ABSTRACT
Harlequin Ducks (Histrionicus histrionicus) were adversely affected by the Exxon Valdez oil spill (EVOS) in Prince William Sound (PWS), Alaska, and some have suggested effects continue two decades later. We present an ecological risk assessment evaluating quantitatively whether PWS seaducks continue to be at-risk from polycyclic aromatic hydrocarbons (PAHs) in residual Exxon Valdez oil. Potential pathways for PAH exposures are identified for initially oiled and never-oiled reference sites. Some potential pathways are implausible (e.g., a seaduck excavating subsurface oil residues), whereas other pathways warrant quantification. We used data on PAH concentrations in PWS prey species, sediments, and seawater collected during 2001–2008 to develop a stochastic individual-based model projecting assimilated doses to seaducks. We simulated exposures to 500,000 individuals in each of eight age/gender classes, capturing the variability within a population of seaducks living in PWS. Doses to the maximum-exposed individuals are ∼400–4,000 times lower than chronic toxicity reference values established using USEPA protocols for sea- ducks. These exposures are so low that no individual-level effects are plausible, even within a simulated population that is orders-of-magnitude larger than exists in PWS. We conclude that toxicological risks to PWS seaducks from residual Exxon Valdez oil two decades later are essentially non-existent.
INTRODUCTION
Harlequin Ducks (Histrionicus histrionicus) are small seaducks that breed along clear, fast-flowing streams and inhabit shallow marine intertidal zones off rocky shorelines during winter (Robertson and Goudie Citation1999). The western North America population of Harlequin Ducks winters along the coast from northern California to British Columbia, southeastern and south-central Alaska, and the Aleutian Islands (Robertson and Goudie Citation1999). Harlequin Ducks are present throughout the year in Prince William Sound (PWS), Alaska. Peak abundances in PWS occur during the fall and winter, when migrating and wintering birds are present (Iverson and Esler Citation2006; authors’ observations). Harlequin Ducks are particularly vulnerable to the direct effects of oil spills because they exhibit high site-fidelity and spend most of their time in intertidal and nearshore waters where they feed on epibenthic invertebrates (King and Sanger Citation1979; Piatt et al. Citation1990; Day et al. Citation1995, Citation1997; Holland-Bartels et al. Citation1998; Esler et al. Citation2000; Iverson and Esler Citation2006).
The Exxon Valdez oil spill (EVOS) in March 1989 released more than 250,000 barrels of crude oil, approximately 40% of which was stranded along ∼783 km (about 16%) of the PWS shoreline (Wolfe et al. Citation1994; Neff et al. Citation1995). Based on late-winter population estimates and retrieval rates of carcasses, an estimated 500–1,000 Harlequin Ducks (approximately 3–7% of the wintering population) were killed as a result of direct exposure to Exxon Valdez oil (EVO) during and immediately after the spill (Esler et al. Citation2002; Rosenberg et al. Citation2005; Rice et al. Citation2007). Within weeks of the oil spill, concerns were expressed about potential persistent adverse toxic effects on Harlequin Ducks from ingesting oiled prey, especially mussels (Patten et al. Citation2000).
The potential for long-term, post-spill effects on Harlequin Duck populations is the subject of continuing discussion. Some studies have reported decreased abundance, linking it to: (1) reduced overwintering survival of females and/or unsuccessful reproduction (Esler et al. Citation2000; Patten et al. Citation2000); (2) degraded habitat quality (Day et al. Citation1995, Citation1997; Irons et al. Citation2000; Wiens et al. Citation2004); or (3) exposure to hydrocarbons and, by inference, continuing toxic effects from EVOS, as indicated by elevated cytochrome P4501A [CYP1A] activity (Trust et al. Citation2000; Esler et al. Citation2002; Esler Citation2008). The Exxon Valdez Oil Spill Trustee Council (EVOSTC) concluded from studies conducted from 1997 through 2005 that Harlequin Ducks are recovering, but have not fully recovered from the effects of the oil spill (EVOSTC 2006, 2010).
Habitat quality has been posited as one possible variable governing the recovery of seaducks in PWS. By using a repeated-measures, multiple-lines-of-evidence approach, Wiens et al. (2004) analyzed habitat use by marine birds, including the Harlequin Duck, during 1989–2001 in ten bays in PWS that spanned a range of initial oiling levels, from unoiled or outside the spill zone to some of the most heavily oiled locations in the Sound (Day et al. Citation1995, Citation1997; Wiens et al. Citation1996; Murphy et al. Citation1997). The habitat-based approach assumes that birds recognize and respond to habitat conditions following a large environmental perturbation such as an oil spill, so the resulting patterns of habitat occupancy and use provide an indirect measure of the health of the environment (Wiens Citation1995; Wiens et al. Citation2004). This habitat-based approach demonstrated that by 1994 all species of marine-oriented birds had reoccupied initially oiled bays in PWS at levels that would be expected in the absence of an oil spill (Wiens et al. Citation1996).
Although interpreting population and demographic data for Harlequin Ducks in PWS has its challenges (e.g., accounting for variation in sampling design among studies, seasonal and interannual variation, absence of pre-spill data for Harlequin Ducks in PWS; Integral 2006), several studies have examined trends in abundance and demography in oiled versus unoiled portions of the spill area (Day et al. Citation1995, Citation1997; Murphy et al. Citation1997; Rosenberg and Petrula Citation1998; Wiens et al. Citation2004; Parker and Wiens Citation2005; Rosenberg et al. Citation2005; McKnight et al. Citation2006; Wiens et al. Citation2010). Wiens et al. (2010) reviewed a compilation of population estimates of Harlequin Ducks in PWS during the months of March, July, and August of 1972–2005 (McKnight et al. Citation2006) and concluded that there was no discernable decline in abundance in the unoiled parts of PWS during this period. McKnight et al. (2006) also found no declining population trends since the spill; however, they concluded that Harlequin Ducks were not fully recovered in the oiled areas because there was not a positive population trend. These differing interpretations of the same data likely derive from differences in the definition of recovery (Parker and Wiens Citation2005).
In a comprehensive analysis of sex- and age-composition during winter, Rosenberg et al. (2005) concluded that Harlequin Duck demographics in oiled areas were similar to elsewhere in their range and that age ratios, which are used as an index of recruitment, were similar between oiled and unoiled areas, indicating that recent productivity is not adversely affected by remnants of EVOS. Further, the higher rates of mortality for females overwintering in oiled parts of PWS that had been documented during the 1990s (Esler et al. Citation2000) have dissipated in more recent years (Bodkin et al. Citation2003), with no difference in winter survival rates in females between oiled and unoiled sites by 2000–2003 (Esler and Iverson Citation2010).
Harlequin Ducks were sufficiently abundant in PWS during mid-summer to permit repeated, within-year analyses for all years of the Wiens et al. (2004) weight-of-evidence study. Those analyses indicated a negative relationship between abundance and oiling level in 1989–1991 that was not present in 1996–2001 ( in Wiens et al. 2004). These results suggested an initial impact of the oil spill on utilization of oiled habitats by Harlequin Ducks that was no longer apparent by 1996. However, when habitat variables were included in the analysis, none of the within-year analyses indicated a negative relationship with oiling level ( in Wiens et al. 2004); instead, the patterns seemed to be associated more closely with habitat differences among the bays than with oiling level. The pre-spill data indicate that in 1984 (and in most post-spill years) Harlequin Ducks were substantially more abundant in those bays that later escaped oiling, and the apparently negative relationship in the oiling analysis disappeared when both habitat and oiling were included in the analysis (Wiens et al. Citation2004). Consequently, the post-spill differences in abundance of Harlequin Ducks between oiled and unoiled areas were not significantly different from those seen prior to the spill. The only exception was in 2001, when the unoiled bays showed a significant relative increase over oiled bays. However, that difference occurred only when the glaciated fjords in the northern part of the Sound were included as part of the reference area. When those glaciated bays were omitted, thereby providing a more appropriate reference area for the parts of the Sound that were oiled by EVOS, the marked increase in abundance in the unoiled region did not emerge (Wiens et al. Citation2010). Thus, the overall habitat assessments suggested that differences among habitats, rather than oiling, were the primary cause of variations in abundances in the bays examined. There was no indication of an impact from EVOS on habitat occupancy by Harlequin Ducks after 1991.
Table 1 Mean body mass ± standard deviations of Harlequin Ducks by gender, age-class, and season in Prince William Sound, Alaska.a
Thus, based on a weight-of-evidence evaluation from the aforementioned studies, Wiens et al. (2010) concluded that there appears to be no indication of continuing population-level impacts, either to abundance or demographics, on Harlequin Ducks from EVOS. On the other hand, while EVOSTC (2010) recognized that no differences in population trends of Harlequin Ducks were observed between oiled and unoiled areas of PWS after 1997, they concluded that there continues to be a risk of exposure to hydrocarbons from EVOS (Esler Citation2008).
The role of biomarkers (i.e., biochemical indicators of exposure), in this case CYP1A, remains an intensely discussed issue in assessing the recovery of Harlequin Ducks. Trust et al. (2000) found statistically significant differences in CYP1A activity in March–April 1998 between Harlequin Ducks sampled on unoiled Montague Island and those sampled in initially oiled sites on Crafton Island and Main Bay, with mean CYP1A at oiled areas exceeding those at reference areas by a factor of ∼3 (205 vs. 71 pmol·min−1·mg−1). Studies by Bodkin et al. (2003) indicated that CYP1A levels in Harlequin populations from oiled and unoiled areas had subsequently converged, although Esler (Citation2008) suggested that statistically different levels returned in 2005, and Esler et al. (2010) reported differences remained during 2005–2009. Overall, there appeared to be a decreasing trend in CYP1A induction through 2005 (Integral 2006).
Interpretation of the CYP1A data, however, is confounded because CYP1A responds more strongly to pyrogenic than petrogenic hydrocarbons (Anderson and Lee Citation2006) and because biomarker-inducing pyrogenic PAHs (e.g., chrysene and benzo(a)pyrene) occur throughout the spill zone (Page et al. Citation1999, Citation2002, Citation2004; Huggett et al. Citation2003). Anderson and Lee (Citation2006) also noted that a number of other factors, including gender, sexual maturity, age, ambient temperature, and season, affect CYP1A levels in marine animals. Moreover, CYP1A induction is not diagnostic of exposure to PAHs; rather, it can be induced in marine animals by exposure to many other chemicals, with a particularly strong CYP-induction response caused by many polychlorinated biphenyl (PCB) congeners (Houde et al. Citation2005). As Trust et al. (2000) and Ricca et al. (2010) reported, CYP1A-inducing PCBs do exist in PWS and are found in Harlequin Duck and sea otter tissues. Moreover, in Trust et al. (2000) shows a threshold response of CYP1A-induction by the PCB congener 138: in all cases where PCB 138 concentrations exceeded ∼2 ppb, CYP1A-induction rates were 50–100% higher than all of the other samples. In fact, the highest CYP1A level occurred for a seaduck at an unoiled site that had the highest concentration of PCB 138. Similarly, Ricca et al. (2010; ) reported that another strong CYP-inducer, PCB congener 81, had more than double the geometric mean concentration in Harlequin Duck blood samples from oiled areas compared to unoiled areas (based on the same 1998 samples reported in Trust et al. Citation2000). These data suggest that the influence of locally elevated PCBs on CYP1A levels cannot be dismissed, irrespective of whether any associated ecological effects occurred. Given the confounding factors discussed above, it is problematic to attribute activation of this biomarker in Harlequin Ducks in PWS solely to exposure from EVOS-derived PAHs.
Nevertheless, there continues to be speculation that remnant subsurface oil residues (SSOR) pose a risk to species such as seaducks that feed within the intertidal zone (ITZ) at northern Knight Island (NKI) (Bodkin et al. Citation2002; Short et al. Citation2006). Esler et al. (2002) and Bodkin et al. (2003) suggested that the foraging behavior of Harlequin Ducks on small invertebrates either attached to or beneath gravel or cobble results in the bioturbation of surficial sediments. According to this argument, this bioturbation would release SSOR, with subsequent oiling of the seaduck's plumage and ingestion of contaminated prey, leading to exposure to PAHs in the residual SSOR. Using data from the NOAA 2003 survey of SSOR, Short et al. (2006; ) estimated probabilities of randomly encountering SSOR from digging into intertidal sediments averaged over all tidal elevations and averaged separately for the upper-half and lower-half of the ITZ (i.e., they did not limit their probability assessment to the tidal zone where sea otters actually forage). Those authors asserted “these encounter probabilities are sufficient to ensure that sea otters and ducks that routinely excavate sediments while foraging within the ITZ would likely encounter subsurface oil repeatedly during the course of a year.” Rice et al. (2007) stated that “population effects on Harlequin Ducks are evident still” and that “poor recovery is probably due to foraging for intertidal invertebrates in oiled sediments.”
Table 3 Chronic toxicity reference valuesa for average daily doses of tpah for Harlequin Ducks (mg·kg body weight–1·day–1)a.
A qualitative ecological risk assessment of the potential continuing effects on Harlequin Ducks from EVOS (Wiens Citation2007) discussed sources of variability in the PWS environment that might affect the seaducks; differences between presence of individual-level biomarkers, such as the CYP1A activity, and effects on populations; and difficulties in defining and assessing recovery, particularly in the presence of uncertainty. Wiens (Citation2007) concluded that the likelihood of continuing effects from EVOS on the PWS Harlequin Duck population seemed remote.
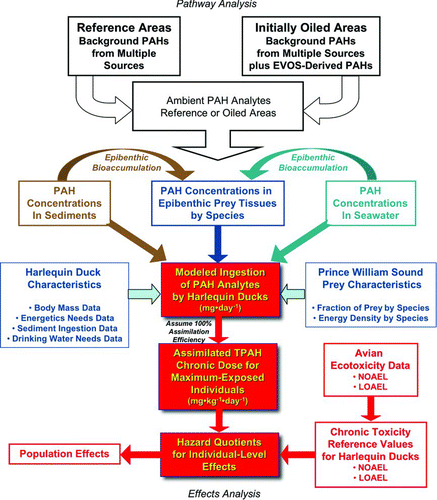
Here we take the next step to conduct a detailed, quantitative, probabilistic ecological risk assessment. Specifically, our objective is to use a risk-based assessment strategy (USEPA 1992, 1998; Gentile et al. Citation1993) to evaluate systematically and quantitatively the question: Is the Harlequin Duck population of Prince William Sound presently at-risk from EVOS-derived PAHs in the environment? The risk-based approach used in this study has as its foundation a conceptual ecological model presented here that describes the suite of exposure pathways by which Harlequin Ducks in PWS could plausibly be exposed to EVOS-derived PAHs in the environment. These exposure pathways are quantified using the most-recent available empirical data and are simulated in a stochastic, predictive model to estimate the total exposure of Harlequin Ducks to PAHs in PWS.
We then forecast the average daily assimilated doses to a hypothetical population of 500,000 Harlequin Ducks, capturing the characteristics of the Harlequin Ducks and the variability of the PAH sources as they exist in PWS. The output from these simulations results in a distribution of doses from which we extract the doses assimilated by the 99.9% quantile (1-in-1,000th) most-exposed individuals. The latter values are compared to appropriate toxicological benchmarks to provide a conservative, quantitative estimate of the current toxicological risks from EVOS to the Harlequin Ducks of PWS, with conservative defined here as resulting in a higher estimate of risk. Further, the projected individual-level effects are extrapolated to provide an assessment of the potential for population-level effects. By following this comprehensive, quantitative ecological risk-based approach and applying conservative data and model assumptions to the assessment, we are able to provide a clear answer to the question whether the Harlequin Duck population of Prince William Sound is presently at-risk from EVOS-derived PAHs.
CHARACTERISTICS OF HARLEQUIN DUCKS USED TO PARAMETERIZE THE MODEL
Several characteristics of Harlequin Ducks were used to parameterize the risk-assessment model (i.e., assign specific values to all of the model parameters) so that it realistically simulates the toxicological risks to Harlequin Ducks in PWS from remnant EVOS-derived PAHs. The characteristics of importance relate to the exposure pathways of Harlequin Ducks to PAHs and to the factors controlling the effects of such exposures. These factors include body mass, energetic requirements, dietary preferences, ingestion of seawater and sediments, and toxic effects from chronic exposure to PAHs. We present information that has been collated and synthesized specifically for Harlequin Ducks and their prey species, whenever possible, with priority given to information from PWS if available and from other areas as necessary. In a few cases, however, we relied upon information characterizing similar species when that information was all that was available. Throughout the model development and parameterization, we followed a conservative approach (i.e., selecting parameters that tended to increase the estimate of risk).
Body Mass
The body mass of Harlequin Ducks changes with age and fluctuates seasonally in response to shifts in energy demands and food availability. When Robertson and Goudie (1999) compiled mean weights of Harlequin Ducks across North America, few data on winter weights were available. Recent studies in PWS (Esler Citation2008) and British Columbia (Bond and Esler Citation2006), as well as in the Aleutian Islands (Fischer Citation1998; Fischer and Griffin Citation2000), have increased our knowledge about seasonal changes in body mass in Harlequin Ducks in western North America.
Female Harlequin Ducks in western and eastern North America are smaller and lighter than males (age classes not distinguished; Robertson and Goudie Citation1999). Esler (Citation2008) reported capture data covering 10 yr (1995–1998, 2000–2002, and 2005–2007), which indicated that weights consistently differed among adults, subadults, and hatching-year birds in both fall and winter. Hatching-year birds increase in weight from fall to winter as they mature but remain the lightest age-class until the following summer. Crowley (Citation1999) reported that the mean body weight of breeding (adult) females during June was greater than that of subadults and that paired subadult females were on average heavier than non-paired females.
The body mass of adult Harlequin Ducks varies seasonally, caused by such factors as ingestion of large amounts of herring eggs (when herring are spawning in large numbers), weight gain prior to migration, weight gain prior to egg-laying, weight loss in females during most of the rest of the breeding season, weight variability associated with molting, and rigors of overwintering life (Rosenberg and Petrula Citation1998; Robertson and Goudie Citation1999; Bond and Esler Citation2006). Because of this variability, we estimated the body biomass for four gender/age classes and two seasons for use in the model ().
Diet
Patten et al. (2000) reported on the marine diet of 89 Harlequin Ducks collected in PWS in 1989–1990. Data were presented as frequency-of-occurrence and as a length-importance index (LII) (considered here to be a surrogate for relative importance index; Robertson and Goudie Citation1999), based on the number and size of organisms consumed. The prey for Harlequin Ducks in PWS were epifaunal species living in rocky-intertidal or nearshore-subtidal habitats. (Note that the diet is quite different when the Harlequin Ducks are in their upland nesting habitat near large streams.) Diet was dominated by mollusks, especially gastropods (including periwinkles [Littorina sp.], chink shells [Lacuna sp.], and limpets [Lottia sp. and Acmaea sp.]), bivalves (especially blue mussels [Mytilus trossulus] and various clam species), and chitons (Tonicella sp.). Fish eggs were next in importance, primarily from Pacific herring (Clupea pallasi) and secondarily from pink salmon (Oncorhynchus gorbuscha). The diet also included crustaceans (e.g., hermit crabs [Pagurus sp.]) and echinoderms of various species (including a sea urchin, sea cucumber, and starfish). One polychaete (Nereis sp.) was the only annelid recorded, and the only fish was one sand lance (Ammodytes sp.).
The Patten et al. (2000) data are consistent with other studies in the northeast Pacific on Harlequin Duck diet (Vermeer Citation1983; Dzinbal and Jarvis Citation1984; Gaines and Fitzner Citation1987; Fischer and Griffin Citation2000). Goudie and Ankney (Citation1986) suggested that because of high energetic needs in winter, seaducks are trophic generalists and opportunists, feeding on high-energy prey when available. Thus, the Patten et al. (2000) data are typical for feeding in coastal marine habitats in winter, and provide an appropriate database with sufficient sample size to describe the PWS Harlequin Duck diet. The approximately 30 species of prey listed in Patten et al. (2000) were aggregated into six prey categories for parameterizing the Harlequin Duck risk model ().
