Abstract
Purpose
Even if migraine is not fatal, it is a common and challenging disease with adverse effects on individuals' lives. The lack of objective diagnostic tools causes delays in diagnosis and treatment initiation. The primary aim of this study is to reveal the diagnostic value of Calcitonin Gene-Related Peptide (CGRP) and Pentraxin-3 (PTX-3) in acute migraine. To this aim, we compared the serum CGRP and PTX-3 levels of migraine patients with acute attacks to those in healthy individuals.
Material and method
A total of 135 individuals (85 patients with migraine attacks with or without aura and 50 healthy controls) participated in the study. Serum CGRP and PTX-3 levels were measured with ELISA analysis. A p value less than 0.05 was considered significant.
Results
Serum CGRP [146.70 (21.52–413.67) vs. 65.90 (3.80–256.60) pg/mL] and PTX-3 levels [12.71 (0.62–33.97) vs. 1.01 (0.06–9.48) ng/mL] were higher in patients with migraine attack than the control group (p < 0.01 and p < 0.01, respectively). ROC analysis showed that the cutoff value for serum CGRP was 121.39 pg/mL (AUC: 0.751, Sen:%61, Spe:%64) whereas the cutoff value for PTX-3 was 4,06 ng/mL (AUC:0.876, Sen:%73, Spe:%76). Serum CGRP levels were positively correlated with pain intensity. Serum CGRP and PTX-3 levels did not differ across gender groups and presence of aura in subgroup analysis.
Conclusion
Patients with acute migraine attacks have higher serum CGRP and PTX-3 levels than controls. Both biomarkers show high potential for the diagnosis of a migraine attack.
Introduction
Migraine continues to be a clinical diagnosis based on the symptoms reported by the patient since it does not have an objective and reliable diagnostic biomarker [Citation1]. A migraine attack, with or without aura, is attributed as one of the most painful conditions a person can experience. For this reason, migraine patients often constitute a cure-seeking group who admit to the emergency departments (EDs) for the relief of pain. The negative reflections of migraine are not limited to headache attacks. Although it is not a fatal disease, its devastating effects on family, social, educational, or business life should not be underestimated [Citation2].
Since ancient times, migraine has been homogeneous enough to be distinguished as a specific syndrome. It was identified based on its unique presentation with features such as unilateralism, vomiting, and visual disturbances even by early physicians [Citation3]. Although thousands of years have passed, its pathophysiology has not been clearly explained [Citation4]. The inability to fully reveal the mechanism makes determining the intervention points difficult. Until the last decades, the widely accepted migraine mechanism has been the ‘vascular theory’ based on arterial dilation [Citation5]. However, studies testing the cause-effect (vasodilation → headache) and effect-cause (headache → vasodilation) relationship of this mechanism led to alternative explanations like neurogenic processes with secondary changes in cerebral perfusion [Citation4,Citation6,Citation7]. The current migraine mechanisms include trigeminal activation, cortical spreading depression, dural perivascular nociceptor activation, and endothelial dysfunction [Citation4,Citation6,Citation8–10]. However, recent reports have revitalized prolonged arterial dilation for migraine pathogenesis [Citation11,Citation12].
One of the most prominent peptides in migraine pathophysiology is Calcitonin Gene-Related Peptide (CGRP). It is a neuropeptide abundant in the nervous system, especially in the perivascular trigeminal sensory afferents and the trigeminal nucleus caudalis [Citation8]. CGRP is a potent vasodilator with a minor role in mast cell degranulation and plasma extravasation [Citation13]. Trigeminal activation, accepted as a common pain pathway in migraine, is one of the critical components of migraine pathophysiology [Citation8,Citation10,Citation14]. The crucial role of CGRP in the mechanism of migraine and its focus on therapeutic targets have been demonstrated by many studies [Citation15–29]. However, its clinical validation as a diagnostic migraine biomarker has not been clarified yet [Citation17–19,Citation24–26,Citation29].
