ABSTRACT
This article presents a critical review of two groups of studies that reported adverse effects to salmon and herring eggs and fry from exposure to 1 μg/L or less of aqueous total polycyclic aromatic hydrocarbons (TPAH), as weathered oil, and a more toxic aqueous extract of “very weathered oil.” Exposure media were prepared by continuously flowing water up through vertical columns containing gravel oiled at different concentrations of Prudhoe Bay crude oil. Uncontrolled variables associated with the use of the oiled gravel columns included time- and treatment-dependent variations in the PAH concentration and composition in the exposure water, unexplored toxicity from other oil constituents/degradation products, potential toxicity from bacterial and fungal activity, oil droplets as a potential contaminant source, inherent differences between control and exposed embryo populations, and water flow rate differences. Based on a review of the evidence from published project reports, peer-reviewed publications, chemistry data in a public database, and unpublished reports and laboratory records, the reviewed studies did not establish consistent dose (concentration) response or causality and thus do not demonstrate that dissolved PAH alone from the weathered oil resulted in the claimed effects on fish embryos at low μg/L TPAH concentrations. Accordingly, these studies should not be relied on for management decision-making, when assessing the risk of very low–level PAH exposures to early life stages of fish.
INTRODUCTION
In the late 1990s, scientists with the National Oceanic and Atmospheric Administration (NOAA) Auke Bay Laboratory (ABL) in Juneau, Alaska, published several studies on the effects of aqueous fractions of weathered crude oil upon salmon and herring eggs and larvae during exposure to water flowing through columns containing oiled-gravel. The investigators interpreted the results of these experiments as evidence of toxicological effects from exposure to 1 μg/L or lower of dissolved total polycyclic aromatic hydrocarbons (TPAH) (Heintz et al. Citation1995, Citation1999; Carls et al. Citation1997, Citation1999). They also concluded that oil toxicity increased with weathering. Their work has been quoted by other authors as representing a “new paradigm” in the evaluation of toxicity of PAH to fish early life stages (Rice et al. Citation2001; Peterson et al. Citation2003), demonstrating that weathered oil is more toxic and that fish embryos are more sensitive to oil than previously thought. Carls and Meador (Citation2009) stated that this work “unambiguously demonstrates that dissolved PAHs from weathered crude oil severely impact fish embryos at low μg/L concentrations.” Although later studies could not replicate these findings (Brannon et al. Citation2006; Sebastian Citation2010), they have been interpreted as requiring “necessary modifications of environmental standards for water quality, storm water control, chronic low-level oil releases, and many other human activities” (Carls et al. Citation2002).
We have reviewed all available information pertaining to the toxicity studies of Heintz et al. (1995, 1999), and Carls et al. (1997, 1999, 2003, 2005) as well as related studies. This review included original chemistry data in the public Exxon Valdez Trustee Council Hydrocarbon Database (EVTHD; EVOSTC 2009), published data in project reports and peer-reviewed papers, and original laboratory notebooks, data, observations, and other information underlying the experiments (Dahlberg Citation1998; Sebastian Citation2010) obtained under the Freedom of Information Act (FOIA). Based on our review, we find that the evidence produced by these studies does not support the conclusions that weathered oil is toxic at low μg/L TPAH concentrations or that oil toxicity increases with weathering. In addition, this review found evidence that these experimental studies were compromised by uncontrolled variables including variation in concentration and composition of exposure media, failure to demonstrate causality, presence of particulate (non-dissolved) oil, microbial activity in the gravel columns, microbial infections, and variation in brood sources that would have limited the conclusions that could be drawn from the studies.
OVERVIEW OF THE OILED-GRAVEL COLUMN EXPERIMENTS
A series of experiments using unfiltered water upwelling through oiled gravel columns to provide an exposure medium were begun at the NOAA Auke Bay Laboratory in Alaska in 1992 to simulate exposure of early life stages of Pacific herring (Clupea pallasi) and pink salmon (Oncorhynchus gorbuscha) to dissolved petroleum constituents in water that had passed through oiled shoreline gravel (Marty et al. Citation1997a,b) Although the experiments were designed to simulate exposure of salmon and herring to oil leaching from intertidal sediments that were contaminated with the Alaska North Slope Crude oil released from the Exxon Valdez, the source of the crude oil used in all the column studies was not identified. Marty et al. (Citation1997b) reported that the oil was collected from the Prudhoe Bay field (PBCO) in 1992. An implicit assumption was made that dissolved PAH and related sulfur heterocyclic compounds were the only toxic components in the aqueous extracts of the artificially weathered crude oil. These oiled gravel column studies include a 1992 salmon study (Marty et al. Citation1997b), a 1993 salmon study (Heintz et al. Citation1995, Citation1999), a 1995 herring study (Carls et al. Citation1997, Citation1999), a 1995 salmon study (Wertheimer et al. Citation1999), a 1998 salmon study (Heintz Citation2000) and a 1999 salmon study (Carls et al. Citation2003, Citation2005). The 1993 salmon study and the 1995 herring study will be discussed in detail here because they form the basis for the conclusions described above as the “new paradigm.”
In the experiments, PBCO was treated to simulate various degrees of weathering. In all cases, the weathered oil to be tested was mixed with gravel that was then placed in vertical cylindrical columns (). In the salmon studies, eggs or larvae to be tested were placed either directly on the gravel or on a perforated plate in the effluent water above the gravel (). In the 1993 salmon study, alternating freshwater (8 h) and seawater (4 h) was flowed through the column from bottom to top to simulate an intertidal environment. Pink salmon (Oncorhynchus gorbusch) are adapted to spawn in estuarine (intertidal) and freshwater reaches of salmon streams. In the other salmon studies, similar variations between fresh and seawater were used to represent tidal flow. In the herring study, eggs were placed on glass slides that were continuously exposed in separate tanks to the seawater effluent from the columns.
Figure 1 Experimental set-up for the oiled-gravel exposure experiments with salmon embryos (Heintz et al. Citation1999) and herring embryos (Carls et al. Citation1999, Citation2005). The column dimensions for the salmon study were 60 cm × 15 cm diameter and were 122 × 30 cm diameter for the herring study.
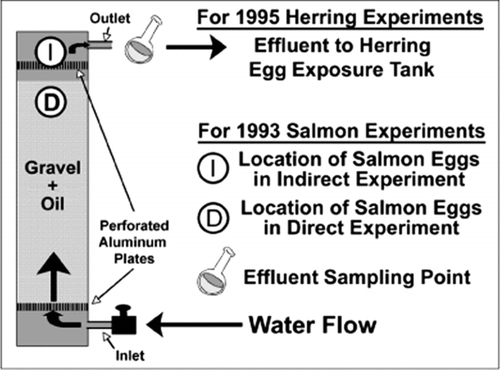
After various periods of exposure, measurements of PAH exposure and organism response to that aqueous extract were made. Response measurements included egg and larva mortality, and selected sublethal effects to larval forms. Chemical measurements included the concentration of total solvent extractable hydrocarbons (mg/kg) in the gravel at the start of the experiment, and the concentrations of 40 individual PAH and alkyl-PAH congener groups, and 23 alkanes in water (μg/L), tissue (μg/kg dw), and oiled gravel (μg/kg dw) for samples taken at time intervals during each experiment. Total PAH (TPAH) were reported as the sum of detected PAH, including some sulfur heterocyclics.
RELATING PAH EXPOSURE TO OBSERVED RESPONSES
The relationship between the exposure concentration of a toxicant and its corresponding effect on an organism is based on 3 key principles (Rand Citation2008; Timbrell Citation2000):
| 1. | the toxic response depends on the concentration of toxicant at the site of action in the organism; | ||||
| 2. | the concentration at the site of action is directly proportional to the external exposure concentration as determined by the toxicokinetics; and | ||||
| 3. | the response is causally related to the toxicant chosen for the dose metric. | ||||
In order to support the authors’ conclusions, the data from the studies reviewed here must follow these three criteria, where dose is defined by a PAH concentration in water or tissue and response is defined by acute mortality, chronic mortality, or sublethal effect. For dose, defined as water concentration, each exposure concentration must be controlled and known to ensure that it accurately reflects the internal concentration of a toxicant in the target tissue of the exposed organism as modified by the toxicokinetics. A major complication in all of the oiled gravel column studies is that the aqueous PAH concentrations declined dramatically over the course of the exposure. Also, the aqueous PAH composition varied between treatments and within treatments during the exposure period. Thus, it impossible to define a water concentration level associated with a given effect (Mathew et al. Citation2008). Internal dose, expressed here as tissue PAH concentration, provides a more realistic assessment of exposure than water PAH concentrations (McCarty Citation1991; Matthew et al. 2008). However, in the gravel column studies, the tissue concentrations and compositions for the various treatments also changed over the course of the exposure, which made it impossible to know the tissue residue concentration and composition of the toxicant(s) associated with an effect at the specific time(s) in the development of the embryo when the effect occurred (Mathew et al. Citation2008).