Table 2 Diet of Harlequin Ducks in Prince William Sound, Alaska, in 1989–1990.a
Bioenergetic Requirements
Because no published studies have quantified the daily bioenergetic requirements of Harlequin Ducks, the estimates used here were based on studies of related bird species. Both Birt-Friesen et al. (1989) and Nagy et al. (1999) summarized information on field metabolic rates (FMR) for a wide range of wild terrestrial vertebrate species as determined by the doubly-labeled-water technique. (In that method, the animal consumes 3H- and 18O-labeled water; the difference in the rate of loss of each radioisotope provides a measure of CO2 production and, therefore, a direct measure of the metabolic rate of the animal; Speakman 1998.) Nagy et al. (1999) estimated the FMR for various groups of mammals, birds, and reptiles using the following allometric equation, and Birt-Friesen et al. (1989) estimated similar allometric equations for FMR of various groups of seabirds:
Seasonal Variation in Bioenergetics
The Harlequin Duck's resting metabolic rate, daily energy expenditure (DEE), and body mass vary seasonally. In PWS, adult females are in coastal habitats from August to early June, specifically during the fall wing-molt (August–September), overwintering (October–April), and the herring-spawning and spring-migration periods (late April and May). Breeding Harlequin Ducks then shift to freshwater habitats for pre-laying, incubation, and brood-rearing (June–August), with males present only during the pre-laying period. Males and non-breeding or failed-breeding females return to coastal environments soon after nest initiation. Confounding factors determining seasonal variation in energetics for wild birds include environmental variation, behavioral modification, and intrinsic seasonal variation in physiology. Seasonal variations in the environment, especially winter ambient air and water temperatures, also affect energetic needs. Water temperature is important, primarily because the rate of thermal transfer in water is about 25 times the rate in air, so the rate of thermal loss increases dramatically in cold waters. In addition, a bird's body temperature is ∼40°C, whereas water temperatures may be at or below 0°C, leading to a great disparity in temperatures and the possibility of rapid energy loss to the environment. As a result, some authors have suggested that water temperatures may be an important factor limiting the wintering distribution of some seaducks (de Leeuw et al. 1999). In winter high winds may increase the DEE of seaducks substantially, and ingesting cold prey further adds to the energetic costs of thermoregulation (Brinkman et al. Citation2003; McKinney and McWilliams Citation2005).
Bioenergetic needs may be reduced behaviorally. For example, seaducks need increased energy to replace all of the feathers in the complete post-nuptial molt; however, they may substantially reduce total energy needs by remaining flightless (Guillemette et al. Citation2007) or by getting out of the water and sitting on rocks to reduce heat loss, both during the molt and in extremely cold temperatures (de Vries and van Eerden 1995; Adams et al. Citation2000). Physiological variability also may cause changes in energetic needs; as one example, body mass declines as the bird enters winter (Guillemette et al. Citation2007) because of reduced feeding, even when food is unlimited and access is unrestricted (Portugal et al. Citation2007). Conversely, between late winter and pre-migration, seaducks store fat and increase mass in preparation for migration and breeding; for example, female Harlequin Duck mass in British Columbia increased 7% during this period (Bond and Esler Citation2006).
No published studies of Harlequin Duck energetics are available, but there is information for two related seaducks, the Common Eider (Somateria mollissima) and the Tufted Duck (Aythya fuligula). Brinkman et al. (2003) developed an energy and heat model for Common Eiders wintering in the Baltic and North seas, estimating DEE at ∼1,550 kJ·day−1 in the summer and ∼3,050 kJ·day−1 during the coldest part of the winter, an increase of 97%. Nehls (1995, cited in Brinkman et al. 2003) estimated DEE as ∼2,100 kJ·day−1 in the summer and ∼3,000 kJ·day−1 in the winter. Brinkman et al. (2003) estimated that summer DEE rates varied by 35%, and de Leeuw et al. (1999) found that DEE rates in Tufted Ducks increased 68% from the summer to the winter. Because these various studies on seasonal variation in bioenergetics show increases ranging from 43% to 97%, we examined the potential importance of this variability through a model sensitivity analysis, discussed below, that doubled the energy needs and, thereby, dietary intake, providing a conservative estimate of the range of natural variability.
Water Intake
Seaducks can drink seawater because they have salt glands that secrete a sodium chloride solution that is more concentrated than seawater (Schmidt-Nielsen and Kim Citation1964). Adult seaducks apparently do not leave the ocean to drink freshwater, although adult females commonly take their broods from the ocean to freshwater to drink (Nystrőm and Pehrsson 1988) because the ducklings have a lower osmoregulatory capacity (Nystrőm and Pehrsson 1988; DeVink et al. Citation2005). Few studies have measured the amount of water consumed by seaducks or other waterfowl, and there is no direct evidence that Harlequin Ducks deliberately ingest seawater. Nevertheless, to be conservative, we added the seawater-ingestion route of exposure to the conceptual model, irrespective of whether such ingestion is done deliberately or inadvertently while feeding. Fletcher and Holmes (Citation1968) measured daily water intake in domestic Mallards (Anas platyrhynchos) in a laboratory and found that the amount of water ingested declined when freshwater was replaced with saline water. At higher salinities, however, one must be cautious in assuming that fluid intake will be similar between domestic Mallards and seaducks because Mallards have a lower physiological capacity for osmoregulation than do seaducks (Bennett and Hughes Citation2003). Further, several studies have shown that birds raised in marine environments and/or with highly developed salt glands have higher salinity thresholds than other birds (Schmidt-Nielsen and Kim Citation1964; Harriman Citation1967; Walter and Hughes Citation1978; Bennett Citation2002; Bennett and Hughes Citation2003; Bennett et al. Citation2003). For example, Barrow's Goldeneyes (Bucephala islandica) are able to drink water of higher salinity without changing fluid intake, whereas Mallards must decrease fluid intake at even moderate salinity levels (Bennett and Hughes Citation2003).
Another approach for estimating bird drinking-water rates uses doubly-labeled-water studies to estimate daily water influx (i.e., water that is drunk + water in food + metabolic water). No studies have been conducted specifically for Harlequin Ducks, but USEPA (1993) recommended the Nagy and Peterson (Citation1988) allometric model for estimating daily water influx based on body mass and taxonomic group:
The Central Science Laboratory (CSL 2007) enhanced the Nagy and Peterson (Citation1988) water-flux model by incorporating data from doubly-labeled-water studies, thereby expanding the number of species analyzed and creating separate models for various categories of 39 species of birds, including seabirds. These two methods (Nagy and Peterson Citation1988; CSL 2007) for estimating the volume of drinking water consumed daily by female Harlequin Ducks produced estimates that differed by only 20%. Here, we selected Fletcher and Holmes’ (Citation1968) estimate of freshwater drinking rates for Mallards to approximate the daily seawater intake for Harlequin Ducks, normalized to body weight. We used this higher value, rather than the value for Mallards drinking saline water, because the latter were under osmotic stress and reduced their daily intake accordingly. In addition, the use of this higher number results in a higher estimated daily intake of water for Harlequin Ducks and thus is more conservative, leading to a higher estimate of risk.
Ingestion of Grit and Sediments
Harlequin Ducks may deliberately ingest sediments for dietary purposes or may consume sediments inadvertently while foraging, particularly in softer substrates. Many bird species ingest grit or sediment to aid in the mechanical breakdown of food items or for nutrients. Several studies (Fischer and Griffin Citation2000; Rodway and Cooke Citation2002) reported the presence of grit in the gizzard, proventriculus, and/or fecal matter of Harlequin Ducks, although none measured the particle size or the volume of grit. Several studies have reported food contents in the gullet (esophagus and proventriculus) of Harlequin Ducks (Kenyon Citation1961; Vermeer Citation1983; Goudie and Ankney Citation1986; Gaines and Fitzner Citation1987; Goudie and Ryan Citation1991; Fischer and Griffin Citation2000; Patten et al. Citation2000), but none includes information on grit characteristics.
The occurrence of grit in Harlequin Duck feces varies seasonally in British Columbia, increasing from 12% (n = 25) of fecal samples tested in the early fall (August–September) to 90% (n = 83) by late winter (April) (Rodway and Cooke Citation2002). During the breeding season, Robert and Cloutier (Citation2001) reported sand in 50% (n = 42) of the Harlequin Duck fecal samples from eastern North American rivers; these authors also classified 21% of the fecal samples containing sand as having “large quantities” of sand, although no quantitative measurements were provided. The percentage of sediment in the diet can be estimated by calculating the acid-insoluble ash (AIA) content of the digesta from both the gizzard and gullet and incorporating information on the digestion rate of food items and the estimated AIA content of ingested sediment. Using this technique, Beyer et al. (2008) estimated that the sediment portion of the diet of seven seaduck species varied from ∼2% to 14% and concluded that grit ingestion in waterfowl was related to diet and substrate. Although Harlequin Ducks are not discussed by Beyer et al. (2008), Common Goldeneyes (Bucephala clangula) were characterized. This species is ecologically similar to Harlequin Ducks in PWS: both species are year-round residents but primarily use PWS for overwintering; both forage in nearshore and intertidal habitats; and both have overlapping diets that include mussels, littorine snails, and crustaceans (Patten et al. Citation2000; Bourget et al. Citation2007; Beyer et al. Citation2008). Beyer et al. (2008) reported that the mean percent grit in the diet of Common Goldeneyes was 5.2% of ingested mass. We regard the latter to be the best estimate of grit consumption for Harlequin Ducks to use in the risk-assessment model; a sensitivity analysis doubles this rate to 10.4%.
Avian Chronic Toxicity Reference Values
Wildlife Toxicity Reference Values (TRVs) are species- and chemical-specific estimates of an exposure or dose concentration that is unlikely to cause adverse ecological effects (e.g., growth, reproduction, or survival) (USARMY 2000; USEPA 2005). The chronic TRV is defined as the dose above which ecologically relevant effects might occur to wildlife species following chronic exposures and below which it is reasonably expected that such effects would not occur (USEPA 2005). There are three categories of TRVs: dose-, concentration-, and tissue-based estimates of exposure that are used to determine the no-observed-adverse-effects level (NOAEL) and the lowest-observed-adverse-effects level (LOAEL). Dose-based TRVs (expressed in units of mg contaminant·kg−1 body wt·day−1) are typically used when evaluating chronic risks to wildlife via ingestion pathways (USEPA 2005) and is the approach followed here.
Guidance for developing TRVs (USARMY 2000; USEPA 2005) provides minimum data criteria that must be satisfied to estimate TRVs. These criteria include, but are not limited to, the following: (1) selecting endpoints that are most clearly linked to population sustainability; (2) employing an oral route of exposure that consists of a suitable control and at least two experimental concentrations that bracket the expected effects levels; (3) continuing exposures for a sufficient duration to qualify as a chronic study; (4) clearly reporting details on the duration of exposure, concentrations applied, and frequency of the dose; and (5) quantifying effects on growth, survival, or reproduction that are most clearly associated with no-to-low adverse effects (i.e., NOAEL and LOAEL). These criteria provide a systematic framework for determining which avian toxicology data are sufficient and appropriate for estimating a TRV for seaducks.
As a component of the USEPA's Ecological Soil Screening Level (Eco-SSL) Guidance, an extensive literature search identified 5,478 papers that contained PAH toxicity data for avian and mammalian species that potentially could be suitable for developing mammalian and avian toxicity reference values (USEPA 2005, Attachment 4-2). Of the 46 potentially suitable papers (i.e., those studies that met all of the USARMY 2000 and USEPA [2005] minimum data criteria), only two contained data for avian species; these were scored following the Eco-SSL guidance (USEPA 2005; Attachment 4-3 and 4-4). Within these two studies, there were data available for only one species exposed to low- and high-molecular-weight PAHs, respectively. Specifically, the low-molecular-weight TRV was for 13-day-old Bobwhite Quail (Colinus virginianus) exposed to naphthalene for a period of 5 days. The reported NOAEL (5620 mg·kg−1 diet) resulted in a TRV of 1653 mg·kg−1·day−1 for both mortality and growth (Landis 1985). The high-molecular-weight TRV was derived from exposing 9-day-old European Starlings (Sturnus vulgaris) for 5 days to 7, 12-dimethylbenz(a)anthracene at 0, 2, or 20 mg·kg−1 body wgt·day−1 (Trust et al. Citation1994). The NOAEL TRV was determined to be 2 mg·kg−1·day−1 and the LOAEL TRV was 20 mg·kg−1·day−1 for growth measured as changes in body weight. However, these two studies failed to meet the minimum criteria developed in the USEPA guidance for TRVs (specifically requiring at least three toxicity values for two species for mortality, growth, or reproduction and at least two chronic LOAELs and one chronic NOAEL). Moreover, additional extrapolation uncertainties limit the use of these data for our study. In particular, these values are for two terrestrial bird species and therefore can only provide general information concerning the magnitude that might be expected for avian TRVs for petroleum hydrocarbons to marine birds. Additionally, these values are for exposures to a single low- or high-molecular-weight PAH and thus cannot be directly converted to the TPAH exposures that are evaluated in this study.
The Mallard Duck, which lives in freshwater, estuarine, or marine environments, is one of the most commonly used species in toxicological studies on avian aquatic species exposure to petroleum hydrocarbons (Hartung and Hunt Citation1966; Holmes et al. Citation1978a,b, 1979; Coon and Dieter 1981; Gorsline and Holmes 1982; Cavanaugh et al. 1983; Holmes and Cavanaugh 1990; Stubblefield et al. 1995a,b). After careful review of these and other studies (including Szaro et al. Citation1978, Citation1981; Albers Citation1979; Patton and Dieter Citation1980; Vanglider and Peterle Citation1980; Hoffman and Gay Citation1981; Cavanaugh and Holmes Citation1982, Citation1987; Rocke et al. Citation1984), we concluded that the studies by Stubblefield et al. (1995a,b) on Mallard Ducks using weathered EVO uniquely provided a reasonable basis for assigning TRV values that are applicable to the Harlequin Duck and to exposure to EVOS-derived PAHs. Accordingly, we followed the USEPA protocols (USEPA 2005, 2007) to derive the TRVs from those studies as follows.
Stubblefield et al. (1995a,b) conducted 22-week dosing experiments with Mallard Ducks that were fed ad libitum a diet containing weathered Exxon Valdez crude oil (WEVC), which was analyzed as having 9.252 mg·g−1 TPAH. Five nominal doses of WEVC were fed to the Mallards: 0, 200, 2,000, or 20,000 mg WEVC·kg−1 diet. These authors measured a suite of endpoints, including two population-relevant parameters of particular importance here: eggshell thickness and eggshell strength. We used the results from the toxicity experiments on these two endpoints to develop gender-specific chronic NOAEL and LOAEL TRVs normalized to body weight (). (Note that none of the other endpoints measured by Stubblefield et al. 1995a,b showed effects at any dose level.) Because the Stubblefield et al. (1995a,b) toxicity experiments used weathered Exxon Valdez crude oil, TRVs could only be derived for TPAH, not for the separate PAH ring classes. TRVs were estimated by converting the nominal WEVC doses to TPAH doses in the feed, then converting the daily feeding rate for each experimental class to a per-weight basis. The no-effects and lowest-effects doses for both males and females for both endpoints were the 2,000 and 20,000 mg nominal dietary exposures, respectively. Differences between the TRVs for males and females reflect slight differences in the actual feeding quantities of the dosed diet.
Although only the Stubblefield et al. (1995a,b) studies are deemed appropriate for developing the TRVs for TPAH exposures to Harlequin Ducks, other studies that failed to meet the USEPA criteria nevertheless provide a relative comparison with our derived TRV values, adding confidence to the appropriateness of our values. Because the release of petroleum hydrocarbons into coastal marine environments is of global importance (NRC 1985), many laboratory and field studies have examined behavioral, biochemical, physiological, morphological, histopathological, hematological, immunological, mortality, growth, and/or reproductive endpoints of coastal species. Studies appropriate for comparison with our TRVs include those on Cassin's Auklets (Ainley et al. Citation1981), Black Oystercatchers (Andres Citation1999), Leach's Storm-Petrels (Butler et al. Citation1988), Sanderlings (Burger and Tsipoura Citation1998), Common Murres (Fry and Lowenstine Citation1985), Wedge-tailed Shearwaters (Fry et al. Citation1986), Black-legged Kittiwakes (Köth and Vauk-Hentzelt Citation1988), and Herring Gulls and Atlantic Puffins (Leighton Citation1993).
Ainley et al. (1981) found that there were no significant differences in the number of eggs laid between controls and Cassin's Auklets (Ptychoramphus aleuticus) fed a single dose of up to 600 mg of Bunker C fuel oil, although there was a significant difference in the percentage of eggs hatched at 1,000 mg. The results of reproductive success studies with Leach's Storm-Petrels (Oceandodroma leucorhoa) exposed in the field to Prudhoe Bay crude oil (PBCO) indicate that hatching success was affected by a single dose of ≥200 mg (Butler et al. Citation1988). Breeding Wedge-tailed Shearwaters (Puffinus pacificus) exposed to a single dose of 2,000 mg weathered Santa Barbara crude oil approximately 30 days prior to egg-laying showed reduced laying, breeding success, and survival of chicks (Fry et al. Citation1986). Peakall et al. (1982) reported that a single dose (∼1,000 mg) of PBCO administered to nestling Herring Gulls (Larus argentatus) reduced bodyweight.
While these studies clearly indicate that petroleum hydrocarbons at certain levels are toxic to a variety of avian species inhabiting coastal waters, the studies do not contain sufficient information to develop a defensible marine avian TRV following the USEPA methodology because of extensive extrapolation uncertainties associated with source, dose, exposure, and/or species-to-species extrapolations. However, using a series of conservative default assumptions for the missing information from these studies, our estimates of potential TRVs from those studies are all greater than the NOAEL and LOAEL TRVs that we calculated for the surrogate species (Mallard Duck) using weathered Exxon Valdez oil (USEPA 2005). Thus, we conclude that the Stubblefield et al. (1995a,b) studies provide conservative estimates for
TRVs.
ASSESSMENT APPROACH
The toxicological risk assessment for the Harlequin Duck is based on a conceptual model describing the potential pathways of PAH exposures in the PWS environment, including potential exposures from PAHs derived from residual EVO (). In developing this conceptual model, we considered these potential exposure pathways: (1) direct encounter with surface oil residues (SOR); (2) direct encounter with subsurface oil residues (SSOR); (3) consumption of prey tissues containing PAHs; (4) consumption of sediments containing PAHs; and (5) consumption of seawater containing PAHs.
Figure 1 Harlequin Duck risk assessment framework. Pathways conceptual model showing: (a) the sources of PAHs in the PWS environment in reference and initially oiled sites; (b) concentrations of PAHs in sediments, seawater, and prey tissues; (c) risk assessment model inputs, including characteristics of Harlequin Ducks in PWS and their prey; (d) elements of the risk assessment model; (e) generation of assimilated doses; (f) development of chronic toxicity reference values for Harlequin Ducks; and (g) assessment of individual- and population-level effects.
Surface and Subsurface Oil Residues from EVOS
With respect to the source material associated with remnant residues from EVOS, much of the EVO on the shore disappeared rapidly after the spill through natural weathering processes, particularly from winter storms, enhanced by intensive shoreline cleanup activities in 1989–1991 (Neff et al. Citation1995). The average annual rate of oil loss from the shore was ∼80% from 1989 to 1992, decreasing to ∼22% from 1992 to 2001 and ∼4% after 2001 (Wolfe et al. Citation1994; Short et al. Citation2004, Citation2007). By 2001 the extent of ITZ shoreline with SOR or SSOR had decreased by ∼99% from the area initially oiled to an estimated 11.3 ha (Short et al. Citation2004), affecting only ≤0.05% of the PWS shoreline length. Further reduction from the 2001 estimates would be expected by 2012, even at the slower degradation rate estimated by Short et al. (2007).