Pentraxin-3 (PTX-3) is an acute-phase protein that differs from C-reactive protein (CRP), another member of the pentraxin family, with its C-terminal and N-terminal domain properties. It is locally released from vascular endothelial cells and macrophages, especially in cases of vascular damage [Citation30,Citation31]. Therefore, it is predominantly studied as a marker of endothelial dysfunction. It can be suggested as a potential biomarker for migraine attacks, considering that several studies show the endothelial dysfunction step in the pathogenesis of migraine [Citation9,Citation32]. Central nervous system (CNS) related studies of PNX-3 are not limited to migraine. It has been evaluated in stroke and traumatic brain injury patients [Citation31,Citation33,Citation34]. Recent reports also show its neuroprotective and neuroregenerative effects [Citation34]. High PTX-3 levels in extra-cranial systems have been demonstrated in renal and coronary vascular and systemic endothelial damage such as sepsis [Citation35–37].
The primary aim of this study is to evaluate the diagnostic value of CGRP and PTX-3 in acute migraine attacks by comparing serum CGRP and PTX-3 levels between migraine patients with acute attacks and healthy controls, men and women, and in terms of the presence of aura. Secondly, it aims to test whether serum CGRP and PTX-3 levels correlate with the duration of migraine headache and severity of pain.
Materials and method
Study design
This study was a prospective case-control study. The Institutional Clinical Ethics Committee approved the study (2017-KAEK-189_2019.01.23_01).
Study participants
The patient group was recruited among the migraine patients admitted to the University ED with acute attacks between January 2020 and June 2021. The patients diagnosed with migraine (with or without aura) by a neurologist at least one year ago and above 18 years old were included in the study. Taking herbal or pharmaceutical drugs before admitting to the ED, not having a definite diagnosis of migraine, intracranial mass, mental retardation, congenital or chromosomal anomaly, other chronic organ diseases, pregnancy, and arterial hypertension were the exclusion criteria. The control group was selected among the healthy volunteers who were the relatives of ED patients with no migraine and hospital staff without any chronic medical conditions like migraine or any history of migraine in the family. Participants in the control group were selected to be similar to those in the patient group regarding gender and age. The written consent forms were obtained from all participants. Demographic data of the patients, migraine subtype, headache severity, and the time between the onset of a migraine attack and blood sampling in minutes were recorded. Blood samples were kept at room temperature for 45–60 min for coagulation and then processed in a centrifuge device at 3000 rpm for 15 min. The serum content was taken into 2 ml Eppendorf tubes to be stored at −80 °C. Serum CGRP and PTX-3 levels were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Bioassay Technology Laboratory, Shanghai, China) according to the manufacturer's instructions. The university research center conducted laboratory analysis blind to clinical information.
Statistical analysis
Data were analyzed using SPSS 25.0 (Statistical Package for the Social Sciences IBM Inc; Chicago, IL, USA). The Kolmogorov-Smirnov test was used to evaluate the normality of the data distributions. Normally distributed data were expressed as mean ± SD, and non-normally distributed data were expressed as median (minimum-maximum). Student t-test or Mann-Whitney U test was used in independent group comparisons depending on data distribution. The Chi-square test was used to compare categorical variables. ROC analysis was performed to reveal the predictive performance of serum biomarker levels. Spearman correlation analysis was used to evaluate the relationship between pain severity and the time elapsed since the onset of pain. A p value <0.05 was considered significant.
Results
A total of 135 participants (85 patients with acute migraine attacks and 50 healthy controls) were included in the study. There was no difference between the patient and the control group regarding age and gender (p = 0.815 and p = 0.859, respectively). Thirty-four (40%) of the patients had migraine with aura (MA), and 51 (60%) had migraine without aura (MO). For the patient group, the severity of pain was measured as 8.76 ± 1.32, while the frequency of pain was 1.23 ± 0.45 days/month. The serum CGRP levels for the patient and control groups were 146.70 pg/mL (21.52–413.67) vs. 65.90 pg/mL (3.80–256.60), and PTX-3 levels were 12.71 ng/mL (0.62–33.97) vs. 1.01 ng/mL (0.06–9.48), respectively. The serum CGRP and PTX-3 levels of the patient group were significantly higher than the control group (p < 0.01 and p < 0.01, respectively). The serum CGRP and PTX-3 levels in subgroup analysis did not differ between MA and MO subgroups (p = 0.467 and p = 0.647, respectively) (). Also, serum CGRP and PTX-3 levels did not differ across gender groups (p = 0.411 and p = 0.661, respectively).
Table 1. Demographic data and biomarker levels of the patient and control group.