Further complicating the treatment of the data from these studies is expression of the results in terms of initial aqueous TPAH concentration. Each treatment had a different mixture of individual PAH, each having a potentially different toxicity, which may vary with endpoint. Therefore, to establish the relationship between exposure and toxic response for complex mixtures of toxicants, selection of the appropriate dose metric is critical. Further, establishing a dose (concentration)–response based on the selected metric will only establish inference about the causative agents and cannot by itself establish causality, because the response may simply be proportional to the toxic agent(s) measured and may not truly describe the actual agent-specific response, depending on what is used as the dose metric. To accomplish proof of causality for mixtures, there must be additional data showing that the response is a direct result of the compounds selected as the dose metric. For instance, proof of causality could come from a toxic unit model based on known toxicity mechanisms and response thresholds.
Heintz, Carls, and co-authors used TPAH as the dose metric, either as a water or tissue concentration. In doing so, they make the implicit assumption that PAH are the only toxic agents in leachates from weathered oil on gravel and that all PAH are contributing to the toxicity with equal potency, for all the endpoints being measured. Because the treatments in these studies have changes in tissue and water PAH concentrations and composition over time, none of the treatments represent simple dilutions of the most concentrated exposure effluent and, unless the PAH are equally toxic, TPAH as a dose metric is not an appropriate representative of the target-specific toxicants. Further, PAH may not be the only toxicants eluted from the oil under these conditions, but were the focus by the authors of the papers under review. Since definitive tests were not performed to conclusively demonstrate that these were the only toxicants under the exposure conditions, conclusions about absolute cause cannot be considered definitive. Under these limitations and knowing that PAH are likely significant, if not the major, contributors to the toxicity, TPAH as an overall dose metric can only be considered an approximation and does not accurately represent the more meaningful metric of individual PAH congener toxicity. The chemistry data we review are: TPAH concentrations in gravel, column effluent water, and in the tissues of eggs and larvae; concentrations of specific PAH analytes in gravel, column effluent water, and tissues; and, PAH compositions (as percentages of individual PAH) in gravel, effluent water, and tissues.
The 1992–1999 Salmon Studies
summarizes the exposure conditions and treatments for the 1992 salmon study (Marty et al. Citation1997a,b), the 1993 salmon study (Heintz et al. Citation1995, Citation1999), and the 1999 salmon study (Carls et al. Citation2003, Citation2005). All used the same oiled gravel column system with different gravel grain sizes and water flow rates to expose salmon eggs and alevins to aqueous extracts of PBCO.
Table 1 Exposure conditions for oiled-gravel column exposure experiments with salmon and herring eggs and larvae. Column size was 60 cm long × 15 cm diameter for the salmon studies and 122 cm long × 30 cm diameter for the herring experiments.
Full details of the 1992 Marty et al. (1997a,b) experiment were not found in published or unpublished project reports. Therefore, this review focuses on the Heintz 1993 salmon study, because a full range of data is available. In this study, two types of PBCO were tested: AWO (artificially weathered oil) applied to the gravel at seven concentrations ranging from 0 to 2,450 mg/kg total solvent extractables and VWO (very weathered oil), with a very different hydrocarbon composition from the AWO. The VWO gravel was re-used, after about 7 months of water-washing in columns and 3 months of open storage, from a similar experiment done a year earlier (Marty et al. Citation1997b) and was heavily biodegraded, as indicated by the almost complete loss of n-alkanes (EVOSTC 2009), compared with the 1993 AWO gravel. Prior to use in 1993, the total extractable oil concentration on the VWO gravel had decreased from 4,510 mg/kg in 1992 to 2,860 mg/kg at the beginning of the1993 study (Heintz et al. Citation1995), consistent with extensive microbial degradation during previous use, as reported by Marty et al. (Citation1997b), and subsequent storage.
Samples of the water, gravel, and eggs/larvae were collected at various time intervals, beginning at day 0 for gravel and day 1 for tissue and water. However, the sampling schedule was not consistent across all matrices and was not done with sufficient frequency during the first 4 weeks of the experiment, when the water and tissue concentrations were changing most rapidly, to identify the magnitude of the peak tissue concentrations and at what stage in embryo development they occurred. This is a key deficiency in this study because the water data (EVOSTC 2009) yield a half life for aqueous TPAH loss of ∼7 days for the higher exposure treatments, indicative of a rapidly declining aqueous concentration. By day 36, when the first egg tissue samples were taken, the aqueous TPAH concentrations were ∼3% of initial values.
The gravel, water, and tissue PAH concentrations in the AWO and VWO treatments did not provide uniform PAH compositions across treatments. For example, the percentage of naphthalenes in the day 0 VWO oiled gravel TPAH was 4.7% and the values for the AWO gravels ranged from 47.2% for the highest concentration to 4.4% for the lowest concentration (EVOSTC 2009). Similarly, the alkane, nC18, was more depleted in the lower oil concentration gravels than those with the higher oil concentrations. Because n-alkane depletion is a measure of biodegradation (NRC 1985), this result is consistent with greater alkane biodegradation in the gravel for the more dilute treatments during the 4 days of column water flow before the initiation of the experiments. Tissue TPAH concentrations provided a better measure of the exposure required to produce a given effect than gravel TPAH concentrations but, as noted above, also contained different PAH compositions among treatments and exposure times. For example, day 36 egg alkyl naphthalenes ranged from 77% of TPAH for the highest exposure concentration to 35% for the lowest exposure concentration (EVOSTC 2009). Thus, the exposures were time variable in terms of both the concentration and composition of PAH such that Heintz et al. (1995, 1999) did not test an oil dilution series but rather a series of different PAH mixtures of differing concentration, complicating interpretation of the results.
Measured lethal effects included the number of dead eggs at the eyeing stage after 36d, the number of salmon fry that failed to emerge from the gravel at 177 d, and the marine survival of post-emergent fry (Heintz et al. Citation2000; Wertheimer et al. Citation1999). While details of sublethal effects were not reported, Heintz et al. (Citation1995) provide a summary figure showing that there was not a significant increase in average% deformities above controls for initial aqueous TPAH concentrations ≤31 μg/L for the AWO treatments, consistent with the lowest observed effects concentration (LOEC) of 31.8 μg/L aqueous TPAH for ascites in emergent fry reported by Marty et al. (Citation1997b).
shows gravel, water, and tissue TPAH concentration data (EVOSTC 2009), as well as whether there was significant egg mortality by the eyeing stage (36 d of exposure) and larval mortality between eyeing and emergence of fry (between 36 and 177 d). The data are anomalous. The PAH-related toxic effect reported by Heintz et al. (1995, 1999) for the VWO treatment occurred at an egg and fry tissue TPAH concentration less than one-third the TPAH concentration that did not produce toxic effects in AWO treatments. Significant egg mortality at the eyeing stage (36 d) and fry mortality (between 36–177 d) were reported for the VWO treatment that resulted in an egg tissue TPAH concentration of 410 μg/kg and a fry tissue TPAH concentration of 380 μg/kg (), whereas no significant egg mortality or fry mortality was reported for AWO treatments with egg tissue TPAH concentrations up to 1,950 μg/kg and fry tissue concentrations up to 1,220 μg/kg ().
Table 2 TPAH concentrations in gravel, water, and egg tissues, and egg/fry mortality in different treatments for the salmon study (Heintz et al. Citation1995, Citation1999; EVOSTC 2009). Tissue TPAH values are rounded to the nearest 10 μg/kg dry wt. Results reported as significantly different (p = .05) from control are identified in boldface and with an asterisk.
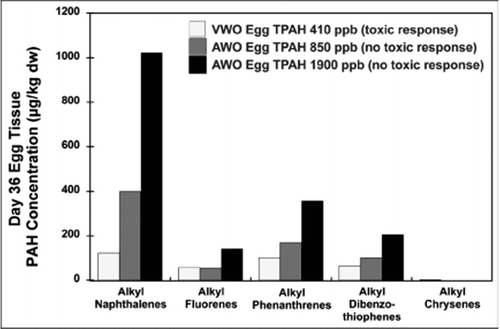
The concentrations of alkyl-PAH congener groups, such as alkyl-phenanthrenes, also follow the dose–response trends shown by the TPAH values in . shows the 36 d egg tissue concentrations of different alkyl-PAH congener groups for three different exposure experiments. The alkyl-phenanthrenes and alkyl-chrysenes are the PAH identified by Heintz et al. (Citation1999) to be mainly responsible for the toxicity and are all substantially lower in the VWO treatment with toxic effects than in AWO treatments that showed no toxic effects. The egg tissue data show that the toxicity in the VWO experiment was not solely PAH-related, because it is impossible to have a PAH-related toxic effect when egg tissue concentrations of all PAH analytes measured are substantially higher in non-toxic treatments than in the toxic VWO treatment. The alevin tissue data plotted in a similar manner to the egg data in give similar results, with non-toxic AWO treatments at higher tissue PAH analyte concentrations than the VWO treatment. These data indicate that there is an absence of a monotonic dose response curve for all the treatments and do not provide support for a cause and effect relationship between the alkyl-PAH or TPAH and the effects observed in the VWO treatment. The fact that alkyl-PAH are considered by the study participants to be the active toxicants, means that the study acknowledges that all PAH are not equipotent; thus, TPAH cannot be considered an appropriate dose metric. Therefore, there is failure to establish an acceptable dose metric on two counts: lack of dose response; and, inability to identify the compounds associated with causality.