Taylor and Reimer (Citation2008) reported that most surveyed sites in PWS in 2002 had very little or no SOR. Where SOR did exist, it had weathered to patches of hard, asphalt “pavement” or highly weathered tar splats, similar to other sites where crude oil had been stranded and exposed to sunlight and biodegradation. Total coverage of weathered SOR was <0.2% of the total surface area of the surveyed sites. SOR was located primarily in middle and upper intertidal zones (MITZ and UITZ, respectively) and up to supratidal zones on beaches characterized by cobble, boulder, and pebble cover in moderate-to-low-wave-energy locations. Because these are not the tidal zones in which Harlequin Ducks feed (Robertson and Goudie Citation1999), the asphaltic residues are very unlikely targets in the Harlequin Duck diet, and the SOR is so highly weathered that their PAHs are essentially not bioavailable (Integral 2006; Taylor and Reimer Citation2008), consumption of SOR is not considered to be a plausible pathway for current PAH exposures to the Harlequin Ducks and therefore was not modeled.
The SSOR has also been surveyed repeatedly since 2001, as discussed in Short et al. (2004, 2006), Michel et al. (2006), Boehm et al. (2008), and Taylor and Reimer (Citation2008). The patches of SSOR occurred primarily in the MITZ and UITZ, with 88.3% of the SSOR found at locations ≥ +0.8 m above mean lower-low water (Short et al. Citation2006). The SSOR was located under a 15–25-cm-thick layer of clean sediments (Hayes and Michel Citation1999; Hayes et al. Citation2010), which in turn were located under a surface covering of stable armor composed of coarse gravel, cobble, and boulders (Hayes and Michel Citation1999; Michel et al. Citation2006; Taylor and Reimer Citation2008; Hayes et al. Citation2010). SSOR rarely occurred in unarmored finer-grained sediments. Thus, within a few years after EVOS, most SSOR was found at locations where physical disturbance of the sediments occurs only rarely (Hayes and Michel Citation1999; Michel et al. Citation2006; Short et al. Citation2007; Taylor and Reimer Citation2008). Consequently, the possibility that a Harlequin Duck would directly excavate the SSOR at the depth at which it exists under surface-armored sediments in the MITZ and UITZ is extremely unlikely because: (1) their diet is largely epibenthic, collected from the lower intertidal zone (LITZ); and (2) Harlequin Ducks do not have the physical capability to dig in such sediments to those depths or under such armoring (see Robertson and Goudie Citation1999; Eadie et al. Citation1995, Citation2000). Consequently, we consider the pathway in which a Harlequin Duck excavated sediments and intercepted SSOR as being too implausible to warrant modeling.
On the other hand, a potential pathway of exposure to these seaducks could occur if bioturbation of SSOR occurred by another organism that brought SSOR to the surface for subsequent contact by seaducks. Two possibilities exist in PWS: fat innkeeper worms and sea otters. The tube-dwelling fat innkeeper worm (Echiurus echiurus alaskensis) lives in the fine-grained LITZ sediments; therefore, it potentially could intersect SSOR when it excavates its burrow. However, the amount of SSOR that could be brought to the surface by fat innkeepers would be very small, as they are microphagous-feeders (Pickford Citation1964; Ax Citation2000) (i.e., collecting food particles suspended in the water or in surface detritus). Unpublished stable-isotope data suggest they are low-level predators, possibly feeding on harpacticoid copepods and constituents of surface detritus, but they are not sediment processors, as their fecal deposits do not contain significant quantities of sediment (RC Highsmith, pers comm). As a result, essentially only the volume of the excavated burrow sediments at SSOR depth would be transported to the surface by the worm. Moreover, almost 90% of SSOR occurs above the LITZ life-zone of the fat innkeepers (Short et al. Citation2006). Consequently, the pathway by which a fat innkeeper worm would bring SSOR to the surface and present an exposure risk to Harlequin Ducks is not sufficiently plausible to warrant modeling.
On the other hand, sea otters regularly excavate sediments to depths of 50 cm while foraging in the LITZ and subtidal zone (STZ) (Shimek and Monk Citation1977; Calkins Citation1978; Hines and Loughlin Citation1980; Kvitek et al. Citation1988) and therefore would appear to be a potential source of bioturbation into SSOR (Harwell et al. Citation2010a). Thus, a potential route of exposure to Harlequin Ducks theoretically could exist if a sea otter excavated a pit that intersected SSOR and a seaduck immediately thereafter collected prey from that pit. A field survey by Boehm et al. (2007a) qualitatively showed that there is a low probability that sea otters would intercept SSOR because of the vertical (tidal) and horizontal spatial separation of the sea otter foraging areas from the SSOR sites. In a detailed quantitative risk assessment, Harwell et al. (2010a) used empirical data from NKI on sea otter foraging habits and the spatial distribution of SSOR to estimate that the rate at which a sea otter pit could intersect SSOR is 1–5 times per 10,000 hr (i.e., an hourly probability of ∼10−4). That rate is also the maximal hourly rate that a seaduck could come into contact with SSOR, but that rate would ensue only if there were a direct association between sea otters and Harlequin Ducks while both are foraging.
Associations between feeding seabirds and feeding marine mammals are known to exist. Harrison (Citation1979) and Grebmeier and Harrison (Citation1992), for example, documented feeding association between at least 13 species of seabirds (but not including Harlequin Ducks) and gray whales (Eschrichtius robustus) in the Bering Sea, whereby infaunal invertebrates stirred up by feeding whales were made available to feeding flocks of seabirds. Similarly, in Alaska, the order of prey selected (urchin, limpet, chiton, barnacle, mussel) by Glaucous-winged Gulls (Larus glaucescens) depends on environmental factors, including competition with sea otters (Trapp Citation1979; Hayward and Verbeek Citation2008); these birds have been observed by one co-author (RHD) feeding with sea otters in both the Aleutians and PWS.
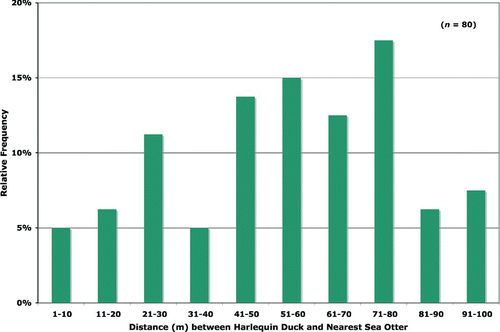
We conducted field studies in PWS that allow us to examine whether such associations exist between sea otters and Harlequin Ducks. Data were collected during nearshore wildlife surveys on 14 research cruises conducted in PWS between 1989 and 2001, covering all seasons of the year (Day et al. Citation1995, Citation1997; Day et al., unpublished data). These surveys were conducted by small boat in ten bays (six oiled and four unoiled) and were designed to count and map the locations of all wildlife occurring within 200 m seaward of the shorelines, in the ITZ, and in the near-shore supratidal zone. For this analysis of co-occurrence, we digitized the mapped locations of all Harlequin Ducks and sea otters, then used GIS software to calculate the nearest distance of a sea otter to each individual or group of Harlequin Ducks when both occurred in the same replicate sample of the same survey. We assigned the presence of both a sea otter and a Harlequin Duck within 100 m as being a potential co-occurrence; this occurred in 80 (∼6.4%) of a total of 1,247 records in which both species were present. Within this 100-m radius, there is no pattern of distribution of Harlequin Ducks relative to sea otters that would suggest an attraction or positive association (). Moreover, in all of our thousands of observations of both sea otters and Harlequin Ducks in PWS, we have never seen a Harlequin Duck immediately forage for food residues from the sea otter or any other indication that Harlequin Ducks have any association with sea otters. Hence, we conclude that there is no feeding relationship or spatial association between Harlequin Ducks and sea otters. As a result, the probability that a Harlequin Duck would contact prey that was recently excavated by a sea otter pit in SSOR is considerably less than 10−4. In fact, that probability is the same as the random frequency distributions of such recently exposed (i.e., by sea otter excavations) contaminated prey across the seaduck feeding area, which would likely be orders of magnitude less than the 10−4 level.
Figure 2 Distribution of co-occurrences of Harlequin Ducks and sea otters in Prince William Sound. Relative frequencies (%) of measured distances (in m) from a Harlequin Duck to its nearest sea otter when both occur simultaneously within a 100-m radius (defined to be co-occurrence). Based on unpublished data collected by Day et al. in several nearshore surveys conducted from 1989 through 2001 and analyzed in GIS framework (n = 80 co-occurrences out of the 1247 observations when both species were present simultaneously).
Even if there were a positive association between the two species, it is extremely unlikely that a Harlequin Duck would be harmed by direct exposure to SSOR that had been recently excavated by a sea otter because: (1) the frequency of a sea otter excavating SSOR is very low (on order of 10−4 h−1); (2) both the sea otter and the Harlequin Duck feed exclusively underwater, either in the subtidal zone or in the intertidal zone when covered by the tide; thus, a recently excavated sea otter pit would not be visible to the Harlequin Duck above the surface; (3) if a Harlequin Duck foraged in a freshly excavated sea otter pit, the amount of contaminated prey collected would be only a small fraction of the prey collected during its daily feeding (i.e., the average surface area of a sea otter pit is about 0.25 m2 [Harwell et al. 2010a], a very small area compared to what the Harlequin Duck would forage over during a day); and (4) any such assimilated dose would be quite low and from a one-time event, so no chronic exposure could occur. Moreover, for there to be a risk to the Harlequin Duck population, there would have to be many thousands of such encounters over a prolonged period of time. Thus, we conclude that there is no plausible risk to Harlequin Ducks from direct exposure to SSOR. Consequently this route is not considered further here.
By contrast, routes of chronic exposure to PAHs in the Sound that occur through prey, sediments, and drinking water do warrant modeling to evaluate the assimilated doses that seaducks could receive. Each route is captured in the pathways conceptual model () and quantified in a simulation model using empirical data on PAH distributions in the PWS ecosystem. We next consider the potential sources of these PAHs.
Sources of PAHs in PWS Prey, Sediments, and Seawater
Hydrocarbons, including PAHs, derived from natural and anthropogenic sources are widespread in marine coastal environments (Brassell and Eglinton Citation1980; Neff Citation2002). Studies conducted in PWS and the Gulf of Alaska (GOA) prior to EVOS indicated that petrogenic PAHs existed in subtidal sediments (Kaplan and Venkatesan Citation1981). Page et al. (1995, 1996) conducted a comprehensive analysis of PAHs in PWS subtidal sediments following EVOS. The natural sources of petrogenic hydrocarbons in PWS include eroding Tertiary sedimentary rocks (petroliferous shales) and associated natural oil seeps in the eastern GOA. Current anthropogenic sources include oil residues from EVOS; oil releases from commercial- and pleasure-vessel operations (e.g., cruise liners, fishing fleet, Alaska Marine Highway ferries, oil tankers) (Page et al. Citation1995; Bence et al. Citation1996, Citation2007); spills of diesel and asphalt refined from Cook Inlet crude and Alaska North Slope Crude (Short et al. Citation1999, Citation2004; Page et al. Citation2002); and heavy Monterey fuel oil and asphalt released into the Sound from storage tanks during the 1964 Great Alaska Earthquake (Kvenvolden et al. Citation1993; USGS 2004). Hydrocarbons from eroding sedimentary rocks and natural seeps contain the same suite of PAH analytes as EVO, confounding interpretation. Fortunately, the relative amounts of individual PAH analytes from different sources are different and therefore can be used to characterize the various sources of PAHs (Bence and Burns Citation1995; Boehm et al. Citation1997; Burns et al. Citation1997).
Pyrogenic inputs of PAHs into PWS include combustion products from burning of coal, fuel oil, and wood; emissions from vessel engines; and atmospheric inputs from forest fires and global industrial sources (Page et al. Citation1995, Citation1999). In addition, historic human-activity sites, including recreation, fish-processing plants, sawmills, and mining activities, have released both petrogenic and pyrogenic hydrocarbons into PWS. For example, Boehm et al. (2004) reported TPAH concentrations of 2–12,056 μg·kg−1 (ppb) in mussel samples from 14 sites affected by past industrial activity but not oiled by EVOS, indicating that residual PAH from historical activities constitute a continuing source of bioavailable PAHs in PWS. Moreover, those authors found that TPAH values from the sampled human activity sites were at least an order of magnitude greater than those from any sampled non-human-activity sites, whether oiled by EVOS or not.
For the purposes of assessing the potential risks from EVO-derived PAHs, we focus on two categories each of prey, sediments, and seawater: (1) those collected on or near shorelines that were initially oiled by EVOS (labeled here oiled), which may or may not continue to have SSOR; and (2) those collected on or near shorelines that did not receive EVOS oiling (labeled reference). Even though the above discussion indicates that multiple sources of PAHs exist in PWS, we conservatively assume for now that any differences in the PAH concentrations between these two categories are attributable only to EVOS. By doing so, an upper bound of the current toxicological risks to PWS Harlequin Ducks from EVOS can be quantitatively and conservatively assessed. However, by quantifying the seaduck exposures to the individual PAH analytes from all pathways, we are able to evaluate the relative frequencies of each PAH within the assimilated TPAH doses. We then use the assimilated PAHs as fingerprints to gain insights into the various sources of PAHs, including EVO, that contribute to the PAH concentrations in the prey, sediments, and seawater, and, in turn, to the doses assimilated by the Harlequin Ducks in the model.
Model Parameterization
A quantitative model was developed to assess the toxicological risks using the simulation language Stella™ (ver. 9.1.2; © isee systems, inc. [www.iseesystems.com];) to simulate each exposure pathway shown in based on empirical data. The risk assessment model developed here is an individual-based model (DeAngelis and Gross Citation1992) in which a Harlequin Duck is assigned characteristics reflective of PWS seaducks and each plausible pathway of exposure is simulated using data that reflect the measured PAH concentrations in the various media in PWS. During each daily time step, the model randomly samples from lognormal distributions that capture the variability of conditions in PWS and calculates the assimilated doses for the individual seaduck from each of the three pathways of exposure to PAHs (prey, sediments, and seawater) (). The Stella™ random lognormal function in the model was chosen because the measured PAH concentrations in prey tissues, sediments, and seawater were bounded by zero, skewed to the right, and shown to be lognormally distributed (Evans et al. Citation1993) when plotted as Q-Q plots (Venables and Ripley Citation2002), a common situation for environmental data.
Each of 40 PAH analytes was modeled separately, and the daily assimilated dose was reported for each PAH and for TPAH. By repeating the simulation for millions of seaduck-days, the full distribution of assimilated doses was projected, capturing the range of variability in daily exposures to the Harlequin Duck population that would be expected in PWS. These daily assimilated doses were converted into average daily doses that are comparable to relevant chronic toxicity reference values in order to assess the potential for individual-level health effects. Several model parameters were also varied in a suite of sensitivity analyses to explore the effects of uncertainty or alternate model parameters.
The risk assessment model was parameterized based on the following information.
Prey
For the route of exposure via ingestion of prey, the relative frequency of prey species in the diet of PWS Harlequin Ducks was taken from the mean percent length-importance index reported in Patten et al. (2000) (). The quantity of prey consumed per day was modeled by: (1) estimating the daily energetics needs of the Harlequin Duck; (2) apportioning that quantity to the prey categories based on their relative frequencies in the diet; and (3) calculating the mass of each prey category needed to contribute the assigned energy required, based on the energy density of each prey species. Once the mass of prey tissues is calculated, the model assigns a value during each time step for the PAH analytes in each prey-tissue category by randomly sampling from a lognormal distribution of TPAH values parameterized using the mean, standard deviation, and PAH distributions from the empirical data for prey tissues collected in PWS at oiled and reference sites (discussed below).
Thus, modeling this pathway required the parameterization of: (1) the body mass of the various categories of ducks (); (2) the a and b fitted-parameters for the FMR (Equation 1) from Nagy et al. (1999) for the marine birds category (specifically a = 14.25, and b = 0.659); (3) the energy density of the prey tissues; and (4) the measured concentrations of each PAH analyte in each prey category.
The energy density (in J·mg dry weight−1) of each prey category was estimated from published data (Cummins and Wuycheck Citation1971; Wacasey and Atkinson Citation1987; Bishop and Green Citation2001; Dean et al. Citation2002; McKinney et al. Citation2004; Elliott and Gaston Citation2008; Cummins pers. comm.) for species identical or similar to those recorded in the Harlequin Duck diet (Patten et al. Citation2000) and aggregated into the six prey categories parameterized in the model ().
Table 4 Energy density of prey categories in the Harlequin Duck risk model.
All analyses of the concentrations of each of 40 PAHs in prey tissues (as well as sediments and seawater, discussed below) were conducted at Battelle Ocean Science, Duxbury, MA, using methods described elsewhere (Page et al. Citation1995; Douglas et al. Citation2004; Neff et al. Citation2006; Boehm et al. Citation2007b). The samples were solvent-extracted, cleaned up by column chromatography (sediment and tissues only), and analyzed by gas chromatography/mass spectrometry (GC/MS) following USEPA SW-846 Method 8270 (USEPA 2001), modified to include the alkylated PAHs and with selected ion monitoring (SIM) to achieve lower detection limits (following e-CFR 2009). Corrections for surrogate recoveries were applied to all analytes. Method Detection Limits (MDL) were calculated following USEPA (2001). If a sample was recorded as non-detected, the PAH concentration was assigned the value of ½ MDL, the best estimate to use following USEPA (1991). The reported TPAH is the sum of the 40 analytes. (Note, we did not include the parent compound non-alkylated or N(0) naphthalene in the TPAH because this analyte is a very common low-level laboratory contaminant, found in many cleaning agents. When sample PAH concentrations are very low, as they are in most prey tissues, the N(0) naphthalene can erroneously become a disproportionate component of TPAH. Not including this analyte in the TPAH values in the model makes no difference in the results or conclusions.) From these data, the means, standard deviations, and relative proportions of each PAH analyte in each pathway were used to parameterize the model. All of the PAH concentration data for prey tissues, sediments, and seawater used in the Harlequin Duck risk assessment model are available at http://www.valdezsciences.com/polycyclic_aromatic_hydrocarbon.cfm.
The PAH concentrations in prey tissues (A) were measured in prey samples collected in 2002–2008; these are the most-recent relevant data available and are conservatively assumed to represent the current PAH concentrations in the environment. The prey samples were collected from oiled and reference sites in PWS as follows: Gastropod data were from samples of Littorina and whelks (Nucella sp.) (Neff et al. Citation2006). Mussel data were measured in Mytilus trossulus samples (Boehm et al. Citation2004; Neff et al. Citation2006). The clam data were derived from two clam species (Protothaca staminea and Saxidomus giganteus). The crustacean data were measured in hermit crab samples (Pagurus beringanus) (Neff et al. Citation2006). The fish egg data were for salmon eggs (Oncorhynchus sp.) (Brannon et al. Citation2007). PAH concentrations in the category other invertebrates were based on polychaete worm samples (Nereis sp.) (Neff et al. Citation2006).
Table 5 Prey, sediments, and seawater tpah data for the Harlequin Duck risk model.a,b
Sediments
The model estimates the PAH doses from the sediment pathway, in which the seaduck consumes sediments at an assigned fraction (5.2% by weight) of the total food ingested. This value was based on the Beyer et al. (2008) measurements of the mean percent sediment in the diet of Common Goldeneyes. A sensitivity analysis doubled this fraction to 10.4%. The PAH concentrations in sediments were based on grid samples collected in the ITZ from oiled and reference sites in PWS (B). The sediment sampling procedure (described in Page et al. Citation2005) for both the oiled and reference sediments was to dig a 50-cm-deep pit (or to bedrock if less than 50 cm), collect the fine-grained fraction of sediments from the full height of the sides of the pit walls, and homogenize these sediments to create a mixture representative of the 50-cm column. Because these are the only appropriate sediment data available, they are used to represent the surface sediments even though most of each sample comes from much greater sediment depths than a Harlequin Duck could contact (and, thus, this sampling procedure provides an overestimate of the concentrations of oil residues that seaducks could encounter). Approximately 300 g (wet weight) of sediment was collected from each pit. The homogenized sediments were aliquoted, and ∼35–50 g of each was extracted and analyzed by GC/MS for PAHs following the laboratory procedures described by Neff et al. (2006).
