inf Model. 2019;59(10):4402–4412.
- Morales-Ruiz M, Lee MJ, Zollner S, et al. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276(22):19672–19677.
- Liu Y, Tie L. Apolipoprotein M and sphingosine-1-phosphate complex alleviates TNF-alpha-induced endothelial cell injury and inflammation through PI3K/AKT signaling pathway. BMC Cardiovasc Disord. 2019;19(1):279.
- Gao JJ, Hu YW, Wang YC, et al. ApoM suppresses TNF-alpha-induced expression of ICAM-1 and VCAM-1 through inhibiting the activity of NF-kappaB. DNA Cell Biol. 2015;34(8):550–556.
- Del Gaudio I, Hendrix S, Christoffersen C, et al. Neonatal HDL counteracts placental vascular inflammation via S1P-S1PR1 axis. Int J Mol Sci. 2020;21(3):789.
- Frej C, Mendez AJ, Ruiz M, et al. A shift in ApoM/S1P between HDL-particles in women with type 1 diabetes mellitus is associated with impaired anti-inflammatory effects of the ApoM/S1P complex. Arterioscler Thromb Vasc Biol. 2017;37(6):1194–1205.
- Kurano M, Tsukamoto K, Shimizu T, et al. Protection against insulin resistance by apolipoprotein M/sphingosine-1-phosphate. Diabetes. 2020;69(5):867–881.
- Zhu Y, Luo G, Jiang B, et al. Apolipoprotein M promotes proliferation and invasion in non-small cell lung cancers via upregulating S1PR1 and activating the ERK1/2 and PI3K/AKT signaling pathways. Biochem Biophys Res Commun. 2018;501(2):520–526.
- Yu M, Pan L, Sang C, et al. Apolipoprotein M could inhibit growth and metastasis of SMMC7721 cells via vitamin D receptor signaling. Cancer Manag Res. 2019;11:3691–3701.
- Kurano M, Tsuneyama K, Morimoto Y, et al. Apolipoprotein M protects lipopolysaccharide-treated mice from death and organ injury. Thromb Haemost. 2018;118(6):1021–1035.