Figure 2 Egg tissue PAH concentrations from Heintz et al. (Citation1999) for the salmon egg/larvae VWO treatment producing toxic responses and the AWO treatments producing no toxic responses.
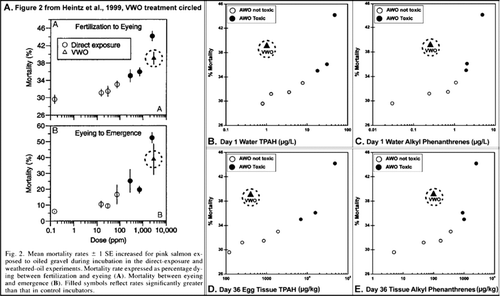
The anomalous behavior of the VWO treatment results documented above is not observed in in Heintz et al. (Citation1999), which shows what appears to be a concentration–response relationship between oil concentrations and mortality of developing eggs and fry. This figure, reproduced here as with the VWO treatment results circled, is misleading because the “dose” for the VWO experiment (2,860 mg/kg total organic extractables on gravel) refers to the total initial oil concentration on gravel in 1993, suggesting that it produced an exposure on a par with the highest AWO exposure (2,450 mg/kg total extractables on gravel). The VWO gravel had a PAH composition that was profoundly different from the oiled gravels used for the AWO experiments. Because the VWO gravel was reused from a 1992 study, it was heavily biodegraded and, consequently, had a higher level of inert solvent-extractable material and a much lower TPAH concentration (4.6 mg/kg) compared with the high concentration AWO treatment (32.7 mg/kg) (Heintz et al. Citation1999). (water TPAH), 3C (water alkyl phenanthrenes), 3D (tissue TPAH) and 3E (tissue alkyl phenanthrenes) show appropriately-drawn concentration–response relationships for egg mortality. If the results of the VWO experiment were PAH-related, the VWO point on each of the four graphs (–) would be displaced to the right with the other black symbols denoting a significant toxic effect in the PAH concentration range where significant toxic effects were observed. Because the TPAH or alkyl-phenanthrenes concentrations for the VWO experiment are in the same range as those showing no toxic effect, the toxicity observed in the VWO experiment was not caused solely by PAH. Thus, the observed mortality must result from either unmeasured compounds or other stressors. Data for emergent fry tissue chemistry plotted in a similar manner give similar results, consistent with the data in . Because the Heintz et al. (Citation1999) dose–response curve in is based on total extractable organics in exposure gravel and not on TPAH concentration in either exposure water or egg tissues, it does not demonstrate causality or even provide a basis for inference as it ignores actual bioavailabile PAH concentrations.
Figure 3 Salmon egg mortality versus dose for five dose metrics. A. Salmon egg mortality compared to the concentration of oil on gravel from Heintz et al. (Citation1999), used with permission; B. and C. Salmon egg mortality compared to aqueous concentrations of TPAH or alkyl-phenanthrenes; D. and E. Salmon egg mortality compared to tissue concentrations of TPAH or alkylphenanthrenes.
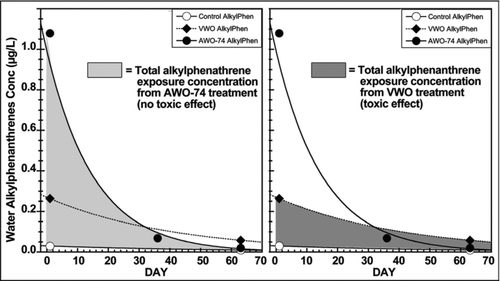
A confounding factor in the oiled gravel column studies is that the water and tissue PAH concentrations and compositions changed dramatically with time, and were not sufficiently measured to define the actual delivered exposure. Mathew et al. (Citation2008) present evidence that the peak tissue PAH concentrations in the 1993 Heintz salmon study likely occurred prior to the first sampling at 36 d. shows that the aqueous alkyl-phenanthrene concentrations measured in the 1993 Heintz salmon study (EVOSTC 2009) declined rapidly, making it impossible to define the true exposure concentration. The total integrated alkyl-phenanthrene exposure, as defined by the area under the curve of the AWO-74 treatment that produced no toxic effect (), is much greater than that for the VWO treatment through day 36 (), indicating that the VWO egg mortality to eyeing at day 36 could not have been caused solely by tissue alkyl-phenanthrenes. Plotting TPAH in a similar manner yields a similar figure. Because the oiled gravel column exposure system produces aqueous PAH concentrations that vary dramatically over time, it is not possible to demonstrate a causal relationship between an observed effect and a chronic aqueous PAH concentration. Because tissue PAH concentrations fluctuate with those in the exposure water, target tissue PAH concentrations also change with time. Further confounding the problem of interpreting the effects of changing PAH concentrations to developing fish embryos, is that the target organism is also changing with time as it develops; the timing and extent of PAH exposure would produce different effects at different times in embryonic development (Timbrell Citation2000). This reality renders the use of average exposure concentrations to describe chronic effects concentrations, such as an LOEC, also inappropriate because the aqueous exposure concentrations and resulting tissue concentrations are not constant.
Figure 4 Comparison of non-toxic AWO and toxic VWO aqueous exposure concentrations of alkyl-phenanthrenes in salmon egg exposures (Heintz et al. Citation1995, Citation1999; EVOSTC 2009). Data are fit using simple exponential decay curves.
Heintz et al. (1995, 1999) reported sublethal responses in embryos exposed to different oil on gravel concentrations of AWO and VWO that were summarized graphically in Heintz et al. (Citation1995), re-drawn here as with a second x axis added showing maximum tissue TPAH concentrations for each treatment (EVOSTC 2009). The LOEC values for deformities, estimated for the treatments with overlapping 95% confidence intervals from , correspond to an initial water TPAH concentration > 31 μg/L, egg tissue TPAH > 15,800 μg/kg, and fry tissue TPAH > 6,140 μg/kg in the AWO exposure (). The single VWO treatment produced significant sublethal effects at an initial water concentration of 1 μg/L, and egg and fry tissue concentrations of 410 and 380 μg/kg, respectively (). The sublethal responses observed could have been caused by, but are not specific to, PAH exposure, and do not correlate with tissue concentrations of TPAH or alkyl-PAH. For example, some of the observed responses could have been caused in part by bacterial toxins (Hamm et al. Citation2006; Berry et al. Citation2007) as discussed later in this article.
Figure 5 Re-drawn from Heintz et al. (Citation1995) with additional descriptive information from Heintz et al. (Citation1999) and EVOSTC (2009) and .
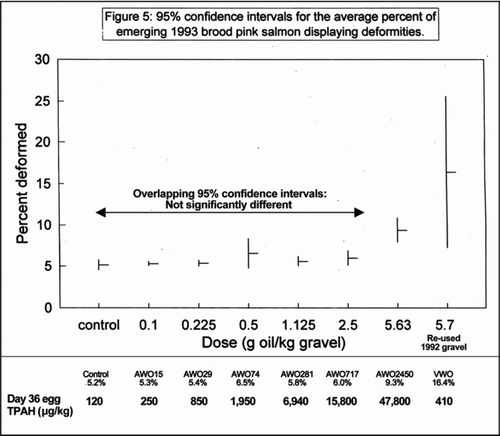
The results of the Heintz et al. (1997, 1999) studies do not support a conclusion that very weathered oil is more toxic than artificially weathered oil. They also do not support a conclusion that weathered oil can cause adverse effects to salmon eggs or fry at water TPAH concentrations of 1 μg/L.
The 1999 salmon study (Carls et al. Citation2003, Citation2005) exhibited lower sensitivity for some endpoints than the AWO results of the earlier salmon studies. This 1999 oiled-gravel column study had 10-fold higher water flow rates () that resulted in a lower control egg mortality of 8.1% compared with a much higher control egg mortality for the 1993 study of 29.6%. In addition, the 1999 salmon study observed no significant egg mortality at any exposure level (), compared with a LOEC for egg mortality, using initial water concentrations as the dose metric, of 18 μg/L TPAH for the 1993 study (Heintz et al. Citation1995, Citation1999). The laboratory records for the 1999 salmon study (Sebastian Citation2010) show that a VWO treatment was included in the study design and data were taken through emergence (∼180 days), but no results have been published or reported (Appendix 1).
Table 3 Key results of the 1999 Carls salmon study (Carls et al. Citation2003) and the 1993 Heintz salmon study (Heintz et al. Citation1999). Treatments are arranged in terms of increasing initial water TPAH concentration.