The oiled sediment samples were taken in 2007 from shoreline segments on Knight, Smith, and Eleanor Islands that had been oiled by EVOS and surveyed for the presence or absence of SSOR. In the surveys, the amount of SSOR oiling present was assigned based on a qualitative visual assessment (e.g., the category dark colors film [HAZMAT 1996] was considered heavy oil residue [HOR]). If no visible oil was seen (i.e., no sheens, pore fillings, or films on sediment particles), then the sampled pit was assigned the NONE category. From the survey data, we selected the NONE category of sediments to represent the oiled site-sediments because the Harlequin Duck risk pathways do not involve direct contact with SSOR; thus, the other categories of SSOR would not be appropriate. These samples are conservative because the PAH concentrations in sediments from shorelines that had SSOR are likely to be higher than those from shorelines in which no SSOR was found. Additionally, the PAH concentrations in the 50-cm sediment column are likely to be higher than the PAH concentrations at the surface where seaducks forage because of the natural loss of oil from near-surface sediments (Hayes and Michel Citation1999). Reference sediment samples were collected in the same manner in 2002 and 2004 from shorelines on Green, Knight, Little Green, and Storey Islands that were never oiled by EVOS.
Seawater
The model also estimates assimilated PAH and TPAH doses contributed from seawater (C) ingested by the Harlequin Duck at the rate of 117 mg·kg body weight−1·day−1. This is a conservative estimate based on the freshwater drinking rate for Mallards measured by Fletcher and Holmes (Citation1968) and is consistent with analyses by CSL (2007).
Seawater samples were collected in 2005 in the NKI area of PWS as 2.8-L samples taken at 1-m depth from nearshore sites (10–50 m offshore) midway through a falling tide when water was flowing off the shore (Boehm et al. Citation2007b). The 1-m depth is appropriate for the potential drinking water as Harlequin Ducks typically dive into the water column when feeding and it is likely that consumption of seawater occurs during feeding. Collecting the water at this depth avoids potential contamination from a surface film or bottom sediments, which are not the likely locations at which seaducks drink seawater. The shorelines at all of the sampling sites had been initially oiled by EVOS, as these were the only appropriate data available (i.e., no data were available from unoiled shorelines). The oiled seawater samples were taken from sites near shorelines that contained SSOR as found in the NOAA 2001 or 2003 surveys (EVOSTC 2008). The reference seawater samples were taken from sites near shorelines that did not contain SSOR as indicated in the joint Exxon–ADEC surveys in 1990 and 1991 (ADNR 1996a,b; ).
Calculating the Assimilated Dose in the Risk Assessment Model
During each daily time step in the model, an individual Harlequin Duck consumes a specified mass of prey tissues, sediments, and seawater, each containing TPAH at model-selected concentrations based on lognormal distributions derived from the empirical data from PWS. This stochasticity allows the simulated population of Harlequin Ducks to be exposed to the TPAHs analogously to how the chemicals are actually heterogeneously distributed in the environment of PWS. The specific mass of each PAH is also calculated by applying the relative frequency of the TPAH contributed by each of 40 PAH analytes, again based on the empirical data for prey tissues, sediment, and seawater from PWS. All ingested PAHs are assumed to be completely bioavailable (i.e., 100% assimilation efficiency), a conservative assumption for sediment-bound PAHs because of solubility and sediment-partitioning constraints (Di Toro and McGrath Citation2000). Using this information, the model calculates the assimilated dose during each time step for each PAH ingested by the seaduck from the prey-, sediment-, and seawater-based routes of
exposure.
We followed this approach to simulate separately a total of 500,000 individuals for each of eight classes of Harlequin Ducks (both summer and winter seasons for adult female, adult male, juvenile female, and juvenile male categories), generating an initial distribution of daily assimilated doses (in mg·kg body weight−1·day−1) for each PAH analyte and for TPAH. From this model output initial distribution, 30 days were randomly sampled with replacement to generate a secondary distribution of average daily assimilated dose for a month in the life of a Harlequin Duck in PWS. This sampling process was repeated 5 times in sets of 100,000 samples each, thereby representing a total population of 500,000 seaducks of each class living under PWS-specific conditions. The purpose of this simulation and post-processing approach is to convert the initial model-generated daily assimilated doses into a month-long period of average daily assimilated doses. The latter is required in order to compare the modeled doses with the chronic TRVs to assess effects. A hazard quotient is the ratio of an assimilated dose to the TRV, where HQ ≥ 1 indicates the exposure could lead to chronic effects and HQ < 1 indicates no ecologically relevant adverse chronic effect would occur.
Using this individual-based modeling approach (DeAngelis and Gross Citation1992) to generate a large population of Harlequin Ducks allows the identification of the maximum-exposed individuals for a conservative assessment of risks, capturing the variability that exists in the PWS environment. The model outputs also allow the assimilated dose to be partitioned into the relative contributions by each of the three exposure pathways and the relative frequency distribution of the PAH analytes assimilated into the Harlequin Ducks. This partitioning provides the quantitative basis to assess the relative importance of each route of exposure.
Each model (i.e., the eight base models and the eight models for each of the eight sensitivity analyses discussed below) was subject to extensive quality assurance (QA) to examine model structure, equations, parameters, data sources, documentation, and each simulation output, following a USEPA-approved QA plan developed by the authors for a previous model. All models, parameters, QA runs, and simulation outputs have been archived for further analysis and reproducibility. Altogether, 72 scenarios were simulated for each of the oiled and reference situations, representing a total modeled population of more than 7 × 106 Harlequin Ducks living under PWS conditions or under variations of the environment to test model
sensitivities.
Sensitivity Analyses
The base models, as described earlier, constitute the conservative, quantitative assessment tool that uses the best-available information on Harlequin Duck characteristics and behavior, the measured concentrations of PAHs as distributed in media within PWS, and the plausible routes of PAH exposures to the seaducks. In order to explore how changes in model parameters would affect the outputs, we conducted a series of sensitivity analyses. In each case, only a single parameter was changed to allow direct comparison with the base model. The following sensitivity analyses were conducted:
| 1. | No MDL. Rather than substitute the value of ½ MDL for non-detects in the analysis of each prey tissue sample, the value of zero was used. This sensitivity analysis allows examination of the effect of differential detection limits across various PAHs on the model-projected distribution of PAHs in the assimilated doses, and illustrates the measured prey-tissue PAH concentrations vis à vis background concentrations. | ||||
| 2. | Full MDL. In this sensitivity analysis, the value of the non-detects was set at the MDL value, providing the upper bound of PAH concentrations in the various media. | ||||
| 3. | Upper 10%. The daily assimilated dose outputs from the base model were rank-ordered and the highest 10% (based on TPAH concentrations) became the distribution from which the average daily assimilated doses were derived. This approach simulates the doses that would be received by individuals who remained at a location that happened to have elevated PAHs compared to other sites, providing a conservative upper-bound estimate of assimilated doses based on larger-scale spatial heterogeneity. | ||||
| 4. | +KN136. To the base-model PAH data set for prey tissues, we added PAH samples collected at the KN136 site, a small (∼0.1 ha), highly non-representative shoreline for PWS (NOAA 2007) of semi-enclosed intertidal peat bog containing residual EVO. Although this area is poor habitat for Harlequin Ducks, and thus is not included in the base model data, this sensitivity analysis was conducted to assess the potential effects on Harlequin Ducks who hypothetically foraged at this site. When the KN136 samples were included in the input data, the oiled prey-tissue PAH concentrations increased 3.1 times for mussels (mean = 142.48 ppb; standard deviation = 507.20), 1.2 times for clams (mean = 90.94 ppb; standard deviation = 138.50), and 2 times for other invertebrates (mean = 214.89 ppb; standard deviation = 566.65); all other prey concentrations remained the same as reported in . | ||||
| 5. | Doubled FMR. The FMR was doubled to simulate the range of energetics needs as discussed previously with respect to environmental, molting stress, or other factors. | ||||
| 6. | Doubled sediments. To examine the effect of uncertainty in the amount of sediments consumed by the seaduck, the proportion of sediments in the diet was doubled (to 10.4% by weight). | ||||
| 7. | Mussels only. To assess the effect of uncertainty in the distribution of prey species in the diet, the prey category Mussels was assigned to be 100% of the diet. This sensitivity analysis is comparable to the assessment presented in Boehm et al. (2004) and is consistent with the concerns of Patten et al. (2000) about PAHs in mussels. It captures the assimilated doses that could result from a Harlequin Duck feeding exclusively in mussel beds, a habitat that typically had higher PAH concentrations than other seaduck habitats after the oil spill. | ||||
| 8. | HQ = 1 Sensitivity analyses. A final set of sensitivity analyses was conducted on the adult female/winter model to explore the range of TPAH concentrations in the PWS environment that would be necessary in order to reach an HQ = 1 for each threshold (i.e., NOAEL and LOAEL). In each sensitivity simulation, a multiplicative factor was applied to the extant PWS environmental TPAH values to generate hypothetical environmental TPAH concentrations for each route of exposure. One series of multiplicative factors was selected to bracket the NOAEL HQ = 1 threshold and another series to bracket the LOAEL HQ = 1 threshold. Because the model-projected doses are essentially linear with the PAH concentrations of the input sources, we used the results of the base-model simulations to estimate the range of multiplicative factors to apply to the extant PWS environmental TPAH values for the sensitivity analyses. A single multiplicative factor was selected for each sensitivity analysis and applied as follows: For each time step and for each exposure source (i.e., sediments, seawater, and each prey category), the base-model-assigned TPAH value was multiplied by the specified factor to create the sensitivity-analysis-assigned TPAH value. The resulting model-generated assimilated dose was converted to individual-level effects for varying fractions of the individuals within the simulated population (ranging from the 99.9% quantile [maximum-exposed individual] down to the 50% quantile [median individual]). The latter allows conversion of the model-generated individual-level effects to expected population-level effects. | ||||
RESULTS AND RISK CHARACTERIZATION
Results of Quantitative Modeling
The results of the individual-based modeling and the post-processing to derive a population of chronic doses of PAHs for each class of Harlequin Ducks are shown in . We report the average daily assimilated dose of TPAH (in mg·kg−1·day−1), showing the mean, median, and 99.9% quantile highest exposures, the latter representing the maximum-exposed individuals (i.e., the 1-in-1,000th highest exposed individuals in the population of 500,000 Harlequin Ducks that was simulated). By focusing on the maximum-exposed individuals, we present a very conservative assessment of potential risks to the Harlequin Duck population of PWS. By reporting the paired results for oiled and reference conditions and conservatively assuming that all differences between the two are attributable solely to EVO-derived PAHs, we obtain an upper-bound of plausible continuing risks attributable to EVOS. The actual risks are likely to be smaller.
Table 6 Harlequin Duck model results (base model) average daily dose TPAH (mg·kg−1·day−1) to simulated population of 500,000 Harlequin Ducks.
In (and the other model-output tables) is reported the coefficient of variation (CV) across the five sets of secondary distributions that were created. These CVs are very low in all scenarios, demonstrating that there is a sufficient number of simulations to represent accurately the variability of PAHs in the environment as captured in the assimilated doses. The distributions across individuals in the population are relatively narrow: the mean and median values are almost identical within each scenario, and the 99.9% quantile value is only modestly higher than the associated mean values (∼33% for oiled and ∼21% for reference). In all cases, the oiled mean and median values are ∼25% higher than the associated reference values, and the 99.9% quantile values are ∼38% higher for the oiled than the paired reference ().
We show in the HQs for each of the NOAEL and LOAEL TRVs for both the modeled oiled and reference conditions for the maximum-exposed Harlequin Duck. The actual threshold of effects is expected to be between the two values, with an HQ based on the NOAEL value being more conservative. The table also shows the multiplicative factor less than the effects threshold for each HQ value (i.e., 1 ÷ HQ).
Table 7 Hazard quotients for maximum-exposed Harlequin Ducks.a,b
These quantitative projections from the base model constitute our conservative best estimate of chronic doses and effects that could occur to the maximum-exposed Harlequin Ducks in PWS. These results () demonstrate that the NOAEL and LOAEL hazard quotients for the 1-in-1,000th highest-exposed individuals at oiled sites are about 2.5 × 10−3 and 2.5 × 10−4, respectively, which is approximately 375–4,500 times lower than the no-effects thresholds. Similarly, the NOAEL and LOAEL HQs for the reference sites are about 1.8 × 10−3 and 1.8 × 10−4, respectively, or approximately 520–6,200 times lower than the no-effects thresholds. Juvenile seaducks have slightly higher exposures than the adults, but there is little variability across age/gender classes or between seasons. Because these HQs are so low and represent the 99.9% quantile highest exposures, it is extremely unlikely that any of the projected exposures represent a health risk to any Harlequin Duck. The differences between reference and oiled doses and effects are modest: in all cases the reference effects are about 72% of the oiled effects (compare the paired Oiled and Reference data in ).
The extremely low probability of individual effects is further demonstrated by examination of the cumulative frequency distribution of the average daily assimilated doses for the adult female in winter across all 500,000 simulations each for the oiled and reference conditions (; similar results occurred for all of the other modeled classes). The full range of the simulations covers only a factor of 2.3 for oiled sites (0.00276–0.00654) and a factor of 1.8 for reference conditions (0.00226–0.00397). This is in marked contrast to the separation of the assimilated doses from the NOAEL and LOAEL TRVs (more than 2–3 orders of magnitude, respectively). Because the maximal single seaduck (i.e., the highest one out of all 500,000 individuals simulated for the adult females in winter) had an assimilated average daily dose of 0.00654 mg·kg·day−1, its NHQ and LHQ were 3.06 × 10−3 and 2.97 × 10−4, respectively, or 327 and 3,367 times lower than the HQ = 1 thresholds. This represents the extreme upper end of the population of 500,000 seaducks.
Figure 3 Cumulative frequency distribution of average daily assimilated doses (in mg·kg−1·day−1) for Harlequin Ducks under oiled and reference conditions. Base model results for adult female Harlequin Duck in winter (n = 500,000) (note: very similar results occurred for all other classes of seaducks). Also shown are the NOAEL and LOAEL toxicity reference values, and the 99.9% quantile of assimilated doses (maximum-exposed individuals). Note that all model input PAH concentration data for PWS prey tissues, sediments, and seawater are available online at http://www.valdezsciences.com/polycyclic_aromatic_hydrocarbon.cfm.
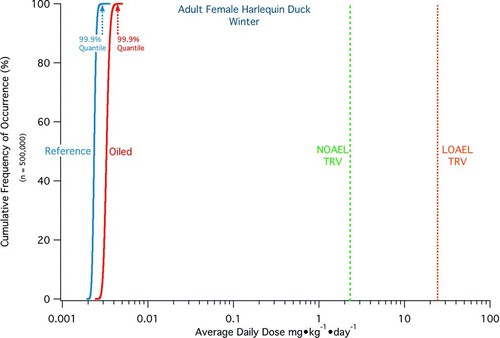
The simulation results for the assimilated average daily doses projected under the various sensitivity analyses are presented in –. Shown in and are the NOAEL and LOAEL HQs for the oiled and reference sensitivity analyses, respectively.
Table 8A Harlequin Duck model results: No MDL sensitivity analysis. Average daily dose TPAH (mg·kg−1·day−1).
Table 8B Harlequin Duck model results: Full MDL sensitivity analysis. Average daily dose TPAH (mg·kg−1·day−1).
Table 8C Harlequin Duck model results: Upper 10% sensitivity analysis. Average daily dose TPAH (mg·kg−1·day−1).
Table 8D Harlequin Duck model results: +KN136 sensitivity analysis. Average daily dose TPAH (mg·kg−1·day−1).
Table 8E Harlequin Duck model results: Doubled FMR sensitivity analysis. Average daily dose TPAH (mg·kg−1·day−1).
Table 8F Harlequin Duck model results: Doubled sediments sensitivity analysis. Average daily dose TPAH (mg·kg−1·day−1).
Table 8G Harlequin Duck model results: Mussels only sensitivity analysis. Average daily dose TPAH (mg·kg−1·day−1).
Table 9 Sensitivity analyses hazard quotients oiled maximum-exposed Harlequin Ducks.
Table 10 Sensitivity analyses hazard quotients reference maximum-exposed Harlequin Ducks.
Allocation of Risks across Pathways
Within each simulation, the model accumulates the PAHs assimilated from each of the three pathways: prey, sediments, and drinking water. By summing across all simulations for a given pathway within a scenario, the relative contributions of the pathways can be determined (). These results demonstrate that consumption of prey constitutes the dominant pathway (∼90%), most of the remainder of the doses derives from sediments (∼10%), and seawater provides only a trivial dose of PAHs (≤0.03%). The values reported here are for adult female Harlequin Ducks; however, results did not differ by age/gender class within a scenario, indicating that the pathway apportionment is independent of the age/gender characteristics of the seaducks. This result is further demonstrated by the close similarity shown between the proportions for the base model and the model with doubled FMR (), representing a sensitivity analysis on a Harlequin Duck characteristic. In contrast, differences appear in the proportions when different source PAH concentrations are used, as in the No MDL, Full MDL, and +KN136 sensitivity analyses.
Table 11 Allocation of assimilated doses of PAHs across exposure pathways for adult female Harlequin Duck in winter with “oiled” exposuresa.
Distribution of PAHs in Assimilated Doses
The relative frequency of the individual PAH analytes is calculated by summing the assimilated dose of each analyte across all 500,000 simulations within a scenario. In essence, the Harlequin Ducks act as integrators of the PAHs from the various sources in PWS, so that the modeled distributions of assimilated PAHs provide diagnostic information on the sources of PAHs that are ingested by the seaducks. Illustrated in – are the PAH distributions under oiled and reference conditions for the assimilated doses in adult females, winter scenario, in the base model and in the No MDL sensitivity analysis (the other age/gender classes have very similar distributions). Those PAHs assimilated from the base-model prey pathway ( and 4B) are graphically distinguished from those derived from the base-model sediments pathway ( and 6B). The PAH distributions from the prey pathways in the No MDL sensitivity analysis are shown in and 5B. Seawater is not shown because of its trivial contribution to the total assimilated dose (Table 11).
Figure 4 Relative frequencies of 40 PAH analytes in assimilated doses from prey pathways (adult female winter base model). Frequencies calculated as portion of TPAH contributed by each PAH analyte. Base model results for adult female Harlequin Duck in winter (n = 500,000) (note: very similar results occurred for all other classes of seaducks). (A) Assimilated PAHs from oiled-site prey pathways only. (B) Assimilated PAHs from reference-site prey pathways only.
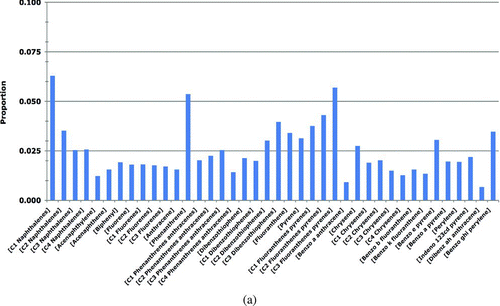
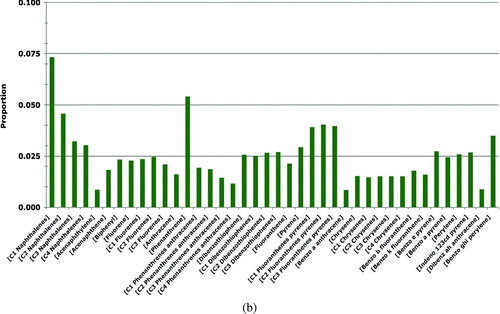
Prey-Pathway PAH Contributions
The close similarity between the oiled and reference distributions of PAHs in the base model's assimilated doses in the Harlequin Ducks from the prey pathway indicates that oiled-site prey () can no longer be distinguished from reference-site prey (). That is, the prey-derived assimilated doses, which constitute ∼90% of the total dose, exhibit concentrations and distributions of PAHs that are essentially identical to background. These two distributions suggest that there was little if any remaining signal from EVOS in prey tissues by the time the samples were collected (2002 through 2007); the small differences between the oiled-site and reference-site distributions do not reflect significant amounts of additional petrogenic PAHs in the oiled sites, whether from EVOS or other petrogenic sources.