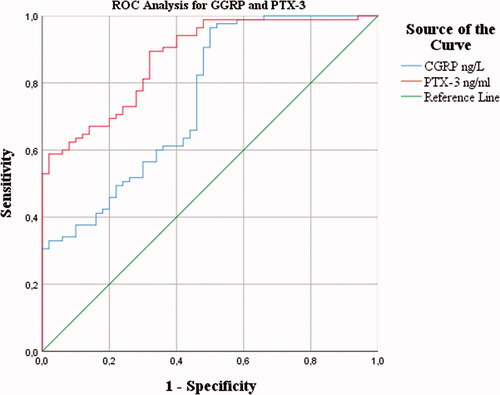
The predictive performance evaluation was evaluated with the ROC analysis for both biomarkers. The ideal cut-off value for CGRP was calculated as 121.39 pg/mL (AUC: 0.751, 95% CI: 0.664-0.837, Sen: 61%, Spe: 64%), while the cut-off value for PTX-3 was 4.06 ng/mL (AUC: 0.876, 95% CI: 0.819-0.933,Sen: 73%, Spe: 76%) ().
Figure 1. ROC analysis of serum CGRP and PTX-3 levels for the diagnosis of migraine with acute attack.
There was a significant correlation between serum CGRP levels and PTX-3 levels (p < 0.01). In addition, serum CGRP and PTX-3 levels were negatively correlated with headache duration (p < 0.01 and p < 0.01, respectively). The serum CGRP level showed a positive relationship with the severity of pain, but a similar relationship was not found with the serum PTX-3 level (p < 0.01 and p = 0.58, respectively) ().
Table 2. Correlation analysis of serum CGRP and PTX-3 levels with the duration of migraine headache and severity of pain.
Discussion
Our results showed that serum CGRP and PTX-3 levels were significantly higher in acute migraine attack patients than in healthy controls. Serum CGRP levels were correlated with PTX-3 levels. Also, serum CGRP levels were correlated with the severity of pain, while PTX-3 levels were not.
The relationship between CGRP and migraine has been studied from many different perspectives through direct and indirect pathways [Citation8–29]. Studies evaluating CGRP levels in migraine patients have reported that it increases both in acute attacks (ictal) and painless periods between attacks (interictal) [Citation15,Citation17,Citation18,Citation21,Citation24–26]. Additionally, higher levels were demonstrated in attack periods. One study testing the hypothesis that lacrimal fluid is a more direct route for peptides secreted from the trigeminal activation pathway found that CGRP levels were much higher than the systemic concentration [Citation19]. Another study showed that CGRP infusion in migraine patients provoked attacks [Citation20]. As it was provoked by other pharmacological agents such as nitroglycerin in migraine patients, higher CGRP levels were found compared to controls. Also, low CGRP was observed in studies where therapeutic agents were administered to patients with migraine attacks [Citation21,Citation22]. Moreover, low CGRP levels have been reported in treated acute migraine attacks and in response to prophylactic treatments in chronic migraine patients [Citation23]. In addition to studies performed on adult patients, Fan et al. showed that CGRP could be used in the pediatric group to distinguish migraine-type headaches from other headache causes and even to determine the response rate to prophylactic treatment [Citation24,Citation25]. Despite many supporting findings, the relationship between CGRP and migraine is not clear yet. For example, one study found no difference between CGRP levels in the external jugular or cubital fossa blood during and outside attacks [Citation38]. Another study evaluating the diagnostic potential of CGRP in chronic migraine reported that it might not be a feasible biomarker [Citation26]. The authors also found higher CGRP levels in the MA group compared to MO, although there were contrary reports [Citation18,Citation20,Citation27]. Our study shows high CGRP levels in migraine patients with acute attacks, which supports the prevailing opinion in the literature. However, we did not find any difference between MA and MO subgroups. We also observed that serum biomarker levels did not differ across gender groups.