Taken together, the data () for these studies, using the initial concentration as the dose metric, suggest an aqueous TPAH no observed effects concentration (NOEC) for embryo mortality of 8 to 16 μg/L, with a less well-defined LOEC with a range of 18 to >45 μg/L. For alevin mortality at emergence, the data indicate an initial aqueous TPAH NOEC ∼8 μg/L and a LOEC ranging from 16 to 18 μg/L. However, when evaluated more appropriately (McCarty et al., Citation2011) based on tissue concentrations of TPAH, the results of these studies are not readily comparable because of the differences in exposure duration at measurement (). In the 1999 study, there was no significant mortality after 55 d exposure at 11,000 μg/kg TPAH in egg tissues, compared to a significant mortality in the 1993 study at 6,940 μg/kg TPAH in egg tissue. For sublethal effects, the Heintz 1993 salmon study () found an initial aqueous TPAH NOEC for % deformed larvae at emergence of >31 μg/L and a LOEC of ∼48 μg/L. However, the results of the 1999 (Carls et al. Citation2005) study were different and reported an initial aqueous TPAH NOEC for mean percent abnormal alevins at emergence of ∼4 μg/L and a LOEC of ∼16.5 μg/L, illustrating the lack of consistency of sublethal effects among the studies. It is worth noting again that, despite suggesting that some adverse effects thresholds based on aqueous TPAH exist, the data from these studies do not confirm that PAH were the causative agents or that some subset of compounds was more important than others (i.e., all PAH are not equally potent). The results of the Heintz et al. (Citation1999) study recently were examined with toxic unit models, as discussed below. Finally, the salmon experiments were not repeatable even within the same laboratory, demonstrating the complications of the dynamic exposure conditions and potential confounding factors such as potential differences in brood stock and microbial action that were not evaluated.
The Carls et al. (1997, 1999) Herring Study
In the 1995 herring egg/larvae experiment of Carls et al. (1997, 1999), herring eggs on glass slides were exposed to the effluent from oiled gravel columns. The treatments involved four levels of artificially weathered oil, mixed with gravel, prepared as in the salmon studies (), plus a control, with the highest gravel loading >10-fold greater than the highest oil loading in Heintz et al. (1995, 1999). The columns for the herring studies were larger, contained more oiled gravel and had higher flow rates than in the columns for the salmon studies (). The 2 treatments consisted of an initial 16 d exposure study with oiled gravel prepared from less weathered oil (LWO, Study 1) and, after a 13 d period during which the columns were drained and stored with no water flow, a second exposure study with the same oiled-gravel columns containing LWO that had been weathered by flowing water for 16 d and damp storage without water flow for 13 d to simulate a more-weathered oil (MWO, Study 2). As was the case with the salmon studies, the LWO was artificially weathered by heating to 70°C and weathered further during the short flushing period before Study 1 began. This resulted in variable concentrations of different PAH among different oil gravel loadings. For example, the Day 2 naphthalenes concentrations in the LWO gravel ranged from 64.1% of the gravel TPAH for the highest treatment concentration to 50.8% for the lowest treatment concentration. For both Studies 1 and 2, herring eggs on glass slides in separate tanks were exposed to column effluents () for 16 d and then transferred to jars in clean seawater.
Table 4 Oil loading (mg/kg total extractable organics) on column gravel at day 0 and PAH concentrations on column gravel on day 2 of the herring eggs study (Carls et al. Citation1997, Citation1999).
Water TPAH concentrations were measured at several time intervals in all treatments and for both studies. Tissue TPAH were analyzed periodically in eggs from all treatments in the Study 2 MWO experiment, but only in the control and high-concentration treatments in the Study 1 LWO experiment, making it impossible to compare exposure and temporal trends in tissue PAH concentrations and compositions for among all LWO and MWO treatments, as was done for the Heintz et al. (1995, 1999) salmon studies. Egg and larval mortality were measured in both studies. In addition, 10 sublethal effects were measured by non-blinded (observers knew the identity of the treatments) visual scoring: median hatch day; peak hatch day; % spinal defects; % effective swimmers; % small jaw; % pericardial edema; % yolk sac edema; yolk volume; spinal curvature; and, length.
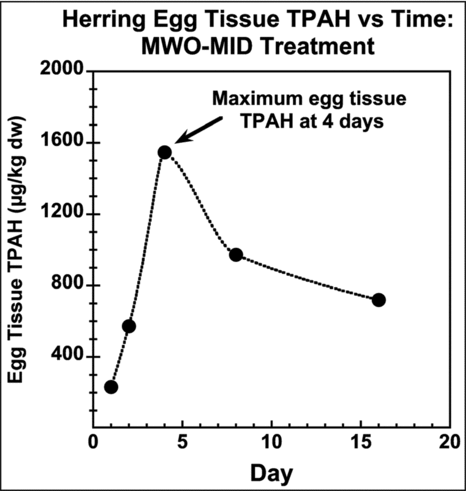
Carls et al. (1997, 1999) used initial water TPAH concentrations as their dose metric to evaluate relationships between PAH exposure and toxicity. Although there is not a full set of tissue PAH data for all treatments and recognizing the limitations posed by variable exposure concentrations and compositions among treatments and during the exposure period, and assuming, as Carls et al. (Citation1999) did, that dissolved PAH are the only toxicants eluted from the columns, we evaluated the tissue TPAH concentration as the more appropriate dose metric. Carls et al. (Citation1999) report tissue data on a wet tissue weight basis. This confounds interpretation of the tissue residue data because the ratio of wet tissue weight/dry tissue weight ranges from 5 to 16 for the herring egg samples as reported in EVTHD (EVOSTC 2009), indicating a large variation in% solids in embryos at different stages of development and from different females. To eliminate the problem of variable water content in the herring eggs, we analyzed only the dry wt. tissue data reported in EVTHD. Our review focuses on data for days 4 and 8. In the MWO study, the tissue TPAH concentration was highest at day 4, as illustrated by the peak egg TPAH concentration for the MWO-mid treatment (). The available tissue data for other MWO treatments and the LWO-high treatments also indicate that peak tissue PAH concentrations were reached by ∼4 d of exposure (EVOSTC 2009).
Figure 6 Bioconcentration curve of TPAH in herring eggs during exposure of the middle treatment concentration of MWO effluent. Maximum dry weight tissue TPAH concentration occurred at day 4 of exposure. From Carls et al. (Citation1999) and summarized in EVOSTC (2009).
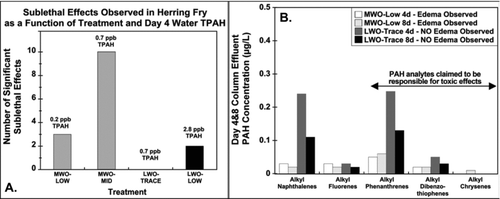
As was the case for Heintz et al. (1995, 1999), the Carls et al. (1997, 1999) study data do not support the conclusion that the TPAH in more weathered oil are more toxic than the TPAH in less weathered oil, because there were significant egg/larvae mortalities in MWO treatments in which day 4 and day 8 aqueous TPAH concentrations were lower than those in LWO treatments with no significant egg/larvae mortalities. illustrates that, while the MWO-mid treatment had significant larvae mortality, the LWO-low treatment had no significant mortality at substantially higher aqueous PAH concentrations for individual alkyl-PAH congener groups, including alkyl-phenanthrenes. illustrates the same phenomenon for sublethal toxicity to herring larvae. shows few significant effects at relatively higher day 4 aqueous TPAH concentrations in the LWO-low treatment and none for the LWO-trace treatment. However, many more significant effects were observed at ∼4 times lower aqueous TPAH concentrations for the MWO-mid and MWO-low treatments. Using yolk-sac edema as a sublethal endpoint, shows no significant increase in observed yolk sac edema for the LWO-trace treatment that had higher aqueous concentrations of TPAH analytes compared with the MWO-low treatment with lower concentrations of all TPAH analytes that had significant yolk sac edema. This lack of concentration–response behavior indicates that the MWO effects observed cannot be causally related to PAH exposure. in Carls et al. (Citation1999) shows two dose–response relationships with the MWO shifted to lower aqueous TPAH concentrations compared with the LWO data. Such shifts in dose response curves clearly suggest that the selected dose metric does not explain all the toxic response for the MWO study. Based on our and , selecting specific PAH, that is, alkyl phenanthrenes, as likely toxicants would not have addressed the difference in sensitivity found in the MWO study, suggesting that other factors, some of which are described below, were significantly contributing to the response of the herring eggs in the MWO study.
Figure 7 Aqueous concentrations of different alkyl-PAH congener groups at day 4 and 8 of exposure of herring eggs to the middle MWO treatment and the low LWO treatment in relation to larvae mortality. Data show that mortality in the MWO treatment occurred at lower aqueous alkyl-PAH concentrations than occurred in the non-toxic LWO treatment. Data from Carls et al. (Citation1999) and EVOSTC (2009).
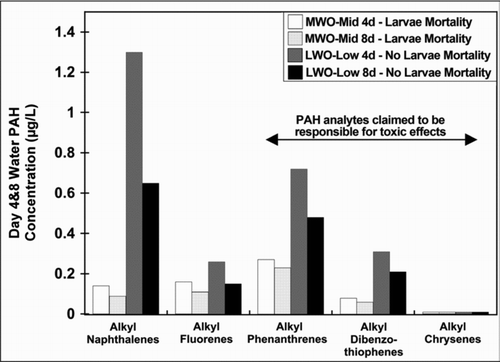
Figure 8 Frequency of sublethal effects in herring larvae versus aqueous TPAH and aqueous alkyl-PAH congener concentrations. A. Frequency of significant sublethal effects in herring larvae exposed to different aqueous TPAH concentrations in LWO and MWO effluents. B. Aqueous concentrations of different alkyl-PAH congener groups in non-toxic trace LWO and toxic low MWO effluents. Frequency of yolk sac edema was significantly increased in herring larvae exposed to low MWO effluent. Data from Carls et al. (1997, 1999) and EVOSTC (2009).