The role of the Method Detection Limits is very important in projecting the assimilated doses from the prey-based pathway (as well as the seawater-based pathway). This is because about 75% of the PAH analytes in the prey samples collected from oiled- and reference-site locations were less than their specific MDL values (), reflecting the fact that the background concentrations of individual PAH analytes in PWS prey are very low, barely above their detection limits. In fact, none of the 87 gastropod, crustacean, or fish samples from either oiled or reference sites had any TPAH values that exceeded their respective detection limits (calculated as the sum of the detection limits of the individual PAHs). Similarly, seawater analyses were more than 90% non-detects. Only in sediment samples, discussed below, did the majority of the analytes exceed the respective detection limits.
Table 12 Frequency of PAH analytes in samples below analytical detection limits.a,b
As a net result of the large fraction of non-detects in the primary route of exposure (i.e., the prey tissues in the Harlequin Duck diet), the modeled assimilated doses to Harlequin Ducks were substantially driven by the values substituted for the non-detects in the input data. A separate analysis of PAH proportions was conducted on prey-tissue PAH concentration data with “0” values for the non-detects (); this was done in order to remove any effect that might ensue from the artifact of differential MDL values across PAH analytes. The PAH distribution for the prey tissues from both oiled sites () and reference sites () is dominated by pyrogenic PAHs, as evidenced by elevated concentrations of parent compounds (fluorene, phenanthrene, fluoranthene/pyrene, and chrysene; labeled with red arrows) relative to their alkylated homologs. The oiled site-prey also demonstrate a minor weathered petrogenic component as evidenced by the progressively increasing homologous series of alkylated dibenzothiophenes and alkylated fluoranthenes/pyrenes (ascending arrows in ). However, the distribution of chrysenes is pyrogenic (C0 > C1 > C2 > C3 > C4) (descending arrow in ) and not that of weathered EVO (e.g., Bence et al. 2007). The relative abundances of the C2- and C3-dibenzothiophenes (labeled Alk Diben) relative to the C2- and C3-phenanthrenes (labeled Alk Phen) suggest a possible correlation to North Slope Alaska crude (Page et al. Citation1995). The distribution of PAHs for the reference-site prey () indicates a minor biogenic component (perylene, labeled Per) and possibly a trace of petrogenic components. Perylene is a naturally occurring 5-ring PAH generated through biogenic processes and through the diagenesis of organic matter in sediments (Venkatesan Citation1988).
Figure 5 Relative frequencies of 40 PAH analytes in assimilated doses from prey pathways only—sensitivity analyses of adult female winter model with No MDL substitutions. Frequencies calculated as portion of TPAH contributed by each PAH analyte. Model results for adult female Harlequin Duck in winter (n = 500,000) without the ½ MDL substitution for non-detects in the input PAH data (i.e., recorded as “0”; see http://www.valdezsciences.com/polycyclic_aromatic_hydrocarbon.cfm) (note: very similar results occurred for all other classes of seaducks). (A) Assimilated PAHs from oiled prey pathways only. Note that C(1)Naphthalenes value = 0.140, and Phenanthrene value = 0.120; y-axis scale here truncated to show other PAHs more clearly. Red arrows represent parent compounds; ascending arrows represent homologous series with increasing concentrations; descending arrow represents homologous series with decreasing concentrations. (B) Assimilated PAHs from reference prey pathways only. Note that C(1)Naphthalenes value = 0.252, and Phenanthrene value = 0.186; y-axis scale here truncated to show other PAHs more clearly. Red arrows represent parent compounds; descending arrow represents homologous series with decreasing concentrations.
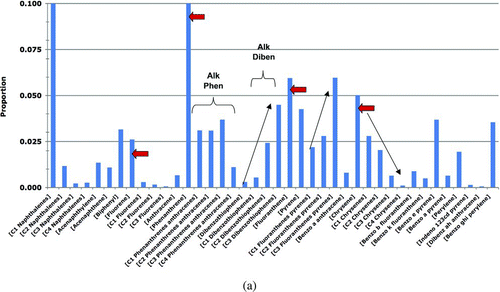
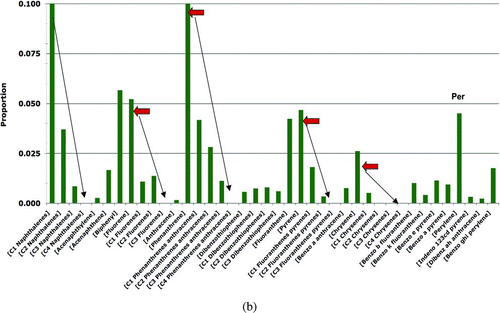
Thus, the analyses using “0” values for non-detects, rather than the ½ MDL, support the previous conclusion based on assessing the PAH distributions derived from the base model: if there remains any EVOS contribution to the prey PAHs, it is very limited and clearly is not now a major component of the PAHs in prey tissues.
Sediment-Pathway PAH Contributions
In contrast to the pattern seen for the prey-derived PAHs, the sediments-derived PAHs are not significantly influenced by detection limits (), and the ½ MDL substitution for the few non-detects that did occur has no effect on either the PAH distributions or the TPAH values in the model-input sediment data. The PAH distribution for the oiled-sediment pathway (), which constitutes <10% of the total assimilated dose (), has both petrogenic and pyrogenic components. The petrogenic component contains abundant alkylated phenanthrenes (labeled Alk Phen in ), dibenzothiophenes (labeled Alk Diben), and C2–C3 fluoranthenes/pyrenes (labeled Alk FP). This component is heavily weathered, as indicated by depleted naphthalenes (labeled Naph) and fluorene homologs (labeled Fluo), depleted C0-phenanthrene and C0-dibenzothiophene, and a heavy PAH [HPAH]/Total PAH ratio of ∼0.7. These sediment-pathway PAHs also show the diagnostic PAH ratios of EVOS-derived residues, specifically C2-dibenzothiophene/C2-phenanthrene and C3-dibenzothiophene/C3-phenanthrene ratios are 0.9–1.13 (Page et al. Citation1995). The pyrogenic component in the oiled sediments contains abundant parent chrysene (labeled C0) and other 4–6-ring PAHs.
Figure 6 Relative frequencies of 40 PAH analytes in assimilated doses from sediment pathways (adult female winter base model). Frequencies calculated as portion of TPAH contributed by each PAH analyte. Base model results for adult female Harlequin Duck in winter (n = 500,000) (note: very similar results occurred for all other classes of seaducks). (A) Assimilated PAHs from oiled-site sediments pathways only. Ascending arrows represent homologous series with increasing concentrations; descending arrows represent homologous series with decreasing concentrations. Alkylated naphthalene homologs labeled Alk Naph; alkylated fluorene homologs labeled Alk Fluo; alkylated phenanthrene homologs labeled Alk Phen; alkylated dibenzothiophene homologs labeled Alk Diben; alkylated fluoranthene/pyrene homologs labeled Alk FP; HPAH = High-Molecular-Weight PAHs (having 4–6 rings). (B) Assimilated PAHs from reference-site sediments pathways only. Note that for the reference sediments, the PAH analyte Perylene value = 0.252; y-axis here truncated to show other PAHs more clearly. C2-dibenzothiophene labeled D2; C2-phenanthrene labeled P2; perylene labeled Per; HPAH = High-Molecular-Weight PAHs (having 4–6 rings). (Continued)
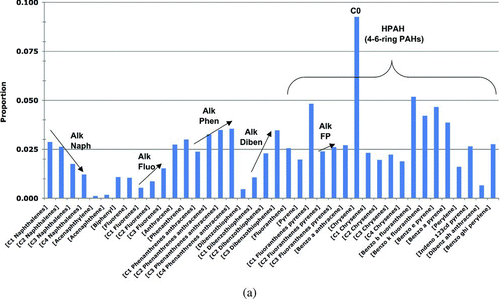
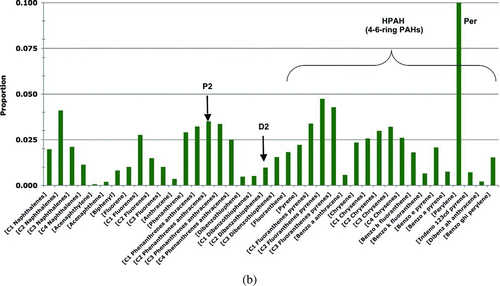
For the reference-site sediments pathway (), the dominant PAH is perylene (∼25% of TPAH). Visual observations indicated that the reference sediment samples had particularly high organic carbon content, which could support high microbial activity, a likely explanation for the very high levels of perylene. The assimilated doses derived from reference-site sediments also have a high HPAH/TPAH ratio (0.58), driven largely by a very high perylene concentration relative to the other 4- to-6-ring PAHs. Reference-site sediments also have a small petrogenic component. The relative proportions of the alkylated phenanthrenes and dibenzothiophenes (e.g., C2-dibenzothiophene [labeled D2]:C2-phenanthrene [labeled P2] ratio ∼0.2) suggest that this component could be related to the natural petrogenic background, rather than to EVOS residues (Page et al. Citation1995; Bence et al. Citation1996).
These results indicate that some of the sediment samples at oiled sites (6 of the 51 sediment samples analyzed) showed an EVOS signal in the constituent PAHs when collected in 2007, with TPAH concentrations on average about double that of the background (non-oiled) sediments (B). However, sediment-derived PAHs contribute less than 10% of the total assimilated dose to the modeled Harlequin Ducks (i.e., the sediment-derived doses, if occurring alone, would result in assimilated doses that are 3 and 4 orders of magnitude less than the NHQs and LHQs, respectively). Thus, the continuing EVOS signal seen in some sediment PAHs is not ecologically significant.
Sensitivity Analyses
The sensitivity analyses (–) illustrate how differing model parameters affect the results of the simulations. The sensitivity analyses in which the concentrations for every non-detectable PAH in the prey tissues were set to zero, rather than the ½ MDL substitution of the base model, resulted in a reduction in the HQs by about one-half for the maximum-exposed Harlequin Ducks in oiled locations and by about two-thirds for the reference locations. Similarly, in the Full MDL sensitivity analyses, in which the non-detects were assigned the MDL values, the results were 50% higher than the base case for oiled and about 75% higher for reference scenarios. These results reflect the fact that the background PAH concentrations in prey tissues, whether in oiled locations or elsewhere in PWS, are very low, essentially close to the detection limits in most cases. Substituting the ½ MDL value for non-detects is the best estimate of the actual concentrations, but they may be anywhere from 0 to up to the full MDL, although, in many cases, PAH concentrations were in fact reported substantially less than the MDL. The significant changes in HQs when not assigning non-zero values to the non-detects indicates how much the estimated risk is directly attributable to the detection-limit issue.
The relative frequencies of PAHs in assimilated doses for all pathways for the Upper 10% adult females (winter scenario) () reveals increasing relative proportions of C1-C3 fluorenes (labeled Alk Fluo), C1-C3 phenanthrenes (labeled Alk Phen), C1-C3 dibenzothiophenes (labeled Alk Diben), C1-C3 fluoranthene/pyrenes (labeled Alk FP), and C1-C4 chrysenes (labeled Alk Chrys). In addition, the ratio C2-dibenzithiophene/C2-phenanthrene is ∼1.2. These trends are consistent with the presence of weathered EVO. A slight increase in the proportions of alkylated chrysenes relative to the base case (i.e., using the base model to simulate the adult female, winter scenario; compare to ) probably reflects a slightly increased contribution from weathered EVO in the Upper 10% scenario. The Upper 10% sensitivity analyses result in an increased risk by ∼88% for the maximum-exposed individuals, similar to the Full MDL sensitivity analyses. The HQs remain about 200–230 times lower than the NOAEL and about 2,000–2,400 times lower than the thresholds of observed effects. This sensitivity analysis result means that even if a Harlequin Duck fed exclusively at a site that was at the upper 10% quantile of PAH concentrations, there would still an additional 2–3 orders-of-magnitude increase in exposures required to cause a significant risk from residual PAHs.
Figure 7 Relative frequencies of 40 PAH analytes in assimilated doses from all pathways under oiled conditions—upper 10% sensitivity analyses. Frequencies calculated as portion of TPAH contributed by each PAH analyte. Model results for adult female Harlequin Duck in winter (n = 500,000) under the Upper 10% scenario (see text for details) (note: very similar results occurred for all other classes of seaducks). Ascending arrows represent increasing homologous series; alkylated fluorene homologs labeled Alk Fluo; alkylated phenanthrene homologs labeled Alk Phen; alkylated dibenzothiophenes homologs labeled Alk Diben; alkylated fluoranthene/pyrene homologs labeled Alk FP; alkylated chrysene homologs labeled Alk Chrys.
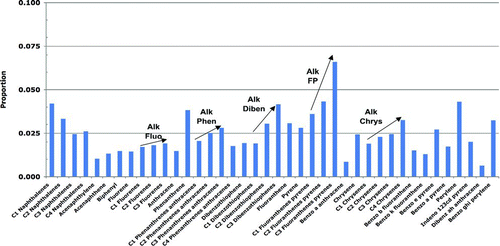
Similarly, including the prey-tissue data from the peat bog site (KN136) results in an increase in risk compared to the base case by a factor of ∼3.7, with the resulting HQs about 100 times and 1,000 times lower than the NOAEL and LOAEL thresholds, respectively. Under that scenario, an additional increase by 2–3 orders of magnitude would still be required to reach an effect of concern. The mean values of exposures and HQs (i.e., the exposures to the 50% quantile individuals) increased by only about 40% above the base case, reflecting the importance of the few higher-PAH concentrations on the upper tail of the distribution of assimilated doses.
The sensitivity analysis in which the metabolic rates for the modeled seaducks were doubled resulted in doubling both the oiled and reference effects, because the individuals in each location consumed twice as much prey and sediments, thereby doubling the assimilated dose. Under this higher-metabolic-rate scenario, the HQs remain about 200 times and 2,000 times lower than NOAEL and LOAEL thresholds, respectively, clearly not changing the conclusions from the base model results. When the proportion of the diet consumed as sediments was doubled, the doses to the maximum-exposed seaducks increased by ∼23%. When the diet was assigned to be 100% mussels, the doses to the maximum-exposed seaducks increased by ∼18%, indicating that the results are not sensitive to variability in the Harlequin Duck's coastal marine diet. Thus, each of these results again shows only a modest elevation in risk from the sensitivity analyses.
The results of the sensitivity analyses that were designed to reach the effects thresholds are shown in and . In these simulations, the input TPAH values were heuristically increased by selected multiplication factors (). Each prey category contributes to the food consumed in proportion to the dietary frequency (), and the assimilated dose contributions of the diet, sediments, and seawater are in the same proportion as the base model (). Therefore, the hypothetical effective mean TPAH concentration in the environment for each multiplicative factor was calculated as weighted contributions from each PAH source ().
Table 13 Multiplicative factors applied in the HQ = 1 sensitivity analyses and associated mean environmental TPAH concentrations.a
Figure 8 Results from the HQ = 1 sensitivity analyses (Hazard Quotients). Plots of Hazard Quotients from adult female winter model with heuristic increase in environmental PAHs (see text for details) (note: very similar results occurred for all other classes of seaducks). Each line represents a different quantile level (from 99.9% quantile [maximum-exposed individuals] down to the 50% quantile [median individuals]) for each of six multiplicative factors applied to the extant PWS environmental PAH levels. See for conversion of multiplicative factors to mean environmental PAH concentrations. Also shown is the HQ = 1 line, at which the assimilated average daily doses equal the toxicity reference values. (A) NOAEL Hazard Quotients. (B) LOAEL Hazard Quotients.
![Figure 8 Results from the HQ = 1 sensitivity analyses (Hazard Quotients). Plots of Hazard Quotients from adult female winter model with heuristic increase in environmental PAHs (see text for details) (note: very similar results occurred for all other classes of seaducks). Each line represents a different quantile level (from 99.9% quantile [maximum-exposed individuals] down to the 50% quantile [median individuals]) for each of six multiplicative factors applied to the extant PWS environmental PAH levels. See Table 13 for conversion of multiplicative factors to mean environmental PAH concentrations. Also shown is the HQ = 1 line, at which the assimilated average daily doses equal the toxicity reference values. (A) NOAEL Hazard Quotients. (B) LOAEL Hazard Quotients.](/cms/asset/8d7cfafb-e4c3-4270-933f-075091c5cae8/bher_a_650582_o_f0008g.jpg)
Figure 9 Results from the HQ = 1 sensitivity analyses (quantile levels). Plot of quantile levels (%) at which HQ = 1 from adult female winter model with heuristic increase in environmental PAHs (ppm) (see text for details) (note: very similar results occurred for all other classes of seaducks). (A) NOAEL Hazard Quotients series. (B) LOAEL Hazard Quotients series.
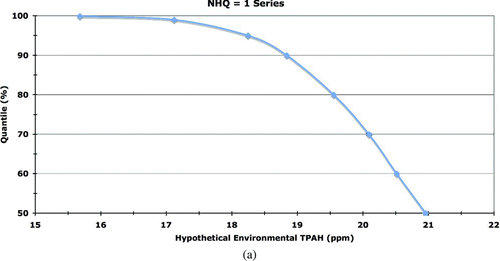
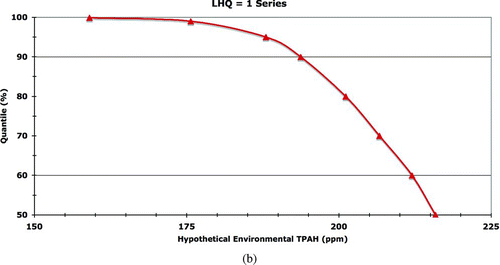
The various quantile lines illustrate the NHQ () and LHQ () values for different fractions of the exposed population. For example, for the 99.9% quantile assimilated dose (maximum-exposed individuals) to reach the HQ = 1 level, the TPAH concentrations would have to be ∼425 times higher than actually exist in the PWS environment for the NOAEL threshold and ∼4,300 times higher for the LOAEL threshold; these correspond to hypothetical environmental TPAH concentrations of ∼16 ppm and ∼160 ppm, respectively (assuming the same relative contributions from each prey category and from prey, sediments, and seawater). Similarly, for the 95% quantile thresholds to be reached, hypothetical environmental TPAH concentrations would have to be ∼18 ppm and ∼185 ppm, respectively; for the 75% quantile, the respective concentrations would be about 20 and 210 ppm.
The purpose of assessing effects at different quantile levels is to assist in translating individual-level effects to population-level effects. For example, even if the PAH exposures to the maximum-exposed individuals were sufficient to reach the TRVs, no population-level effect would result, as still only 1 out of 1000 members of the population would be affected, a level clearly insufficient to be detected in the population. Conversely, if the 50% quantile reached the effects threshold, then half the members of the population would be affected, and a population-level effect would likely be anticipated.
There is no generic threshold value that can be used to determine when population-level effects occur, but we can examine alternate levels of what could be considered to constitute a population-level effect. For example, USFWS (2001) suggested that for the Spectacled Eider (Somateria fischeri), a species listed as Threatened in Alaska, a 5% increase in mortality (in this case, from native Alaskan harvesting) would not lead to the loss of the population but might impede recovery. This corresponds to the 95% quantile results in our study, or ∼18 ppm for the NOAEL and ∼185 ppm for the LOAEL HQ = 1 thresholds. Alternatively, the natural interannual variability of the population could be used as a guide for selecting the quantile level at which population-level effects would be expected. Examination of the available Harlequin Duck population data reported in Wiens et al. (2010) yields a CV of ∼20–30% (which corresponds to our 75% quantile level). The mean environmental TPAH concentrations for this level would need to be ∼20 ppm and 210 ppm, respectively ( and B). Other values could be selected, but the relative closeness of the family of curves indicates that this is not a sensitive parameter (see and ). Thus, we conclude that somewhere between 15–20 ppm (no-observed-effects threshold) and 150–200 ppm (lowest-observed-effects threshold) TPAH in the environment would lead to population-level effects being anticipated.