Although many studies indicate a positive relationship between migraine and migraine subtypes in various populations, the reports showing CGRP molecule is not a very useful biomarker for chronic migraine should not be ignored [Citation26,Citation28,Citation29]. Lee et al. emphasized that technical factors related to CGRP might affect their contradicting findings [Citation26]. The short half-life of the CGRP molecule, the concentration difference between CNS and the systemic circulation, the changes caused by serum and plasma sampling, and the inconstancy of ELISA kits from different manufacturers might be the variables that should be considered by researchers working in this field. Probably due to the mentioned variability, literature reports show a 10–100-fold differentiation between CGRP levels detected in migraine patients [Citation17,Citation19,Citation26]. The downward trend in concentration detected in sequential blood samples points to a rapid half-life for CGRP, which should also be considered when planning a study based on this biomarker [Citation21]. Another concern might be the sampling source. We used venous blood from the antecubital area for the present study. To obtain serum from the samples, we paid attention not to exceed a one-hour waiting period for coagulation to the centrifuge step. We also tried to lower the bias by recruiting a similar control group to the patient group regarding age and gender. However, the single-center nature of our study hinders its generalizability across populations.
PTX-3 is not a widely studied biomarker as CGRP in migraine patients. The limited number of reports show that it increases in migraine attacks and is higher in migraine patients without attack than in the control group [Citation9,Citation23,Citation32]. Therefore, some authors emphasize that it may be helpful as a diagnostic migraine biomarker [Citation32]. The serum PTX-3 level, an effective diagnostic biomarker according to our findings, has been suggested in another study as a biomarker for determining Onabotulinumtoxin A treatment candidates in chronic migraine [Citation23]. In the same study, the other evaluated biomarker was CGRP, and the authors concluded that CGRP levels could also be used for diagnostic purposes like PTX-3. Ceylan et al. reported that PTX-3 levels were lower in patients with more than five years of migraine disease and an acute attack longer than 12 h than in the control groups. They argued that their findings suggest a temporal change in vascular inflammation during pathological processes of migraine because it represents endothelial dysfunction [Citation32]. However, their conclusion could be interpreted as a severe limitation for a diagnostic biomarker. On the other hand, the correlation between serum PNX-3 level and CGRP in our study can be considered a reliable basis for further questioning its role in the pathogenesis of migraine.
In conclusion, our results show that serum CGRP and PTX-3 levels are higher in patients with migraine attacks than in the control group and can be used as valuable biomarkers to diagnose migraine attacks. In addition, we observed that CGRP and PTX-3 levels did not differ according to migraine type (whether with aura or not) or gender. While serum CGRP level was positively correlated with pain severity, no significant correlation was found for PTX-3. However, further studies are needed to evaluate CGRP and PTX-3 molecules' diagnostic value as biomarkers for migraine attacks.
Ethical approval
The study approval was obtained from the clinical ethics committee of the University Medical Faculty (2017-KAEK-189_2019.01.23_01).
Author contributions
SV made substantial contributions to conception and design. SV and LA supervised data collection and managed the data. SV provided advice on study design. SV and LA analyzed the data. SV and LA take responsibility for the paper as a whole.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia. 2018;38(1):1–211.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990e2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–e1259.
- Pearce JM. Historical aspects of migraine. J Neurol Neurosurg Psychiatry. 1986;49(10):1097–1103.
- Qubty W, Patniyot I. Migraine pathophysiology. Pediatr Neurol. 2020;107:1–6.
- Graham JR, Wolff HG. Mechanism of migraine headache and action of ergotamine tartrate. Arch NeurPsych. 1938;39(4):737e763.
- Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extra-cranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 2013;12(5):454e461–454e461.
- Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28(3):226e236–226e236.
- Khan S, Amin FM, Christensen CE, et al. Meningeal contribution to migraine pain: a magnetic resonance angiography study. Brain. 2019;142(1):93–102.
- Gokdemir MT, Nas C, Gokdemir GS. Pentraxin 3 level in acute migraine attack with aura: patient management in the emergency department. Am J Emerg Med. 2020;38(1):38–42.
- Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36.
- Pellesi L, Al-Karagholi MA, Chaudhry BA, et al. Two-hour infusion of vasoactive intestinal polypeptide induces delayed headache and extracranial vasodilation in healthy volunteers. Cephalalgia. 2020;40(11):1212–1223.
- Pellesi L, Al-Karagholi MA, De Icco R, et al. Effect of vasoactive intestinal polypeptide on development of migraine headaches: a randomized clinical trial. JAMA Netw Open. 2021;4(8):e2118543.
- Theoharides TC, Donelan J, Kandere-Grzybowska K, et al. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev. 2005;49(1):65–76.