A confounding factor in the Carls et al. (1997, 1999) herring study is the source of the herring used in the LWO and MWO studies. The herring were collected from different locations on different dates with the collections for the LWO experiment occurring on April 11, 1995, at a location 200 miles south of where fish for the MWO experiment were collected on May 13, 1995. The differences between the eggs produced by the 2 populations of herring are evident in , redrawn from Carls et al. (Citation1999), which shows embryo mortality as a function of initial water TPAH concentrations. The mean control egg mortality was approximately 5% in the LWO study and 20% in the MWO study (), indicating that the eggs used in the MWO study were not as healthy as those used in the LWO study. The use of different eggs for the two tests confounds the results of Carls et al. (1997, 1999). Further potential confounding factors are discussed later in this article.
Figure 9 Relationship between initial aqueous TPAH concentrations and% mortality of herring eggs during exposure to LWO (squares) and MWO (circles) effluents. Filled symbols represent values significantly difference from controls. Figure from Carls et al. (Citation1999), used with permission.
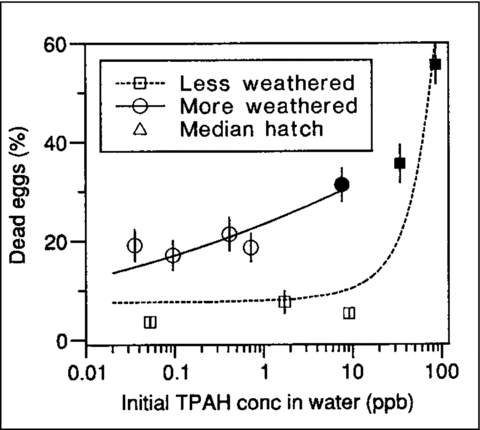
The conclusion of Carls et al. (Citation1999), that <1 μg/L of dissolved TPAH from more weathered oil (MWO) causes mortality and sublethal effects in herring eggs and larvae is contradicted by their own exposure water chemistry data. Toxicity cannot be attributed to TPAH exposure in one experiment when associated experiments show no toxicity for exposure to higher levels of TPAH analytes (Carls et al. Citation1997, Citation1999). The dose–response relationships for MWO treatments show a lack of causality with respect to aqueous TPAH because there were two separate dose–response relationships based on TPAH instead of a single dose–response relationship. In summary, the conclusion of Carls et al. (1997, 1999) that oil toxicity increases as it weathers is not supported by their data. Their argument for the higher toxicity of MWO is based on the increase in the relative concentrations of higher molecular weight alkyl-PAH in the MWO effluent than in the LWO effluent. However, measured concentrations of high molecular weight PAH were lower in the more toxic day 0 MWO-high and MWO-mid effluent treatments than in the LWO effluent concentration with greater TPAH concentrations (). More important, their data also do not support a conclusion that weathered oil can cause adverse effects to herring eggs or larvae at water TPAH concentrations of 1 μg/L. The authors’ data indicate that PAH exposure alone was not responsible for the effects observed during exposure to MWO. The data do, however, support the inference that initial aqueous TPAH concentrations greater than ∼34 μg/L can cause adverse effects to herring eggs or fry under the experimental conditions used. Because of the absence of supporting data such as definitive tests with only PAH present or a clear mechanistic model, the best that can be attributed to these tests is inference and not causality. However, as with the salmon study, an attempt was made post-publication to examine causality via toxic unit models as described later.
Table 5 Day 0 (initial) concentrations of TPAH and individual alkyl-PAH congener groups in column effluent water (EVOSTC 2009) and response data for the LWO-Low concentration treatment and the MWO-High and MWO-Mid concentration treatments of the herring egg studies (Carls et al. Citation1997, Citation1999).
ERRORS IN CHEMICAL ASSESSMENTS
Heintz et al. (1995, 1997) and Carls et al. (1997, 1999) report that oil toxicity is associated with higher molecular weight (MW) PAH and that oil becomes more toxic as it weathers because the proportion of higher MW PAH in the oil increases with weathering. However, their conclusions are based on a comparison of the relative concentrations of PAH (% TPAH) (i.e., fractions) in each of the treatments; they ignore the absolute concentrations of TPAH and individual PAH. In addition, they ignore any compounds other than PAH as potential contributors to the observed response as described below.
As oil weathers, the higher MW PAH comprise an increasing proportion of the remaining PAH residues because lower MW PAH are lost more rapidly (NRC 1985; Di Toro et al. 2007). For the Heintz et al. (Citation1999) AWO-74 (not toxic) treatment, the day 1 water TPAH had 13.9% alkylphenanthrenes as compared to 26.2% alkylphenanthrenes in the day 1 water TPAH for the VWO (toxic) treatment. However, the absolute concentrations of all PAH analytes decreased with weathering, reducing the overall toxicity as shown for the alkyl-phenanthrenes in and . For the AWO-74 (not toxic) treatment, the day 1 water alkylphenanthrenes concentration was 1.1 μg/L and for the day 1 VWO (toxic) it was 0.26 μg/L. Alkyl-phenanthrenes were considered the most toxic PAH by Heintz et al. (1995, 1999). However, as shown above and in and , AWO treatments with no significant egg mortality at 36 d contained higher concentrations of alkyl-phenanthrenes than the VWO treatment that showed toxicity. Toxic effects depend on absolute concentrations of analytes in exposure water and tissue, not their relative concentrations. Therefore, Heintz et al. (1995, 1999) are not correct in considering the alkyl-phenanthrenes (“persistent PAH”) to be mainly responsible for the VWO toxicity they observed. Nor, as previously discussed, are they correct in concluding from their data that weathering increases oil toxicity. Further, Heintz et al. (1995, 1999) did no definitive tests to support their supposition that these compounds were in fact the toxic agents. Such support should include tests with only the suspected toxicants to show that they can produce toxicity in the range identified or mechanistic models based on known toxic thresholds. Thus, while inference may be attributed to the AWO data for toxicity by PAH, causality has not been established for either the AWO or VWO data.
Similar errors were made by Carls et al. (1997, 1999), who also interpreted their herring toxicity data in terms of the relative amounts of higher MW PAH, not absolute concentrations. Carls et al. (Citation1999) observed greater toxicity in MWO treatments, but failed to consider the fact that this toxicity occurred at lower absolute and relative concentrations of higher MW PAH compared with the non-toxic LWO treatments as illustrated by the data in . Thus, the apparent enhanced MWO toxicity observed was not solely PAH-related.
Carls et al. (1997, 1999) and Heintz et al. (1995, 1999) misinterpreted their data in concluding that oil becomes more toxic as it weathers. Carls and Meador (Citation2009) failed to correct this misinterpretation and thus perpetuated the incorrect conclusions of these studies that very weathered oil is more toxic than less weathered oil. Confounding factors involving unmeasured compounds or stressors other than PAH were likely partly or completely responsible for the toxicity observed to both herring and salmon eggs/larvae, as discussed below.
CONFOUNDING FACTORS
The Carls et al. (1997, 1999) and Heintz et al. (1995, 1999) studies focused on dissolved oil, that is, PAH dissolved in the water not in droplets or associated with particles, but the analytical methods used did not distinguish between dissolved and particulate oil. They did not consider the possibility (Brannon et al. Citation2006) of direct contact with oil droplets during exposure, when interpreting their toxicity data, because they concluded that phytane, a marker for non-dissolved oil, was absent from all tissue and water samples. In the Heintz et al. (Citation1999) salmon study, the eggs were placed in direct contact with the oiled gravel in the AWO and VWO treatments, or were exposed to just the aqueous effluents from the AWO columns. It is counterintuitive to assume that the oil phase in the oiled gravel was not directly transferred to the eggs in some proportion of the total accumulation during the direct exposure. However, the eggs exposed to just the aqueous effluent contained similar tissue PAH concentrations and compositions to the eggs exposed directly to oiled gravel. Thus, the influence of oil-coated particles and direct contact with oil remains in question.