For context, it is useful to compare these hypothetical values with the actual TPAH concentrations measured in PWS prey tissues shortly after EVOS. Two databases are relevant: (1) the data collected for the Natural Resource Damage Assessment (NRDA) conducted by NOAA, the US Departments of Interior and Agriculture, and the State of Alaska Departments of Law, Fish & Game, and Environmental Conservation, labeled PWSOIL (see the Exxon Valdez Trustees Database [EVTHD], reported in Short and Nelson 1998); and (2) the ad hoc Oil Spill Health Task Force (OSHTF) assessments conducted by scientists from federal, state, and local agencies (including public health) and from Exxon (cited in Bence and Burns Citation1995).
The OSHTF data (reported on a wet-weight basis only, with water content not reported) for 1989 show: 1 (∼2%) mussel out of 42 sampled had a TPAH of 18.5 ppm wet weight (or ∼180 ppm dry weight assuming a 90% water content); 3 (∼7%) had about 2–4 ppm (∼20–40 ppm dry weight); and the rest were less than 1 ppm (∼10 ppm dry weight). In 1990, a total of 11 (∼8%) mussels out of 140 sampled had TPAH between 1.7 and 5.5 ppm dry weight (∼17–55 ppm dry weight), and the rest were less than 1 ppm; and none of the 180 mussels sampled in 1991–1993 exceeded 1 ppm (∼10 ppm dry weight). The OSHTF database for clams showed none exceeding 1 ppm wet weight (∼10 ppm dry weight) at any time following the spill.
The PWSOIL/EVTHD mussel data (dry-weight basis; n = 970 for 1989, 1990, and 1991) showed 18 (∼2%) of the samples collected in 1989 in the 10–40 ppm range, and ∼12% between 1 and 10 ppm; however, no mussels exceeded 10 ppm dry weight at any point in time beyond 90 days after the oil spill. Moreover, no mussels exceeded 1 ppm in 1991. For clams, the PWSOIL database (dry-weight basis; n = 347 for 1989, 1990, and 1991) showed 6 clams in the 10–35 ppm range about 6 weeks after the spill, but none exceeding 10 ppm later in 1989 or in 1990, and none exceeding 1 ppm in 1991.
These data and the results of the HQ = 1 sensitivity analyses demonstrate that even as early as 90 days after EVOS, the mean measured environmental TPAH levels in PWS did not reach concentrations sufficient to cause individual-level chronic-exposure toxicological effects on Harlequin Ducks.
SYNTHESIS
This quantitative assessment of the potential for continuing toxicological risks to Harlequin Ducks in PWS caused by EVOS-derived PAHs has been conducted to evaluate the plausibility of published suggestions that continuing effects do exist on that Harlequin Duck population and that those effects are attributable to EVOS, even two decades after the event (Bodkin et al. Citation2002, Citation2003; Esler et al. Citation2002; Short et al. Citation2006; Esler Citation2008; EVOSTC 2010). In contrast to those assertions, Wiens et al. (2004) concluded that any population-level effect may be explained more readily by differences in habitats between the oiled sites in western PWS and the unoiled sites on Montague Island that were used for comparisons. Moreover, in a qualitative assessment of the plausibility of current risks from remnant EVO, Wiens (Citation2007) concluded that it is highly unlikely that the spill currently has continuing effects on Harlequin Ducks through ingestion of hydrocarbon-contaminated mussels in spill-affected shorelines of PWS.
In the present study, we use the USEPA ecological risk assessment framework (USEPA 1992, 1998; Gentile et al. Citation1993) to evaluate systematically and quantitatively the hypothesis that the Harlequin Duck population of Prince William Sound continues to be at-risk from EVOS. This quantitative toxicological risk assessment compares the PAH exposures from initially oiled areas with those from never-oiled areas in PWS in order to evaluate whether those differences could plausibly lead to effects on individual Harlequin Ducks, with the highly conservative assumption that any such differences are solely attributable to EVOS.
By quantifying all plausible pathways by which PAHs from oiled sites could get into an individual Harlequin Duck living under PWS conditions, we have confidence that this risk assessment has captured the key components of risk. Using the most recent actual measured PAH concentrations in prey, sediments, and seawater in the oiled and reference sites of PWS, we base the analyses on empirical data that capture the range of existing toxicological conditions in PWS. By developing a stochastic model that explicitly uses the distributions derived from empirical data, we capture the variability that is expected among the doses assimilated by PWS Harlequin Ducks. By simulating a population of 500,000 individual ducks within each of four age/gender classes of Harlequin Ducks and two seasons, we have captured the variability of the Harlequin Duck population characteristics as they exist in PWS. In essence, we let the prey and sediments integrate the various sources of PAHs, whether from initially oiled or from never-oiled sites in PWS. Similarly, the modeled Harlequin Ducks integrate the PAHs from the various prey, sediment, and seawater sources. Consequently, the final model projections give the best insights into actual risks to Harlequin Ducks in PWS from PAHs at the present time.
By consistently incorporating conservative assumptions into the risk-assessment model, we have confidence that the estimates of risk are at the upper end of the range of plausible values. Those conservative assumptions include: (1) attributing any PAH differences between oiled sites and reference sites solely to EVO-derived PAHs; (2) assigning the modeled Harlequin Duck to feeding solely within the oiled areas; and (3) assuming that all ingested PAHs are completely bioavailable and assimilated as dose, including those that are tightly bound to sediments (e.g., Kow values up to 10−6; Durrell et al. 2006). By conservatively focusing the effects assessments on the 1-in-1,000th most-exposed individuals, we have even greater confidence that the actual risks to the Harlequin Duck population in PWS are considerably less than the projected values. By conducting sensitivity analyses on selected model parameters, we demonstrate that the model projections are robust. By following a carefully designed and implemented QA regime, we are confident that the model projections are reliable and reproducible.
The results of the quantitative modeling clearly demonstrate that there currently is no plausible risk from PAHs in the oiled areas of PWS to Harlequin Ducks. Since the chronic assimilated doses of TPAH to the maximum-exposed individuals at the oiled sites are about 400 times lower than the no-observed-adverse-effects threshold and about 4,000 times lower than the lowest-observed-adverse-effects threshold, it is unreasonable to expect any individual health effects attributable to the oiled sites and, by implication, to continuing EVOS effects. Because the various sensitivity analyses also are at least two orders of magnitude less than NOAEL effects, and at least three orders of magnitude less than LOAEL effects, this conclusion is robust. Moreover, the sensitivity analysis of not substituting ½ MDL for the non-detectable PAH concentrations in prey tissues showed just how low the background PAH concentrations actually are in PWS, whether at oiled sites or elsewhere in the Sound. This result highlights the very low risks associated with PAHs remaining from residual EVOS sources, driven by the fact that both the oiled-site and reference-site prey tissues are essentially at or less than detection limits. Moreover, the analyses of the distributions of PAHs in the assimilated doses indicate that the differences between the prey at oiled sites and the reference sites do not point to an enhanced EVOS- or other petrogenic-based signal in the oiled sites; rather, the additional PAHs seem to be pyrogenic or biogenic in origin and, thus, clearly are not attributable to EVOS.
Some of the sediments from oiled sites, on the other hand, do demonstrate a signal that is consistent with residual EVO that is not seen in the reference-site sediments. However, because the assimilated dose to the Harlequin Ducks from sediments is projected to be no more than 10% of the total assimilated dose, the sediments from oiled shorelines contribute a dose to the most highly exposed seaducks that is more than 3–4 orders of magnitude less than NOAEL and LOAEL values. As a result, while the EVOS signal is detectable in PWS sediments, it does not constitute a risk to the Harlequin Ducks because of the extremely low assimilated doses.
Some authors have suggested that in 2009 there continued to be a difference between the exposure biomarker CYP1A in PWS Harlequin Ducks at oiled sites compared to reference sites (Esler et al. Citation2010), although as discussed previously, attributing CYP1A responses solely to EVOS-derived PAHs is problematic. However, whether or not an exposure biomarker difference exists in PWS is not the issue: As Anderson and Lee (2006) and (Esler et al. Citation2010) correctly pointed out, biomarkers only reflect that an exposure has occurred, but do not indicate either the magnitude of the exposure or the ecological effects that could potentially occur. Similarly, Esler and Iverson (Citation2010) suggested that while presently there may be sufficient exposure to trigger biomarker induction, evidence indicates that demographic effects no longer exist.
By contrast, the risk assessment conducted here provides a complete risk characterization (USEPA 1992, 1998), including quantification of both the amount of the exposure that occurs and the potential for any effects that might result from that exposure. Based on our quantitative risk assessment results, the magnitude of the exposures to PAHs that are presently occurring in PWS are well below any possibility of health effects to the Harlequin Ducks, irrespective of whether that exposure would be sufficient to cause CYP1A induction.
Moreover, in order for there to be a population-level effect caused by differences in PAH concentrations between oiled and reference sites, there would have to be individual-level effects on literally hundreds or thousands of Harlequin Ducks in PWS. However, since the ecotoxicological risk model demonstrates that not even a single Harlequin Duck out of the millions of individuals simulated would experience an assimilated dose that is closer than 3 orders of magnitude less than the threshold for observable adverse toxicological effects, that eventuality is not plausible based on the model projections.
The results also show that the assimilated doses to both the average and the maximum-exposed individuals increase linearly with concentrations of environmental PAHs. In one set of sensitivity analyses, the model was modified heuristically to assign TPAH concentrations sufficient to cause a health-effects risk. Under this hypothetical scenario, the mean TPAH concentrations in the PWS environment would have to be at least ∼16 ppm for the maximum-exposed individuals to reach the NOAEL threshold, and increase by yet another order of magnitude (i.e., ∼160 ppm) for the LOAEL threshold. Additionally, population-level effects on PWS Harlequin Ducks would require continuous exposures to mean environmental TPAH concentrations on the order of between 15–20 and 150–200 ppm, irrespective of what threshold one chose between 0.1% and 30% for population-level effects to occur. Such mean environmental TPAH concentrations are higher than have been reported in the PWS environment since 1990 and an order-of-magnitude higher than any reported since the clean-up was completed in 1991. Consequently, the results from this risk assessment are clear: PAH concentrations in PWS now two decades after the oil spill could not plausibly lead to any adverse health effects on any individual Harlequin Duck, much less on the PWS population.
Based on this quantitative toxicological risk assessment, we conclude that the residual PAHs derived from the Exxon Valdez oil spill that occurred two decades ago cannot be a cause of adverse effects on any individual Harlequin Duck and, as a result, cannot be affecting the PWS population of Harlequin Ducks. Consequently, if there are any subpopulation-level differences for Harlequin Ducks in PWS, they may be attributable to habitat variation across the Sound, breeding conditions, or other pre-existing conditions in the PWS ecosystem. Many other stressors also affect the PWS ecosystem. In particular, those stressors deriving from climate and oceanographic variability fundamentally affect the entire ecosystem through altering primary and secondary productivity and the PWS trophic structure (as shown in the PWS conceptual model presented in Harwell et al. 2010b). In light of these results and the other environmental factors operating in PWS, the risk to the Harlequin Ducks of PWS that can be attributable to EVOS is essentially non- existent.
AUTHOR-DIRECTED PEER REVIEW
This manuscript was prepared under HERA's author-directed peer review process, wherein a manuscript's authors nominate proposed peer reviewers to HERA's Managing Editor for approval or revision. The following persons reviewed and approved the publication of this manuscript:
| • | Dr. Donald DeAngelis, theoretical ecologist and ecological modeler, U.S. Geological Survey, Department of Biology, University of Miami, Coral Gables, FL, USA; | ||||
| • | Dr. Jean-Pierre L. Savard, migratory bird research scientist, Environment Canada, Canadian Wildlife Service Office, Sainte-Foy, Quebec, Canada; | ||||
| • | Dr. Christopher Teaf, toxicologist and risk assessment specialist, Associate Director, Center for Biomedical & Toxicological Research, Florida State University, Tallahassee, FL, USA | ||||
GLOSSARY OF ABBREVIATIONS
| ADEC: | = |
Alaska Department of Environmental Conservation |
| ADNR: | = |
Alaska Department of Natural Resources |
| AIA: | = |
acid-insoluble ash |
| ASTM: | = |
American Society of Testing and Materials |
| CV: | = |
coefficient of variation |
| CYP1A: | = |
biomarker for cytochrome P4501A |
| DEE: | = |
daily energy expenditure |
| Doubled FMR: | = |
sensitivity analysis with field metabolic rate doubled |
| Doubled Sediments: | = |
sensitivity analysis with fraction of diet consumed as sediments doubled |
| e-CFR: | = |
electronic Code of Federal Regulations; available online at: http://ecfr.gpoaccess.gov |
| EcoSSL: | = |
Ecological Soil Screening Level; from USEPA (2005, 2007) |
| EVO: | = |
Exxon Valdez oil |
| EVOS: | = |
Exxon Valdez oil spill |
| EVOSTC: | = |
Exxon Valdez Oil Spill Trustee Council |
| FMR: | = |
field metabolic rate |
| Full MDL: | = |
sensitivity analysis with MDL value entered for non-detects in PAH analyses |
| GC/MS: | = |
gas chromatography/mass spectrometry |
| GOA: | = |
Gulf of Alaska |
| HAZMAT: | = |
NOAA Hazardous Materials Response and Assessment Division |
| HOR: | = |
heavy oil residue (highest visual oiling level for SSOR) |
| HPAH: | = |
heavy PAH |
| HQ: | = |
hazard quotient; ratio of assimilated dose to TRV |
| ITZ: | = |
intertidal zone |
| Kow: | = |
octanol/water partitioning coefficient |
| KN136: | = |
peat bog site on Knight Island |
| +KN136: | = |
sensitivity analysis adding prey data from KN136 site |
| LITZ: | = |
lower intertidal zone |
| LOAEL: | = |
lowest-observed-adverse-effects level |
| MDL: | = |
Method Detection Limit |
| MITZ: | = |
middle intertidal zone |
| Mussels Only: | = |
sensitivity analysis with diet consisting of 100% mussels |
| NKI: | = |
northern Knight Island |
| No MDL: | = |
sensitivity analysis with “0” entered for non-detects in PAH analyses |
| NOAA: | = |
U.S. National Oceanic and Atmospheric Administration |
| NOAEL: | = |
no-observed-adverse-effects level |
| NRC: | = |
National Research Council |
| PAHs: | = |
polycyclic aromatic hydrocarbons |
| PWS: | = |
Prince William Sound |
| QA: | = |
quality assurance |
| SIM: | = |
selected ion monitoring |
| SOR: | = |
surface oil residues |
| SSOR: | = |
subsurface oil residues |
| TPAH: | = |
total PAH |
| TRV: | = |
toxicity reference value |
| UITZ: | = |
upper intertidal zone |
| Upper 10%: | = |
sensitivity analysis using upper 10% of modeled TPAH daily doses to generate secondary distributions to calculate average daily doses |
| USEPA: | = |
U.S. Environmental Protection Agency |
| USFWS: | = |
U.S. Fish & Wildlife Service |
| USGS: | = |
U.S. Geological Survey |
| WEVC: | = |
weathered Exxon Valdez crude oil |
| ½ MDL: | = |
sensitivity analysis with ½ MDL value entered for non-detects in PAH Analyses |
ACKNOWLEDGMENTS
The authors acknowledge the participants in the workshops that were convened to develop the pathways conceptual model and the data for the parameterization of the quantitative toxicological risk assessment model, specifically: Drs. Paul Boehm, John Brown, Susan Kane Driscoll, David Garshelis, Ray Highsmith, Rick Johnson, and David Page. We also thank these scientists for their comments and suggestions on earlier drafts of the article. Additionally, Dr. Highsmith provided very useful insights and unpublished information on the fat innkeeper in Prince William Sound. Dr. Ken Cummins provided very useful insights on measurements of energy density in tissues of prey species relevant to the risk assessment. We particularly appreciate the careful reviews and suggestions of three peer reviewers on the pre-submission draft of the article—Drs. Ken Dickson, David Weinstein, and Terry Young, and the formal peer reviewers selected by the editors of Human and Ecological Risk Assessment—Drs. Donald DeAngelis, Jean-Pierre Savard, and Christopher Teaf. Finally, we acknowledge the financial support provided by ExxonMobil Corporation for the time needed to prepare this article.
The opinions and conclusions expressed herein are strictly those of the authors and do not necessarily represent the opinions of ExxonMobil.
REFERENCES
- Adams , P A , Robertson , G J and Jones , I L . 2000 . Time-activity budgets of Harlequin Ducks molting in the Gannet Islands, Labrador . Condor , 102 ( 3 ) : 703 – 8 .
- ADNR (Alaska Department of Natural Resources) . 1996a . Exxon Valdez Oil Spill–Shoreline Oiling-PWS Fall89–Spring/90 , Anchorage, AK , , USA : Alaska Department of Natural Resources . Available at http://dnr.alaska.gov/SpatialUtility/SUC?cmd=vmd&layerid=1281
- ADNR . 1996b . Exxon Valdez Oil Spill–Shoreline Oiling–PWS Spring 91. , Anchorage, AK , , USA : Alaska Department of Natural Resources . Available at http://dnr.alaska.gov/mdfiles/evos_oiling_pws_1991.html
- Ainley , D G , Grau , C R Roudybush , T E . 1981 . Petroleum ingestion reduces reproduction in Cassin's Auklets . Marine Pollut Bull , 12 ( 9 ) : 314 – 7 .
- Albers , PH. 1979 . Transfer of crude oil from contaminated water to bird eggs . Environ Res , 22 ( 2 ) : 307 – 14 .
- Anderson , J W and Lee , R F . 2006 . Use of biomarkers in oil spill risk assessment in the marine environment . Hum Ecol Risk Assess , 12 ( 6 ) : 1192 – 222 .
- Andres , BA. 1999 . Effects of persistent shoreline oil on breeding success and chick growth in Black Oystercatchers . Auk , 116 ( 3 ) : 640 – 50 .
- Ax , P. 2000 . “ Multicellular Animals, vol II. The Phylogenetic System of the Metazoa ” . Heidelberg , , Germany : Springer-Verlag .
- Bence , A E and Burns , W A . 1995 . “ Fingerprinting hydrocarbons in the biological resources of the Exxon Valdez spill area ” . In Exxon Valdez , Edited by: Wells , P G , Butler , J N and Hughes , J S . 84 – 140 . Oil Spill: Fate and Effects in Alaskan Waters . ASTM Special Technical Publication No. 1219. American Society of Testing and Materials, Philadelphia, PA, USA
- Bence , A E , Kvenvolden , K A and Kennicutt , M C . 1996 . Organic chemistry applied to environmental assessments of Prince William Sound, Alaska, after the Exxon Valdez oil spill—A review . Org Geochem , 24 ( 1 ) : 7 – 42 .
- Bence , A E , Page , D S and Boehm , P D . 2007 . “ Advances in forensic techniques for petroleum hydrocarbons: The Exxon Valdez experience ” . In Oil Spill Environmental Forensics, Fingerprinting and Source Identification , Edited by: Wang , Z and Stout , S A . 449 – 87 . San Diego , , CA, USA : Academic Press .
- Bennett , DC. 2002 . “ Effect of Saline Intake on Osmotic Homeostasis in Ducks. Ph.D. dissertation ” . Vancouver , , BC, Canada : University of British Columbia .
- Bennett , D , Gray , D and Hughes , M . 2003 . Effect of saline intake on water flux and osmotic homeostasis in Pekin ducks (Anas platyrhynchos) . J Comp Physiol Part B , 173 ( 1 ) : 27 – 36 .
- Bennett , D C and Hughes , M R . 2003 . Comparison of renal and salt gland function in three species of wild ducks . J Exp Biol , 206 : 3273 – 84 .
- Beyer , W N , Perry , M C and Osenton , P C . 2008 . Sediment ingestion rates in waterfowl (Anatidae) and their use in environmental risk assessment . Integrated Environ Assess Manage , 4 ( 2 ) : 246 – 51 .