- Iyengar S, Johnson KW, Ossipov MH, et al. CGRP and the trigeminal system in migraine. Headache. 2019;59(5):659e681–659e681.
- Ashina M, Bendtsen L, Jensen R, et al. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain. 2000;86(1-2):133–138.
- Ashina H, Schytz HW, Ashina M. CGRP in human models of primary headaches. Cephalalgia. 2018;38(2):353–360.
- Cernuda-Morollón E, Larrosa D, Ramón C, et al. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81(14):1191–1196.
- Latif R, Rafique N, Al Asoom L, et al. Diagnostic accuracy of serum calcitonin gene-related peptide and apolipoprotein E in migraine: a preliminary study. Int J Gen Med. 2021;14:851–856.
- Kamm K, Straube A, Ruscheweyh R. Calcitonin gene-related peptide levels in tear fluid are elevated in migraine patients compared to healthy controls. Cephalalgia. 2019;39(12):1535–1543.
- Hansen JM, Hauge AW, Olesen J, et al. Calcitonin generelated peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–1186.
- Juhasz G, Zsombok T, Modos EA, et al. NO-induced migraine attack: Strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106(3):461–470.
- Juhasz G, Zsombok T, Jakab B, et al. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25(3):179e183–179e183.
- Domínguez C, Vieites-Prado A, Pérez-Mato M, et al. CGRP and PTX3 as predictors of efficacy of onabotulinumtoxin type a in chronic migraine: an observational study. Headache. 2018;58(1):78–87.
- Fan PC, Kuo PH, Chang SH, et al. Plasma calcitonin gene-related peptide in diagnosing and predicting paediatric migraine. Cephalalgia. 2009;29(8):883–890.
- Fan PC, Kuo PH, Lee MT, et al. Plasma calcitonin gene-related peptide: a potential biomarker for diagnosis and therapeutic responses in pediatric migraine. Front Neurol. 2019;10:10.
- Lee MJ, Lee SY, Cho S, et al. Feasibility of serum CGRP measurement as a biomarker of chronic migraine: a critical reappraisal. J Headache Pain. 2018;19(1):53.
- Hansen JM, Ashina M. Calcitonin gene-related peptide and migraine with aura: a systematic review. Cephalalgia. 2014;34(9):695–707.
- Schain AJ, Melo-Carrillo A, Stratton J, et al. CSD-Induced arterial dilatation and plasma protein extravasation are unaffected by fremanezumab: implications for CGRP's role in migraine with aura. J Neurosci. 2019;39(30):6001–6011.
- Pérez-Pereda S, Toriello-Suárez M, Ocejo-Vinyals G, et al. Serum CGRP, VIP, and PACAP usefulness in migraine: a case-control study in chronic migraine patients in real clinical practice. Mol Biol Rep. 2020;47(9):7125–7138.
- Liu S, Qu X, Liu F, et al. Pentraxin 3 as a prognostic biomarker in patients with systemic inflammation or infection. Mediators Inflamm. 2014;2014:421429.
- Ryu WS, Kim CK, Kim BJ, et al. Pentraxin 3: a novel and independent prognostic marker in ischemic stroke. Atherosclerosis. 2012;220(2):581–586.
- Ceylan M, Bayraktutan OF, Becel S, et al. Serum levels of pentraxin-3 and other inflammatory biomarkers in migraine: association with migraine characteristics. Cephalalgia. 2016;36(6):518–525.
- Gullo JdS, Bertotti MM, Silva CCP, et al. Hospital mortality of patients with severe traumatic brain injury is associated with serum PTX3 levels. Neurocrit Care. 2011;14(2):194–199.
- Zhou C, Chen H, Zheng JF, et al. Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice. Neural Regen Res. 2020;15(12):2318–2326.
- Tong M, Carrero JJ, Qureshi AR, et al. Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol. 2007;2(5):889–897.
- Chu Y, Teng J, Feng P, et al. Pentraxin-3 in coronary artery disease: a meta-analysis. Cytokine. 2019;119:197–201.
- Song J, Park DW, Moon S, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the sepsis-3 definitions. BMC Infect Dis. 2019;19(1):968.
- Tvedskov JF, Lipka K, Ashina M, et al. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58(4):561–568.