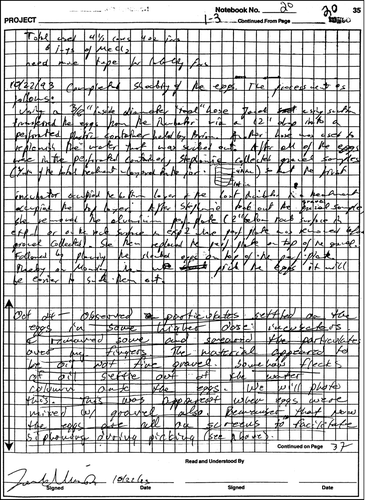
The hydrocarbon chemistry data for these studies indicate the presence of non-dissolved oil in the water and on the eggs. Most tissue and water samples for both studies contained highly insoluble saturated hydrocarbons, including phytane (EVOSTC 2009). A total of 29 of 31 exposure water samples collected in the Heintz et al. (1995, 1999) salmon study contained phytane; 55 of 69 exposure water samples collected in the Carls et al. (1997, 1999) herring study contained phytane. The presence of phytane indicates that non-dissolved oil was in the aqueous effluent from the columns for these studies. Marty et al. (Citation1997b) also confirmed the presence of phytane in effluent water from the 1992 oiled-gravel column experiments and Pearson (2005) and Brannon et al. (Citation2006) confirmed the presence of oil droplets in effluents from oiled-gravel columns similar to those used by Heintz et al. (Citation1999) and Carls et al. (Citation1999). The presence of non-dissolved oil on eggs was also recorded in laboratory notebook pages from these studies (Dahlberg Citation1998). For example (Appendix 2), the Heintz 1993 Pink Salmon Genetics Lab Notebook entry from October 24, 1993 reads: “Oct 24—observed particulates settled on the eggs in some higher dose incubators. I removed some and smeared the particulates over my fingers. The material appeared to be oil not fine gravel. Somehow flecks of oil settle out of the water column onto the eggs. We will photo this. This was apparent when eggs were mixed w/ gravel also. Remember that now the eggs are all on screens to facilitate siphoning during picking.” Similarly (Appendix 3), the Heintz 93 BY Incubation-Env Monitor Laboratory Notebook 12/5/93 reads: “While removing the eggs I observed that the yellow treatment (1.125 g of oil) had oil dusting on the eggs.” These findings indicate the presence of non-dissolved oil in the water and on the eggs during oil gravel column experiments. Thus, uptake and toxicity of PAH cannot be attributed solely to the dissolved fraction of the oil. These observations also may explain why salmon in direct contact with oiled gravel and those exposed to column effluent alone contained similar concentrations and compositions of PAH.
Microbial colonization, including fungal and bacterial infections, is common in egg and fry culture of fish (Barnes et al. Citation2002; Marty and Heintz Citation2010). It is not surprising, therefore, that we found examples of fungal and bacterial growth in oiled gravel treatments recorded in the laboratory records (Dahlberg Citation1998) of the reviewed studies: gram-negative bacterial growth in jars containing incubating herring eggs that had previously been exposed to oil-rock column effluent (Carls et al. Citation1999, Citation1995 herring study; Appendix 4); and examples of fungal growth on salmon eggs (Heintz et al. Citation1999, 1993 brood year pink salmon study; Appendix 3). Further, a review of the literature shows that there are clearly documented examples of microbial contamination in similar oiled rock column experiments performed by others (Clarke et al. Citation2004; Hustad Citation2008) in which the infections confounded the results.
Microbes can affect embryo mortality directly through an infectious process or indirectly affect mortality and other endpoints through production of toxic metabolites. Microbial contaminants can produce toxic metabolites through the biodegradation of weathered oil, particularly when the systems are nutrient enriched. For example, Middaugh et al. (Citation2002) showed that bacteria isolated from PWS Alaska degraded Alaskan North Slope crude oil and further that the degradation products were more toxic and teratogenic than the unmetabolized fractions of oil. Middaugh et al. (Citation1998) also showed that exposure of embryonic and larval Pacific herring to water-soluble microbial degradation products of weathered petroleum produced significant embryo mortality or teratogenic responses. Similarly, Middaugh et al. (Citation1996), Shelton et al. (Citation1999), Hamdoun et al. (Citation2002), and Sepič et al. (2003) also demonstrated that products of microbial degradation of petroleum hydrocarbons can contribute to oil toxicity.
Oiled-gravel columns can act as bioreactors in which microbial colonization results in the metabolism of organic matter, with infertile eggs and dead embryos providing a nutrient source for microbial metabolism, in addition to the weathered oil. Thus, there is the clear potential for increased mortality and abnormalities caused by toxic bacterial biodegradation products to be mistakenly ascribed to PAH exposure. Such a mis-attribution seems a particular risk in the VWO treatment that re-used oiled gravel from a prior experiment and was shown by Marty et al. (Citation1997b) to have undergone extensive microbial degradation even before long-term storage and reuse in 1993 column experiments.
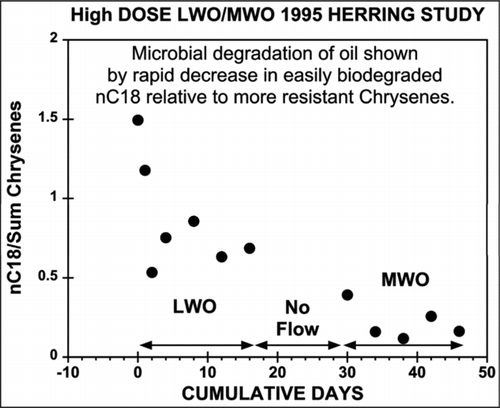
In view of this published literature and the data from these studies, we disagree with the claim made by Carls and Meador (Citation2009) that the toxicity of oiled-rock column effluent is independent of microbial activity. It is impossible to know with certainty the extent to which the oiled-rock column experiments reviewed here were contaminated and affected by microbial colonization. It is clear, however, from our review of these studies that microbial contamination was an observed and uncontrolled variable in those experiments. Hydrocarbon chemistry data from both the 1993 salmon study and the 1995 herring study (EVOSTC 2009) demonstrate extensive microbial activity in the oiled gravel columns. illustrates this for the 1995 herring study, showing the rapid loss of the easily biodegraded alkane nC18, where ∼50% of the nC18 is lost during the 0–16 d LWO period, another ∼25% was lost during the period between experiments and a final ∼15% was lost from the remaining nC18 during the MWO 30–46 d period. The sum of chrysenes was used as a reference point to assess extent of oil biodegradation (Short et al. 2007), because their degradation is very slow. Similar biodegradation occurred in the 1993 salmon study as reflected in the gravel hydrocarbon chemistry data (EVOSTC 2009). Review of the published literature demonstrates that this is a potentially important variable that can dramatically affect the results of the experiments. That such microbial contamination occurred is not surprising, but failure to document and provide at least analytical, if not operational, control of this confounding factor is a substantial flaw in the reviewed work.
Figure 10 Ratios of C18 versus sum of chrysenes (an indicator of biodegradation) with time in effluent water from the high-concnetration LWO and MWO treatments in the herring egg studies of Carls et al. (1997, 1999). Chemistry data are from (EVOSTC 2009).
The morphological responses evaluated by Heintz et al. (Citation1999) and by Carls et al. (Citation1999) are not specific to PAH exposure, and cannot be reliably attributed to PAHs. Mortality and teratogenesis in early fish life stages can result from exposure to a number of natural and anthropogenic toxicants, including by-products of microbial activity. In fact, herring larvae used as controls in laboratory experiments often show a high proportion of the deformities reported by Carls et al. (Citation1999) for unexplained reasons (Griffin et al. Citation2009; Hershberger et al. Citation2005; Vines et al. Citation2000; Kocan et al. Citation1996a,b; Pearson et al. 1995). Examples of factors that cause morphological and other deformities in fish (e.g., skeletal and spinal deformities, vascular and cardiac deformities and dysfunction, edema of various organs including the yolk sac, neurologic signs and abnormal hatch rate and size) include hypoxia (Shang and Wu Citation2004), bacterial toxins (Grisolia et al. Citation2009; Osswald et al. Citation2009; Hamm et al. Citation2006; Berry et al. Citation2007; Liu et al. Citation2002) and saxitoxin (Lefebvre et al. Citation2004).
INVESTIGATING CAUSALITY
Carls et al. (Citation1999) and Heintz et al. (Citation1999) concluded, without verification, that either dissolved TPAH or alkyl-phenanthrenes were the responsible causative agents for the toxic responses to exposure to effluents containing MWO or VWO. Subsequent to the publication of these papers, Barron et al. (Citation2004) undertook a modeling study to investigate the possible causes of the toxicity of oiled gravel column effluents. Tissue TPAH and individual PAH concentrations were used as the dose metrics and the data were fit to four different models, a sum of PAH model based on non-polar narcosis for chronic mortality, an aryl hydrocarbon receptor (AhR) model which is identified with induction of cytrochrome P450, a toxic unit model for alkyl-phenanthrenes (AP), and a model combining the toxic units from narcosis, AhR, and AP models. Barron et al. (Citation2004) used wet wt tissue PAH data for the herring egg study. As discussed earlier, wet wt/dry wt ratios in herring eggs vary widely during development and in eggs from different females, confounding interpretation of dose–response relationships. In addition, there was not a complete tissue data set available for the LWO experiment precluding equal comparison between the two experiments. Therefore, because the modeling of the herring data was compromised by both the wide variability in the wet weight-based tissue PAH data used and the absence of a complete data set, only the modeling results for the Heintz et al. (Citation1999) salmon study will be discussed here.