- Birt-Friesen , V L , Montevecchi , W A Cairns , D K . 1989 . Activity-specific metabolic rates of free-living Northern Gannets and other seabirds . Ecology , 70 : 357 – 67 .
- Bishop , M A and Green , S P . 2001 . Predation on Pacific herring (Clupea pallasi) spawn by birds in Prince William Sound, Alaska . Fisheries Oceanography , 10 ( Suppl 1 ) : 149 – 58 .
- Bodkin , J L , Ballachey , B E Dean , T A . 2002 . Sea otter population status and the process of recovery from the 1989 “Exxon Valdez” oil spill . Mar Ecol Progr Ser , 241 : 237 – 53 .
- Bodkin , J , Ballachey , B Esler , D . 2003 . “ Patterns and Processes of Population Change in Selected Nearshore Predators. EVOS Project No. 423. Report prepared for the Exxon Valdez Oil Spill Trustee Council ” . Delta , , BC, Canada : US Geological Survey, Alaska Science Center and Centre for Wildlife Ecology, Simon Fraser University .
- Boehm , P D , Douglas , G S Burns , W A . 1997 . Application of petroleum hydrocarbon chemical fingerprinting and allocation techniques after the Exxon Valdez oil spill . Mar Pollut Bull , 34 ( 8 ) : 599 – 613 .
- Boehm , P D , Page , D S Brown , J S . 2004 . Polycyclic aromatic hydrocarbon levels in mussels from Prince William Sound, Alaska, USA, document the return to baseline conditions . Environ Toxicol Chem , 23 ( 12 ) : 2916 – 29 .
- Boehm , P D , Page , D S Neff , J M . 2007a . Potential for sea otter exposure to remnants of buried oil from the Exxon Valdez oil spill . Environ Sci Technol , 41 ( 19 ) : 6860 – 7 .
- Boehm , P D , Neff , J M and Page , D S . 2007b . Assessment of polycyclic aromatic hydrocarbon exposure in the waters of Prince William Sound after the Exxon Valdez oil spill: 1989–2005 . Mar Pollut Bull , 54 ( 3 ) : 339 – 67 .
- Boehm , P D , Page , D S Brown , J S . 2008 . Distribution and weathering of crude oil residues on shorelines 18 years after the Exxon Valdez spill . Environ Sci Technol , 42 ( 24 ) : 9210 – 6 .
- Bond , J C and Esler , D . 2006 . Nutrient acquisition by female Harlequin Ducks prior to spring migration and reproduction: Evidence for body mass optimization . Can J Zool , 84 ( 9 ) : 1223 – 9 .
- Bourget , D , Savard , J-L and Guillemette , M . 2007 . Distribution, diet, and dive behavior of Barrow's and Common Goldeneyes during spring and autumn in the St. Lawrence estuary . Waterbirds , 30 ( 2 ) : 230 – 40 .
- Brannon , E L , Collins , K C Cronin , M A . 2007 . Risk of weathered residual Exxon Valdez oil to pink salmon embryos in Prince William Sound . Environ Toxicol Chem , 26 ( 4 ) : 780 – 6 .
- Brassell , S C and Eglinton , G . 1980 . “ Environmental chemistry—An interdisciplinary subject. Natural and pollutant organic compounds in contemporary aquatic sediments ” . In Analytical Techniques in Environmental Chemistry , Edited by: Algaiges , J . 1 – 22 . Oxford , , UK : Pergamon Press .
- Brinkman , A G , Ens , B J and Kats , R . 2003 . “ Modelling the Energy Budget and Prey Choice of Eider Ducks ” . Alterra-rapport No. 839 Alterra, Wageningen , , The Netherlands
- Burger , J and Tsipoura , N . 1998 . Experimental oilings of sanderlings (Calidris alba): Behavior and weight changes . Environ Toxicol Chem , 17 ( 6 ) : 1154 – 8 .
- Burns , W A , Mankiewicz , P J Bence , A E . 1997 . A principal-component and least-squares method for allocating polycyclic aromatic hydrocarbons in sediments to multiple sources . Environ Toxicol Chem , 16 ( 6 ) : 1119 – 31 .
- Butler , R G , Harfenist , A Leighton , F A . 1988 . Impact of sublethal oil and emulsion exposure on the reproductive success of Leach's Storm Petrels: Short and long-term effects . J Appl Ecol , 25 : 125 – 43 .
- Calkins , DG. 1978 . Feeding behavior and major prey species of the sea otter, Enhydra lutris, in Montague Strait, Prince William Sound, Alaska . Fish Bull , 76 ( 1 ) : 125 – 31 .
- Cavanaugh , K P and Holmes , W N . 1982 . Effects of ingested petroleum on plasma levels of ovarian steroid hormones in photostimulated mallard ducks . Arch Environ Contam Toxicol , 11 ( 4 ) : 503 – 8 .
- Cavanaugh , K P and Holmes , W N . 1987 . Effects of ingested petroleum on the development of ovarian endocrine function in photostimulated ducks (Anas platyrhychos) . Arch Environ Contam Toxicol , 16 ( 2 ) : 247 – 53 .
- Cavanaugh , K P , Goldsmith , A R Holmes , W N . 1983 . Effects of ingested petroleum on the plasma prolactin levels during incubation and on the breeding success of paired mallard ducks . Arch Environ Contam Toxicol , 12 ( 2 ) : 335 – 341 .
- Coon , N C and Dieter , M P . 1981 . Responses of adult Mallard Ducks to ingested south Louisiana crude oil . Environ Res , 24 ( 2 ) : 309 – 14 .
- Crowley , DW. 1999 . “ Productivity of Harlequin Ducks breeding in Prince William Sound, Alaska ” . Edited by: Goudie , R I , Petersen , M R and Robertson , G J . Ottawa , , ON, Canada : Behaviour and Ecology of Sea Ducks, pp 14–20. Canadian Wildlife Service Occasional Paper Series No. 100 .
- CSL (Central Science Laboratory) . 2007 . “ Improved Estimates of Food and Water Intake for Risk Assessment. SID5 Research Project Final Report, PS2330 ” . London , , UK : Department for Environment, Food and Rural Affairs .
- Cummins , K W and Wuycheck , J C . 1971 . “ Caloric Equivalents for Investigations in Ecological Energetics ” . Stuttgart , , Germany : International Association of Theoretical and Applied Limnology .
- Day , R H , Murphy , S M Wiens , J A . 1995 . “ Use of oil-affected habitats by birds after the Exxon Valdez oil spill ” . In Exxon Valdez , Edited by: Wells , P G , Butler , J N and Hughes , J S . Philadelphia , , PA, USA : Oil Spill: Fate and Effects in Alaskan Waters, pp 726–61. ASTM Special Technical Publication No. 1219. American Society of Testing and Materials .
- Day , R H , Murphy , S M Wiens , J A . 1997 . Effects of the Exxon Valdez oil spill on habitat use by birds in Prince William Sound, Alaska . Ecol Appl , 7 ( 2 ) : 593 – 613 .
- de Leeuw , J J , van Eerden , M R and Visser , G H . 1999 . Wintering Tufted Ducks Aythya fuligula diving for zebra mussels Dreissena polymorpha balance feeding costs within narrow margins of their energy budgets . J Avian Biol , 30 : 182 – 92 .
- de Vries , J and van Eerden , M R . 1995 . Thermal conductance in aquatic birds in relation to the degree of water contact, body mass, and body fat: Energetic implications of living in a strong cooling environment . Physiol Zool , 68 ( 6 ) : 1143 – 63 .
- Dean , T A , Bodkin , J L Fukuyama , A K . 2002 . Food limitation and the recovery of sea otters following the Exxon Valdez oil spill . Mar Ecol Progr Ser , 241 : 255 – 70 .
- DeAngelis , D L and Gross , L J . 1992 . “ Individual-Based Models and Approaches in Ecology: Populations, Communities and Ecosystems ” . New York , , NY, USA : Chapman and Hall .
- DeVink , J MA , Gilchrist , H G and Diamond , A W . 2005 . Effects of water salinity on growth and survival of Common Eider (Somateria mollissima) ducklings . Auk , 122 ( 2 ) : 523 – 9 .
- Di Toro , D M and McGrath , J A . 2000 . Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria: II. Mixtures and sediments . Environ Toxicol Chem , 19 ( 8 ) : 1971 – 82 .
- Douglas , G S , Burns , W A Bence , A E . 2004 . Optimizing detection limits for the analysis of petroleum hydrocarbons in complex environmental samples . Environ Sci Technol , 38 ( 4 ) : 3958 – 64 .
- Durrell , G , Utvik , T R Johnsen , S . 2006 . Oil well produced water discharges to the North Sea. Part I. Comparison of deployed mussels (Mytilus edulis), semi-permeable membrane devices, and the DREAM model predictions to estimate the dispersion of polycyclic aromatic hydrocarbons . Mar Environ Res , 62 ( 3 ) : 194 – 223 .
- Dzinbal , K A and Jarvis , R L . 1984 . “ Coastal feeding ecology of Harlequin Ducks in Prince William Sound, Alaska, during summer ” . In Marine Birds: Their Feeding Ecology and Commercial Fisheries Relationships , 6 – 10 . Ottawa , , ON, Canada : Canadian Wildlife Service Special Publication .
- e-CFR (Electronic Code of Federal Regulations) . 2009 . “ Title 40 Protection of Environment. Part 300 National Oil and Hazardous Substances Pollution Contingency Plan ” . Appendix C Swirling Flask Dispersant Effectiveness Test, Revised Standard Dispersant Toxicity Test, and Bioremediation Agent Effectiveness Test. Section 4.6.3.2. GC/MS analysis procedure . Available at http://ecfr.gpoaccess.gov
- Eadie , J M , Mallory , M L and Lumsden , H G . 1995 . “ Common Goldeneye (Bucephala clangula) ” . Edited by: Poole , A . NY, USA : The Birds of North America Online, Cornell Lab of Ornithology Ithaca . Available at http://bna.birds.cornell.edu/bna/species/170
- Eadie , J M , Savard , J-P L and Mallory , M L . 2000 . “ Barrow's Goldeneye (Bucephala islandica) ” . Edited by: Poole , A . NY, USA : The Birds of North America Online, Cornell Lab of Ornithology Ithaca . Available at http://bna.birds.cornell.edu/bna/species/548 doi:10.2173/bna. 548
- Elliott , K H and Gaston , A J . 2008 . Mass-length relationships and energy content of fishes and invertebrates delivered to nestling Thick-billed Murres Uria lomvia in the Canadian Arctic, 1981–2007 . Marine Ornithology , 36 ( 1 ) : 25 – 34 .
- Esler , D. 2008 . “ Quantifying Temporal Variation in Harlequin Duck Cytochrome P4501A Induction ” . In Exxon Valdez , Delta, BC , , Canada : Oil Spill Trustee Council Restoration Project Final Report (Restoration Project 050777), Centre for Wildlife Ecology, Simon Fraser University .
- Esler , D and Iverson , S A . 2010 . Female harlequin duck winter survival 11 to 14 years after the Exxon Valdez oil spill . J Wildlife Manage , 74 ( 3 ) : 471 – 8 .
- Esler , D , Schmutz , J A Jarvis , R L . 2000 . Winter survival of adult female Harlequin Ducks in relation to history of contamination by the Exxon Valdez oil spill . J Wildlife Manage , 64 : 839 – 47 .
- Esler , D , Bowman , T D Trust , K A . 2002 . Harlequin Duck population recovery following the Exxon Valdez oil spill: Progress, process and constraints . Mar Ecol Progr Ser , 241 : 271 – 86 .
- Esler , D , Trust , K A Ballachey , B E . 2010 . Cytochrome P450A biomarker indication of oil exposure in harlequin ducks up to 20 years after the Exxon Valdez oil spill . Environ Toxicol Chem , 29 ( 5 ) : 1138 – 45 .
- Evans , M , Hastings , N AJ and Peacock , J B . 1993 . “ Statistical Distributions, 3rd edit ” . New York , , NY, USA : John Wiley & Sons .
- EVOSTC (Exxon Valdez Oil Spill Trustee Council) . 2006 . “ Update on Injured Resources and Services 2006 ” . In Exxon Valdez , Anchorage , , AK, USA : Oil Spill Trustee Council .
- EVOSTC . 2008 . “ Exxon Valdez Hydrocarbon Database ” . Juneau, AK , , USA : NOAA National Marine Fisheries Service, Auke Bay Laboratories . Available at http://www.afsc.noaa.gov/ABL/Habitat/ablhab_exxonvaldez_hydrocarbon_database.htm
- EVOSTC . 2010 . “ Exxon Valdez Oil Spill Trustee Council, 2010 Update. Injured Resources and Services ” . In Exxon Valdez , Anchorage, AK , , USA : Oil Spill Trustee Council . Available at http://www.evostc.state.ak.us/recovery/status.cfm)
- Fischer , JB. 1998 . “ Feeding Behavior, Body Condition, and Oil Contamination of Wintering Harlequin Ducks (Histrionicus histrionicus) at Shemya Island, Alaska. M.S. thesis ” . Amherst , , MA, USA : University of Massachusetts .
- Fischer , J B and Griffin , C R . 2000 . Feeding behavior and food habits of wintering Harlequin Ducks at Shemya Island, Alaska . Wilson Bull , 112 ( 3 ) : 318 – 25 .
- Fletcher , G L and Holmes , W N . 1968 . Observations on the intake of water and electrolytes by the duck (Anas platyrhynchos) maintained on fresh water and on hypertonic saline . J Exp Biol , 49 : 325 – 9 .
- Fry , D M and Lowenstine , L J . 1985 . Pathology of Common Murres and Cassin's Auklets exposed to oil . Arch Environ Contam Toxicol , 14 ( 6 ) : 725 – 37 .
- Fry , D M , Swenson , J Addiego , L A . 1986 . Reduced reproduction of wedge-tailed shearwaters exposed to weathered Santa Barbara crude oil . Arch Environ Contam Toxicol , 15 ( 4 ) : 453 – 63 .
- Gaines , W L and Fitzner , R E . 1987 . Winter diet of the Harlequin Duck at Sequim Bay, Puget Sound, Washington . Northwest Sci , 61 ( 4 ) : 213 – 5 .
- Gentile , J H , Harwell , M A van der Schalie , W . 1993 . Ecological risk assessment: A scientific perspective . J Hazard Mater , 35 ( 2 ) : 241 – 53 .
- Gorsline , J and Holmes , W N . 1982 . Ingestion of petroleum by breeding mallard ducks: Some effects on neonatal progeny . Arch Environ Contam Toxicol , 12 ( 2 ) : 147 – 53 .
- Goudie , R I and Ankney , C D . 1986 . Body size, activity budgets, and diets of sea ducks wintering in Newfoundland . Ecology , 67 ( 6 ) : 1475 – 82 .
- Goudie , R I and Ryan , P C . 1991 . Diets and morphology of digestive organs of five species of sea ducks wintering in Newfoundland . J Yamashina Institute of Ornithology , 22 ( 1 ) : 1 – 8 .
- Grebmeier , J M and Harrison , N M . 1992 . Seabird feeding on benthic amphipods facilitated by gray whale activity in the northern Bering Sea . Mar Ecol Progr Ser , 80 : 125 – 33 .
- Guillemette , M , Pelletier , D Grandbois , J M . 2007 . Flightlessness and the energetic cost of wing molt in a large sea duck . Ecology , 88 ( 11 ) : 2936 – 45 .
- Harriman , AE. 1967 . Laughing gulls offered saline in preference and survival tests . Physiol Zool , 40 ( 3 ) : 273 – 8 .
- Harrison , CS. 1979 . The association of marine birds and feeding gray whales . Condor , 81 ( 1 ) : 93 – 5 .
- Hartung , R and Hunt , G S . 1966 . Toxicity of some oils to waterfowl . J Wildl Manage , 30 ( 3 ) : 564 – 70 .
- Harwell , M A , Gentile , J H Johnson , C B . 2010a . A quantitative ecological risk assessment of the toxicological risks from Exxon Valdez subsurface oil residues to sea otters at Northern Knight Island, Prince William Sound, Alaska . Hum Ecol Risk Assess , 16 ( 4 ) : 727 – 61 .
- Harwell , M A , Gentile , J H Cummins , K W . 2010b . A conceptual model of the natural and anthropogenic drivers and their influence on the Prince William Sound, Alaska, ecosystem . Hum Ecol Risk Assess , 16 ( 4 ) : 672 – 726 .
- Hayes , M O and Michel , J . 1999 . Factors determining the long-term persistence of Exxon Valdez oil in gravel beaches . Mar Pollut Bull , 38 ( 2 ) : 92 – 101 .
- Hayes , M O , Michel , J and Betenbaugh , D V . 2010 . The intermittently exposed, coarse-grained gravel beaches of Prince William Sound, Alaska: Comparison with open-ocean gravel beaches . J Coastal Research , 26 ( 1 ) : 4 – 30 .
- Hayward , J L and Verbeek , N A . 2008 . “ Glaucous-winged Gull (Larus glaucescens) ” . Edited by: Poole , A . Ithaca, NY , , USA : The Birds of North America Online, Cornell Lab of Ornithology . Available at http://bna.cornell.edu/bna/species/059 doi:10.2173/bna.59
- HAZMAT (NOAA Hazardous Materials Response and Assessment Division) . 1996 . “ Aerial Observations of Oil at Sea. HAZMAT Report 96-7, April 1996 ” . Seattle , , WA, USA : National Oceanic and Atmospheric Administration Office of Ocean Resources Conservation and Assessment .
- Hines , A H and Loughlin , T R . 1980 . Observations of sea otters digging for clams at Monterey Harbor, California . Fish Bull , 78 ( 1 ) : 159 – 63 .
- Hoffman , D J and Gay , M L . 1981 . Embrotoxic effects of benzo(a)pyrene, chrysene, and 7,12-dimethylbenz(a)anthracene in petroleum hydrocarbon mixtures in mallard ducks . J Toxicol Environ Health Part A , 7 ( 5 ) : 775 – 87 .
- Holland-Bartels , L , Ballachey , B Bishop , M A . 1998 . “ Mechanisms of Impact and Potential Recovery of Nearshore Vertebrate Predators, Exxon Valdez Oil Spill Annual Report (Restoration Project 97025) ” . In Exxon Valdez , Anchorage , , AK, USA : Oil Spill Trustee Council .
- Holmes , W N and Cavanaugh , K P . 1990 . Some evidence for an effect of ingested petroleum on the fertility of the mallard drake (Anas platychynchos) . Arch Environ Contam Toxicol , 19 ( 6 ) : 898 – 901 .
- Holmes , W N , Cavanaugh , K P and Cronshaw , J . 1978a . The effects of ingested petroleum on oviposition and some aspects of reproduction in experimental colonies of mallard ducks (Anas platyrhynchos) . J Reproduction Fertility , 54 : 335 – 47 .
- Holmes , W N , Cronshaw , J and Gorsline , J . 1978b . Some effects of ingested petroleum on seawater-adapted ducks (Anas platyrhynchos) . Environ Res , 17 ( 2 ) : 177 – 90 .
- Holmes , W N , Gorsline , J and Cronshaw , J . 1979 . Effects of mild cold stress on the survival of seawater-adapted Mallard ducks (Anas platyrhynchos) maintained on food contaminated with petroleum . Environ Res , 20 ( 2 ) : 425 – 44 .
- Houde , M , Hoekstra , P F Solomon , K R . 2005 . Organohalogen contaminants in delphinoid cetaceans . Rev Environ Contam Toxicol , 184 : 1 – 57 .
- Huggett , R J , Stegeman , J J Page , D S . 2003 . Biomarkers in fish from Prince William Sound and the Gulf of Alaska: 1999–2000 . Environ Sci Technol , 37 ( 18 ) : 4043 – 51 .
- Integral (Integral Consulting Inc.) . 2006 . “ Information Synthesis and Recovery Recommendations for Resources and Services Injured by the Exxon Valdez Oil Spill ” . Anchorage, AK , , USA : Restoration Project 060783 Final Report, Exxon Valdez Oil Spill Trustee Council .