Tissue PAH concentrations after 35 d of exposure in the salmon study (Heintz et al. Citation1999) were used for the modeling by Barron et al. (Citation2004). Although, this tissue PAH concentration was not the peak concentration for all treatments based on the data in of Heintz et al. (Citation1999) or based on the modeling of PAH in tissue that included accounting for biotransformation (Mathew et al. Citation2008), it provided a consistent exposure duration. The narcosis model at the highest concentration only yielded 0.243 toxic units and so narcosis was ruled out as the main driving force for the observed toxicity. The AhR model only yielded 0.139 toxic units at the highest concentration. The best single model was the AP model, but the toxic VWO treatment had lower toxic units than the non-toxic AWO treatments, consistent with the concentrations in tissues shown in . Thus, the model was mostly tracking the AWO data and the VWO data were an anomaly. The range of toxic units for the AP model ran from 0.113 to 6.205 across the range of treatments, not including the control. Predicted toxic units did not correlate well with measured toxicity of the different AWO and VWO treatments. The modeling activity supports the conclusions of Heintz et al. (Citation1999) that alkyl-phenanthrenes likely dominated the observed response in the AWO treatments but cannot account for the enhanced toxicity of the VWO treatment, suggesting that either other unmeasured compounds or non-chemical stressors were contributing to the toxic response in addition to the PAH. While the modeling activity is encouraging, tests with the suggested toxicants in the ranges found in these studies would be required to definitively identify causality.
Overall, Barron et al. (Citation2004) provide some very suggestive data that alkyl-phenanthrenes probably are substantial contributors to the observed egg/larvae toxicity in the salmon experiments, but modeling results are somewhat uncertain because of the use of trout embryo data as opposed to species-specific data for the toxic unit determination and the use of only one alkyl-phenanthrene, retene (a C4-phenanthrene not present in crude oil), instead of compound specific potency information for toxic unit determination. However, these data clearly demonstrate that the use of TPAH, either as an aqueous concentration or tissue concentration, as a dose metric is not appropriate for evaluating oil toxicity.
CONCLUSIONS
The conclusions of Heintz et al. (1995, 1999) and Carls et al. (1997, 1999) that weathered oil is more toxic than unweathered oil and that such toxicity occurs at about 1 μg/L dissolved TPAH are not supported by the data, based on our analyses of their published and unpublished data. The toxicity to developing eggs and larvae of salmon and herring observed by these investigators cannot be ascribed solely to dissolved PAH, at least at the lower concentrations tested. Confounding factors, including oil droplets, microbial contamination and microbial toxins, and other unmeasured compounds in weathered oil likely contributed to observed effects in eggs and larvae. The salmon and herring egg studies have not demonstrated that crude oil toxicity increases as it weathers in the environment. Rice et al. (Citation2001) are incorrect in describing, based on Heintz et al. (Citation1999) and Carls et al. (Citation1999), a “new paradigm” that weathered petroleum is more toxic than previously thought.
APPENDICES 1–4
Pages reproduced from FOIA material providing examples that are pertinent to points in the text.
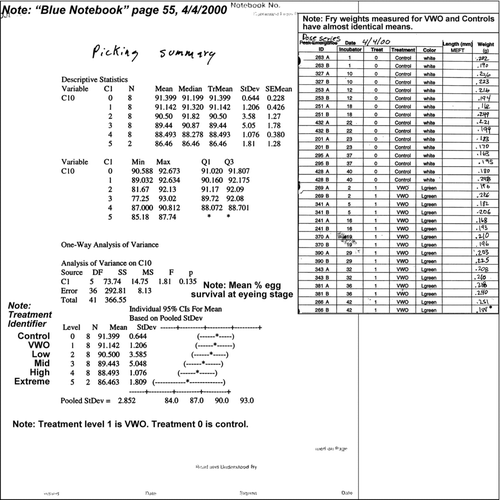
Appendix 1 NOAA FOIA 2010-0138 material (Sebastian Citation2010) showing that data were taken for a very weathered oil (VWO) treatment in the Carls et al. (Citation1999) salmon study. The FOIA file “Blue notebook PWS-344.pdf,” p. 55, shows the workup of embryo survival at eyeing data. This notebook entry shows that VWO treatment response measures were assessed on a date not given, but between 11/13/99 and 12/9/99. The VWO mean (Level 1) and the control mean Level 0) are almost identical. The inset shows data from FOIA 2010-0138 file “mass.pdf,” for weights at emergence (∼180 days) that were measured for the VWO treatment and control treatment emergent fry. The control mean is 0.201 ± 0.027 g and the VWO mean is 0.207 ± 0.021 g.
Appendix 2 Heintz 1993 Pink Salmon Genetics Lab Notebook (Dahlberg Citation1998), p. 35, October 24, 1993 recorded observations of eggs with oil particles on surface.
Appendix 3 Heintz laboratory record: ‘93 BY Incubation-Env Monitor, December 5, 1993–December 17, 1993 (Dahlberg Citation1998). Note clear evidence of non-dissolved oil on eggs and also white fungus noted for all treatments.

Appendix 4 Carls Herring Study notebook June 5, 1995, p. 28 (Dahlberg Citation1998) recorded observations of bacterial growth and problems with flow system. The levels of bacterial contamination, as evidenced by cloudy water and gram negative bacteria, illustrate potentially significant effects on herring embryos/larvae from microbial contamination.

ACKNOWLEDGMENT
The authors thank Ms. Karen Humphrey of Aquatechnics Inc. for her excellent critical review of a draft of this article. Funding for this work was provided by Exxon Mobil Corporation, Houston, TX. However, the conclusions are those of the authors and do not necessarily represent those of ExxonMobil.
Notes
aValue is the mean (n = 2) for the AWO-74 indirect treatment.
REFERENCES
- Barnes , M E , Sayler , W A and Cordes , R J . 2002 . Survival of rainbow trout sac fry subjected to various formalin and hand-picking regimes during rearing in vertical-flow tray incubators . N Am J Aquacult. , 64 : 129 – 135 .
- Barron , M G , Carls , M G Heintz , R . 2004 . Evaluation of fish early life-stage toxicity models for chronic embryonic exposures to complex polycyclic aromatic hydrocarbon mixtures . Toxicol Sci , 78 : 60 – 7 .
- Berry , J P , Gantar , M Gibbs , PDL . 2007 . The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae . Comp Biochem Physiol C , 145 : 61 – 72 .
- Brannon , E L , Collins , K M Brown , J S . 2006 . Toxicity of weathered Exxon Valdez crude oil to pink salmon embryos . Environ Toxicol Chem , 25 : 962 – 72 .
- Carls , M G and Meador , J P . 2009 . A perspective on the toxicity of petrogenic PAHs to developing fish embryos related to environmental chemistry . Hum Ecol Risk Assess , 15 : 1084 – 98 .
- Carls , M G , Johnson , S W Thomas , R E . 1997 . “ Health and Reproductive Implications of Exposure of Pacific Herring (Clupea) Adults and Eggs to Weathered Crude Oil, and Reproductive Condition of Herring Stock in Prince William Sound Six Years after the Exxon Valdez Oil Spill. Exxon Valdez Oil Spill Restoration Final Project Report (Restoration Project 95074) ” . Juneau , , AK, USA : National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Auke Bay Laboratory .
- Carls , M G , Rice , S D and Hose , J E . 1999 . Sensitivity of fish embryos to weathered crude oil: Part I. Low level exposure during incubation causes malformations, genetic damage, and mortality in larval Pacific herring (Clupea pallasi) . Environ Toxicol Chem , 18 : 481 – 93 .
- Carls , M G , Marty , G D and Hose , J E . 2002 . Synthesis of the toxicological impacts of the Exxon Valdez oil spill on Pacific herring (Clupea pallasi) in Prince William Sound, Alaska, USA . Can J Fish Aquat Sci , 59 : 153 – 72 .
- Carls , M G , Heintz , R A and Rice , S D . 2003 . “ Have wild pink salmon and their habitat recovered from persistent Exxon Valdez oil contamination? Exxon Valdez Oil Spill. Restoration Project Final Report (Restoration Project 00454) ” . Juneau , , AK, USA : National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Auke Bay Laboratory .
- Carls , M G , Heintz , R A Marty , G D . 2005 . Cytochrome P4501A induction in oil-exposed pink salmon Oncorhynchus gorbuscha embryos predicts reduced survival potential . Mar Ecol Progr Ser , 301 : 253 – 65 .
- Clarke , L MJ , Khan , C W Akhtar , P . Comparative toxicity of four crude oils to the early life stages of rainbow trout (Oncorhynchus mykiss) . Proceedings of the 27th Arctic and Marine Oilspill Program (AMOP) Technical Seminar, June 8–10, 2004, Edmonton, AL, Canada . Ottawa , ON , Canada. pp. 785 – 92 . Environment Canada .
- Dahlberg , ML. 1998 . “ NOAA FOIA production #1998-0327: 12 boxes of material produced pertaining to Auke Bay Laboratory Salmon & Herring toxicological studies ” . Juneau , , AK, USA : National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Auke Bay Laboratory .
- DiToro , D M , McGrath , J A and Stubblefield , W A . 2007 . Predicting the toxicity of neat and weathered crude oil: toxic potential and the toxicity of saturated mixtures . Environ Toxicol Chem , 26 : 24 – 36 .
- EVOSTC . 2009 . “ Exxon Valdez Hydrocarbon Database (EVTHD). Exxon Valdez Oil Spill Trustee Council (EVOSTC), Anchorage, AK, USA, Project #090290 ” . The current version of EVTHD is available at http://www.afsc.noaa.gov/ABL/Habitat/ablhab_exxonvaldez_hydrocarbon_database.htm Copies of the data files extracted from EVTHD and used in our review are available at http://www.valdezsciences.com
- Griffin , F J , Smith , E H Vines , C A . 2009 . Impacts of suspended sediments on fertilization, embryonic development, and early larval life stages of the Pacific herring, Clupea pallasi . Biol Bull , 216 : 175 – 87 .
- Grisolia , C K , Oliveira , R Domingues , I . 2009 . Genotoxic evaluation of different δ-endotoxins from Bacillus thuringiensis on zebrafish adults and development in early life stages . Mutat Res , 672 : 119 – 23 .
- Hamdoun , A M , Griffin , F J and Cherr , G N . 2002 . Tolerance to biodegraded crude oil in marine invertebrate embryos and larvae is associated with expression of a multixenobiotic resistance transporter . Aquat Toxicol , 61 : 127 – 40 .
- Hamm , E E , Voth , D E and Ballard , J D . 2006 . Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication . PNAS , 103 : 14176 – 81 .
- Heintz , RA. 2000 . “ Effects of Oiled Incubation Substrate on Pink Salmon Reproduction. Exxon Valdez Oil Spill Restoration Project Annual Report (Restoration Project 99476) ” . Juneau , , AK, USA : US Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Auke Bay Fisheries Laboratory .
- Heintz , R , Rice , S and Short , J . 1995 . “ Injury to Pink Salmon Eggs and Preemergent Fry Incubated in Oiled Gravel (Laboratory Study), Exxon Valdez Oil Spill Restoration Project Annual Report (Restoration Project 94191-2) ” . Juneau , , AK, USA : National Marine Fisheries Service . Available at http://www.evostc.state.ak.us/projects/ProjectInfo.cfm?project_id=1496
- Heintz , R A , Short , J W and Rice , S D . 1999 . Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon (Oncorhynchus gorbuscha) embryos incubating downstream from weathered Exxon Valdez crude oil . Environ Toxicol Chem , 18 : 494 – 503 .
- Heintz , R A , Rice , S D Wertheimer , A C . 2000 . Delayed effects on growth and marine survival of pink salmon after exposure to crude oil during embryonic development . Mar Ecol Progr Ser , 208 : 205 – 16 .
- Hershberger , P K , Elder , N E Wittouck , J . 2005 . Abnormalities in larvae from the once-largest Pacific herring population in Washington State result primarily from factors independent of spawning location . Trans Am Fish Soc , 134 : 326 – 37 .
- Hustad , A. 2008 . “ Effects of Crude Oil Contaminated Sediment on the Early Life Stages of Lumpsucker (Cyclopterus lumpus L.) MSc Thesis ” . 51 Tromsø , , Norway : University of Tromsø . pp. Available at http://munin.uit.no/munin/handle/10037/1960
- Kocan , R M , Hose , J E Brown , E D . 1996a . Pacific herring (Clupea pallasi) embryo sensitivity to Prudhoe Bay petroleum hydrocarbons: Laboratory evaluation and in situ exposure at oiled and unoiled sites in Prince William Sound . Can J Fish Aquat Sci , 53 : 2366 – 75 .
- Kocan , R M , Marty , G D Okihiro , M S . 1996b . Reproductive success and histopathology of individual Prince William Sound Pacific herring 3 years after the Exxon Valdez oil spill . Can J Fish Aquat Sci , 53 : 2388 – 93 .
- Lefebvre , K A , Trainer , V L and Scholz , N L . 2004 . Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin . Aquat Toxicol , 66 : 159 – 70 .
- Liu , Y , Song , L Xiaoyu , L . 2002 . The toxic effects of microcystin-LR on embryo-larval and juvenile development of loach, Misguruns mizolepis Gunthe . Toxicon , 40 : 395 – 99 .
- Marty , G D and Heintz , R A . 2010 . Ruptured yolk sacs and visceral fungi in emergent pink salmon alevins: Histopathology and relation to marine survival . Dis Aquat Org , 88 : 115 – 26 .
- Marty , G D , Heintz , R A and Hinton , D E . 1997a . Histology and teratology of pink salmon larvae near the time of emergence from gravel substrate in the laboratory . Can J Zool , 75 : 978 – 988 .
- Marty , G D , Short , J W Dambach , D M . 1997b . Ascites, premature emergence, increased gonadal cell apoptosis, and cytochrome P4501A induction in pink salmon larvae continuously exposed to oil-contaminated gravel during development . Can J Zool , 75 : 989 – 1007 .
- Mathew , R , McGrath , J A and Di Toro , D M . 2008 . Modeling polycyclic aromatic hydrocarbon bioaccumulation and metabolism in time-variable early life-state exposures . Environ Toxicol Chem , 27 : 1515 – 25 .
- McCarty , LS. 1991 . “ Toxicant body residues: Implications for aquatic bioassays with some organic chemicals ” . In Aquatic Toxicology and Risk Assessment: Fourteenth Volume. ASTM STP 1124 , Edited by: Mayes , M A and Barron , M G . 183 – 92 . Philadelphia , , PA, USA : American Society for Testing and Materials .
- McCarty , L S , Landrum , P F Luoma , S N . 2011 . Advancing environmental toxicology through chemical dosimetry: External exposures versus tissue residues . Integr Environ Assess Manag , 7 : 7 – 27 .
- Middaugh , D P , Chapman , P J and Shelton , M E . 1996 . Responses of embryonic and larval inland silversides, Menidia beryllina, to a water-soluble fraction formed during biodegradation of artificially weathered AlaskaNorth Slope crude oil . Arch Environ Contam Toxicol , 31 : 410 – 9 .
- Middaugh , D P , Shelton , M E McKenney , C L . 1998 . Preliminary observations on responses of embryonic and larval Pacific herring, Clupea pallasi, to neutral fraction biodegradation products of weathered Alaskan North Slope oil . Arch Environ Contam Toxicol , 34 : 188 – 96 .
- Middaugh , D P , Chapman , P J Shelton , M E . 2002 . Effects of fractions from biodegraded Alaska North Slope crude oil on embryonic inland silversides, Menidia beryllina . Arch Environ Contam Toxicol , 42 : 236 – 43 .
- NRC (National Research Council) . 1985 . Oil in the Sea: Inputs, Fates, and Effects , Washington , , DC, USA : National Academies Press .
- Osswald , J , Carvalho , A P Claro , J . 2009 . Effects of cyanobacterial extracts containing anatoxin-a and of pure anatoxin-a on early developmental stages of carp . Ecotoxicol Environ Saf , 72 : 473 – 8 .
- Pearson , W H , Moksness , E and Skalski , J R . 1995 . “ A field and laboratory assessment of oil spill effects on survival and reproduction of Pacific herring following the Exxon Valdez spill ” . In Exxon Valdez , Edited by: Wells , P G , Butler , J N and Hughes , J S . 626 – 661 . Philadelphia , , USA : oil spill: fate and effects in Alaskan waters. ASTM STP 1219, American Society for Testing and Materials .
- Peterson , C H , Rice , S D Short , J W . 2003 . Long-term ecosystem response to the Exxon Valdez oil spill . Science , 302 : 2082 – 6 .
- Rand , GM. 2008 . “ Fish toxicity studies ” . In The Toxicology of Fishes , Edited by: Di Giulio , R T and Hinton , D E . 659 – 81 . Boca Raton , , FL, USA : CRC Press .
- Rice , S D , Thomas , R E Carls , M G . 2001 . Impacts to pink salmon following the Exxon Valdez oil spill: Persistence, toxicity, sensitivity, and controversy . Rev Fish Sci , 9 : 165 – 211 .
- Sebastian , E. 2010 . “ Response to NOAA FOIA Request #2010-0138, March 8, 2010 and Supplemental Response to NOAA FOIA Request #2010-0138 ” . May 24, 2010
- Sepič , E , Bricelj , M and Leskovsek , H . 2003 . Toxicity of fluoranthene and its biodegradation metabolites to aquatic organisms . Chemosphere , 52 : 1125 – 33 .
- Shang , E H and Wu , R SS . 2004 . Aquatic hypoxia is a teratogen and affects fish embryonic development . Environ Sci Technol , 38 : 4763 – 7 .
- Shelton , M E , Chapman , P J Foss , S S . 1999 . Degradation of weathered oil by mixed marine bacteria and the toxicity of accumulated water-soluble material to two marine Crustacea . Arch Environ Contam Toxicol , 36 : 13 – 20 .
- Timbrell , JA. 2000 . Principles of Biochemical Toxicology , 3rd ed , London , , UK : Taylor & Francis .
- Vines , C A , Robbins , T Griffin , F J . 2000 . The effects of diffusible creosote-derived compounds on development in Pacific herring (Clupea pallasi) . Aquat Toxicol , 51 : 225 – 39 .
- Wertheimer , A C , Heintz , R A Thedinga , J F . 1999 . “ Effects of Oiled Incubation Substrate on Survival and Straying of Wild Pink Salmon. Exxon Valdez Oil Spill Restoration Project Final Report (Restoration Project 98076) ” . Juneau , , AK, USA : National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Auke Bay Laboratory .