- Irons , D B , Kendall , S J Erickson , W P . 2000 . Nine years after the Exxon Valdez oil spill: Effects on marine bird populations in Prince William Sound, Alaska . Condor , 102 ( 4 ) : 723 – 37 .
- Iverson , S A and Esler , D . 2006 . Site fidelity and the demographic implications of winter movements by a migratory bird, the Harlequin Duck Histrionicus histrionicus . J Avian Biology , 37 ( 3 ) : 219 – 28 .
- Kaplan , I R and Venkatesan , M I . 1981 . “ Characterization of Organic Matter in Sediments from Gulf of Alaska, Bering and Beaufort Seas ” . Boulder , , CO, USA : Final Report to Outer Continental Shelf Environmental Assessment Program, US Department of Commerce and US Department of Interior .
- Kenyon , KW. 1961 . Birds of Amchitka Island, Alaska . Auk , 78 : 305 – 26 .
- King , J G and Sanger , G A . 1979 . “ Oil vulnerability index for marine oriented birds ” . In Conservation of Marine Birds of Northern North America , Edited by: Bartonek , J C and Nettleship , D N . 227 – 39 . Washington, DC , , USA : Wildlife Research Report No. 11. US Fish and Wildlife Service .
- Köth , T and Vauk-Hentzelt , E . 1988 . Influence of plumage and stomach oiling on body and organ growth in young Kittiwakes . Mar Pollut Bull , 19 ( 2 ) : 71 – 3 .
- Kvenvolden , K A , Hostettler , F D Rapp , J B . 1993 . Hydrocarbon in oil residues on beaches of islands of Prince William Sound, Alaska . Mar Pollut Bull , 26 ( 1 ) : 24 – 9 .
- Kvitek , R G , Fukuyama , A K Anderson , B S . 1988 . Sea otter foraging on deep-burrowing bivalves in a California coastal lagoon . Mar Biol , 98 ( 2 ) : 157 – 67 .
- Landis (Landis Associates Inc) . 1985 . “ A Dietary LC50 Study in the Bobwhite Quail with Naphthalene (Final Report) ” . Doc #86-870000551, Ref ID: 27393 Washington, DC , , USA : Report to the US Environmental Protection Agency, EPA/OTS .
- Leighton , FA. 1993 . The toxicity of petroleum oils to birds . Environ Rev , 1 : 92 – 103 .
- McKinney , R A and McWilliams , S R . 2005 . A new model to estimate daily energy expenditure for wintering waterfowl . Wilson Bull , 117 ( 1 ) : 44 – 55 .
- McKinney , R A , Glatt , S M and McWilliams , S R . 2004 . Allometric length-weight relationships for benthic prey of aquatic wildlife in coastal marine habitats . Wildlife Biol , 10 ( 4 ) : 214 – 49 .
- McKnight , A , Sullivan , K Irons , D B . 2006 . “ Marine Bird and Sea Otter Population Abundance of Prince William Sound, Alaska: Trends Following the T/V Exxon Valdez Oil Spill, 1989–2005 ” . In Exxon Valdez , Anchorage , , AK, USA : Oil Spill Restoration Project Annual Report (Restoration Projects 040159/050751), Exxon Valdez Oil Spill Trustee Council .
- Michel , J , Nixon , Z and Cotsapas , L . 2006 . “ Evaluation of Oil Remediation Technologies for Lingering Oil from the Exxon Valdez Oil Spill in Prince William Sound, Alaska ” . Columbia, SC , , USA : Restoration Project 050778 Final Report. Research Planning Inc. .
- Murphy , S M , Day , R H Wiens , J A . 1997 . Effects of the Exxon Valdez oil spill on birds: Comparisons of pre- and post-spill surveys in Prince William Sound, Alaska . Condor , 99 ( 2 ) : 299 – 313 .
- Nagy , K A and Peterson , C C . 1988 . “ Scaling of water flux rate in animals ” . Vol. 120 , 131 – 72 . University of California Publications in Zoology .
- Nagy , K A , Girard , I A and Brown , T K . 1999 . Energetics of free-ranging mammals, reptiles, and birds . Ann Rev Nutr , 19 : 247 – 77 .
- Neff , JM. 2002 . “ Polycyclic aromatic hydrocarbons in the ocean ” . In Bioaccumulation in Marine Organisms, Effects of Contaminants from Oil Well Produced Water , Edited by: Neff , J M . 241 – 318 . Amsterdam , , The Netherlands : Elsevier Science Publishers .
- Neff , J M , Owens , E H Stoker , S W . 1995 . “ Shoreline oiling conditions in Prince William Sound following the Exxon Valdez oil spill ” . In Exxon Valdez , Edited by: Wells , P G , Butler , J N and Hughes , J S . Philadelphia , , PA, USA : Oil Spill: Fate and Effects in Alaskan Waters, pp 312–45. ASTM Special Technical Publication No. 1219. American Society of Testing and Materials .
- Neff , J M , Bence , A E Parker , K R . 2006 . Bioavailability of polycyclic aromatic hydrocarbons from buried shoreline residues thirteen years after the Exxon Valdez oil spill: A multispecies assessment . Environ Toxicol Chem , 25 ( 4 ) : 947 – 61 .
- Nehls , G. 1995 . “ Strategien der Ernahrung und ihre Bedeutung fur Energiehaushalt und Okologie der Eiderente. Ph.D. dissertation ” . Germany : University of Kiel .
- NOAA (National Oceanic and Atmospheric Administration) . 2007 . “ ESI Atlas, Prince William Sound, Digital Data Release, April 2007 ” . Seattle, WA , , USA : National Oceanic and Atmospheric Administration, Office of Response and Restoration .
- NRC (National Research Council) . 1985 . “ Oil in the Sea ” . Washington, DC , , USA : National Academy Press .
- Nyström , K GK and Pehrsson , O . 1988 . Salinity as a constraint affecting food and habitat choice of mussel-feeding diving ducks . Ibis , 130 ( 1 ) : 94 – 110 .
- Page , D S , Boehm , P D Douglas , G S . 1995 . “ Identification of hydrocarbon sources in the benthic sediments of Prince William Sound and the Gulf of Alaska following the Exxon Valdez oil spill ” . In Exxon Valdez , Edited by: Wells , P G , Butler , J N and Hughes , J S . Philadelphia , , PA, USA : Oil Spill: Fate and Effects in Alaskan Waters, pp 41–83. ASTM Special Technical Publication No. 1219. American Society of Testing & Materials .
- Page , D S , Boehm , P D Douglas , G S . 1996 . The natural petroleum hydrocarbon background in subtidal sediments of Prince William Sound, Alaska, USA . Environ Toxicol Chem , 15 ( 8 ) : 1266 – 81 .
- Page , D S , Boehm , P D Douglas , G S . 1999 . Pyrogenic polycyclic aromatic hydrocarbons in sediments record past human activity: A case study in Prince William Sound, Alaska . Mar Pollut Bull , 38 ( 4 ) : 247 – 60 .
- Page , D S , Bence , A E Burns , W A . 2002 . A holistic approach to hydrocarbon source allocation in the subtidal sediments of Prince William Sound, Alaska, embayments . Environ Forensics , 3 : 331 – 40 .
- Page , D S , Huggett , R J Stegeman , J J . 2004 . Polycyclic aromatic hydrocarbon sources related to biomarker levels in fish from Prince William Sound and the Gulf of Alaska . Environ Sci Technol , 38 ( 19 ) : 4928 – 36 .
- Page , D S , Boehm , P D Brown , J S . 2005 . Mussels document loss of bioavailable polycyclic aromatic hydrocarbons and the return to baseline conditions for oiled shorelines in Prince William Sound, Alaska . Mar Environ Res , 60 ( 4 ) : 422 – 36 .
- Parker , K R and Wiens , J A . 2005 . Assessing recovery following environmental accidents: Environmental variation, ecological assumptions, and strategies . Ecol Appl , 15 ( 6 ) : 2037 – 51 .
- Patten , S M Jr. , Crowe , T Gustin , R . 2000 . “ Assessment of Injury to Sea Ducks from Hydrocarbon Uptake in Prince William Sound and the Kodiak Archipelago, Alaska, Following the Exxon Valdez Oil Spill, vol 1, pp 1–167. Bird Study Number 11 Final Report ” . Anchorage , , AK, USA : Alaska Department of Fish and Game .
- Patton , J F and Dieter , M P . 1980 . Effects of petroleum hydrocarbons on hepatic function in the duck . Comparative Biochem Physiol Part C , 65 ( 1 ) : 33 – 6 .
- Peakall , D B , Hallett , D J Bend , J R . 1982 . Toxicity of Prudhoe Bay crude oil and its aromatic fractions to nestling herring gulls . Environ Res , 27 ( 1 ) : 206 – 15 .
- Piatt , J F , Lensink , C J Butler , W . 1990 . Immediate impact of the “Exxon Valdez” oil spill on marine birds . Auk , 107 : 387 – 97 .
- Pickford , G E . 1964 . “ Spoon, a worm ” . Vol. 7 , 898 – 901 . Encyclopedia Britannica . Available at http://www.stelio.net/archive/sp-worm.html
- Portugal , S J , Gree , J A and Butler , P J . 2007 . Annual changes in body mass and resting metabolism in captive barnacle geese (Branta leucopsis): The importance of wing moult . J Exp Biol , 210 : 1391 – 7 .
- Ricca , M A , Miles , A K Ballachey , B E . 2010 . PCB exposure in sea otters and harlequin ducks in relation to history of contamination by the Exxon Valdez oil spill . Mar Poll Bull , 60 ( 6 ) : 861 – 72 .
- Rice , S D , Short , J W Carls , M G . 2007 . “ The Exxon Valdez oil spill ” . Edited by: Spies , R B . 419 – 520 . Elsevier, Amsterdam , , The Netherlands : Long-Term Ecological Change in the Northern Gulf of Alaska .
- Robert , M and Cloutier , L . 2001 . Summer food habits of Harlequin Ducks in eastern North America . Wilson Bull , 113 ( 1 ) : 78 – 84 .
- Robertson , G J and Goudie , R I . 1999 . “ Harlequin Duck (Histrionicus histrionicus) ” . Edited by: Poole , A . The Birds of North America Online, Cornell Lab of Ornithology Ithaca, NY, USA . Available at http://bna.birds.cornell.edu/bna/species/466
- Rocke , T E , Yuill , T M and Hinsdill , R D . 1984 . Oil and related toxicant effects on Mallard immune defenses . Environ Res , 33 ( 2 ) : 343 – 52 .
- Rodway , M S and Cooke , F . 2002 . Use of fecal analysis to determine seasonal changes in the diet of wintering Harlequin Ducks at a herring spawning site . J Field Ornithology , 73 ( 4 ) : 363 – 71 .
- Rosenberg , D H and Petrula , M J . 1998 . “ Status of Harlequin Ducks in Prince William Sound, Alaska, after the Exxon Valdez Oil Spill, 1995–1997, pp 1–64 + appendices ” . Exxon Valdez Oil Spill Restoration Project Final Report (Restoration Project 97427) Anchorage, AK , , USA : Exxon Valdez Oil Spill Trustee Council .
- Rosenberg , D H , Petrula , M J Hill , D D . 2005 . “ Harlequin Duck Population Dynamics: Measuring Recovery from the Exxon Valdez Oil Spill, pp 1–47 ” . Exxon Valdez Oil Spill Restoration Project Final Report (Restoration Project 040407) Anchorage, AK , , USA : Exxon Valdez Oil Spill Trustee Council .
- Schmidt-Nielsen , K and Kim , Y T . 1964 . The effect of salt intake on the size and function of the salt gland of ducks . Auk , 81 : 160 – 72 .
- Shimek , S J and Monk , A . 1977 . Daily activity of sea otters off the Monterey Peninsula, California . J Wildl Manage , 41 ( 2 ) : 277 – 83 .
- Short , J W and Nelson , B D . 1998 . “ Hydrocarbon Data Analysis, Interpretation and Database Maintenance for Restoration and NRDA Environmental Samples Associated with the Exxon Valdez Oil Spill ” . In Exxon Valdez , Juneau, AK , , USA : Oil Spill Restoration Project Annual Report (Restoration Project 97290). National Oceanic and Atmospheric Administration, National Marine Fisheries Service .
- Short , J W , Kvenvolden , K A Carlson , P R . 1999 . Natural hydrocarbon background in benthic sediments in Prince William Sound, Alaska: Oil vs coal . Environ Sci Technol , 33 ( 1 ) : 34 – 42 .
- Short , J W , Lindeberg , M R Harris , P M . 2004 . Estimate of oil persisting on the beaches of Prince William Sound 12 years after the Exxon Valdez oil spill . Environ Sci Technol , 38 ( 1 ) : 19 – 25 .
- Short , J W , Maselko , J M Lindeberg , M R . 2006 . Vertical distribution and probability of encountering intertidal Exxon Valdez oil on shorelines of three embayments within Prince William Sound, Alaska . Environ Sci Technol , 40 ( 12 ) : 3723 – 9 .
- Short , J W , Irvine , G V Mann , D H . 2007 . Slightly weathered Exxon Valdez oil persists on Gulf of Alaska beach sediments after 16 years . Environ Sci Technol , 41 ( 4 ) : 1245 – 50 .
- Speakman , J R . 1998 . The history and theory of the doubly labeled water technique . Am J Clin Nutr , 68 ( Suppl ) : 932S – 938S .
- Stubblefield , W A , Hancock , G A Ford , W H . 1995a . Acute and subchronic toxicity of naturally weathered Exxon Valdez crude oil in mallards and ferrets . Environ Toxicol Chem , 14 ( 11 ) : 1941 – 50 .
- Stubblefield , W A , Hancock , G A Prince , H H . 1995b . Effects of naturally weathered Exxon Valdez crude oil on mallard reproduction . Environ Sci Technol , 14 ( 11 ) : 1951 – 60 .
- Szaro , R C , Dieter , M P Heinz , G H . 1978 . Effects of chronic ingestion of south Louisiana crude oil on mallard ducklings . Environ Res , 17 ( 3 ) : 426 – 36 .
- Szaro , R C , Hensler , G and Heinz , G H . 1981 . Effects of chronic ingestion of no. 2 fuel oil on mallard ducklings . J Toxicol Environ Health Part A , 7 ( 5 ) : 789 – 99 .
- Taylor , E and Reimer , D . 2008 . Oil persistence on beaches in Prince William Sound—A review of SCAT surveys conducted from 1989 to 2002 . Mar Pollut Bull , 56 ( 3 ) : 458 – 74 .
- Trapp , J L . 1979 . Variation in summer diet of Glaucous-winged Gulls in the western Aleutian Islands: An ecological interpretation . Wilson Bull , 91 ( 3 ) : 412 – 9 .
- Trust , K A , Fairbrother , A and Hopper , M J . 1994 . Effects of 7,12-dimethylbenz[A]anthracene on immune function and mixed-function oxygenase activity in the European starling . Environ Toxicol Chem , 13 ( 5 ) : 821 – 30 .
- Trust , K A , Esler , D Woodin , B R . 2000 . Cytochrome P4001A induction in sea ducks inhabiting nearshore areas of Prince William Sound, Alaska . Mar Poll Bull , 40 ( 5 ) : 397 – 403 .
- USARMY (US Army) . 2000 . “ Standard Practice for Wildlife Toxicity Reference Values ” . Washington, DC , , USA : Technical Guide No. 254. US Army Center for Health Promotion and Preventive Medicine, Environmental Health Risk Assessment Program .
- USEPA (US Environmental Protection Agency) . 1991 . “ EPA Region 3 Guidance on Handling Chemical Concentration Data Near the Detection Limit in Risk Assessments ” . Philadelphia, PA , , USA : U.S. Environmental Protection Agency Region 3 .
- USEPA . 1992 . “ Framework for Ecological Risk Assessment. EPA/630/R-92/001 ” . Washington, DC , , USA : Risk Assessment Forum .
- USEPA . 1993 . “ Wildlife Exposure Factors Handbook, vol I. Office of Development and Research ” . Washington, DC , , USA
- USEPA . 1998 . “ Guidelines for Ecological Risk Assessment. EPA/630/R-95/002F ” . Washington, DC , , USA : Risk Assessment Forum .
- USEPA . 2001 . “ Test Methods for Evaluating Solid Wastes—Physical and Chemical Methods. Laboratory Methods, vol 1B (SW-846) ” . Washington, DC , , USA : Office of Solid Waste and Emergency Response .
- USEPA . 2005 . “ Guidance for Developing Ecological Soil Screening Levels. OSWER Directive 9285.7-55 ” . Washington, DC , , USA : Office of Solid Waste Emergency Response .
- USEPA . 2007 . “ Ecological Soil Screening Levels for Polycyclic Aromatic Hydrocarbons ” . Washington, DC , , USA : Interim Final OSWER Directive 9285.7-78. Office of Solid Waste and Emergency Response .
- USFWS (US Fish & Wildlife Service) . 2001 . Endangered and threatened wildlife and plants; final determination of critical habitat for the spectacled eider. Final rule. 50 CFR 17 . Fed Reg , 66 ( 25 ) : 9146 – 85 .
- USGS (US Geological Survey) . 2004 . “ Large Earthquakes in the United State and the World. Prince William Sound, Alaska. USGS Earthquake Hazards Program, US Department of the Interior, Washington DC, USA ” . Available at http://neic.usgs.gov/neis/eq_depot/usa/1964_03_28.html
- Vanglider , L D and Peterle , T J . 1980 . South Louisiana crude oil and DDE in the diet of mallard hens: Effects on reproduction and duckling survival . Bull Environ Contam Toxicol , 25 : 23 – 8 .
- Venables , W N and Ripley , B D . 2002 . “ Modern Applied Statistics with S+ 4th edit ” . New York , , NY, USA : Springer Science + Business Media .
- Venkatesan , M I . 1988 . Occurrence and possible sources of perylene in marine sediments—A review . Mar Chem , 25 ( 1 ) : 1 – 27 .
- Vermeer , K . 1983 . Diet of the Harlequin Duck in the Strait of Georgia, British Columbia . Murrelet , 64 : 54 – 7 .
- Wacasey , J W and Atkinson , E G . 1987 . Energy values of marine benthic invertebrates from the Canadian Arctic . Mar Ecol Prog Ser , 39 : 243 – 50 .
- Walter , A and Hughes , M R . 1978 . Total body water volume and turnover rate in fresh water and sea water adapted Glaucous-winged gulls, Larus glaucescens . Comp Biochem Physiol A , 61 : 233 – 7 .
- Wiens , J A . 1995 . “ Recovery of seabirds following the Exxon Valdez oil spill: An overview ” . In Exxon Valdez , Edited by: Wells , P G , Butler , J N and Hughes , J S . Philadelphia, PA , , USA : Oil Spill: Fate and Effects in Alaskan Waters, pp 854–893. ASTM Special Technical Publication No. 1219. American Society of Testing and Materials .
- Wiens , J A . 2007 . Applying ecological risk assessment to environmental accidents: Harlequin ducks and the Exxon Valdez oil spill . BioScience , 57 ( 9 ) : 769 – 77 .
- Wiens , J A , Crist , T O Day , R H . 1996 . Effects of the Exxon Valdez oil spill on marine bird communities in Prince William Sound, Alaska . Ecol Appl , 6 ( 3 ) : 828 – 41 .
- Wiens , J A , Day , R H Murphy , S M . 2004 . Changing habitat and habitat use by birds after the Exxon Valdez oil spill, 1989–2001 . Ecol Appl , 14 ( 6 ) : 1806 – 25 .
- Wiens , J A , Day , R H Murphy , S M . 2010 . Assessing cause-effect relationships in environmental accidents: Harlequin Ducks and the Exxon Valdez oil spill . Current Ornithology , 17 : 131 – 189 .
- Wolfe , D A , Hameedi , M J Galt , J A . 1994 . The fate of the oil spilled from the Exxon Valdez . Environ Sci Tech , 28 ( 13 ) : 560A – 8A .