ABSTRACT
During 1985–1990, two groups of killer whales in Prince William Sound, Alaska, experienced unusually high rates of mortality, while seven others did not. Those affected were AB pod, part of the southern Alaska population of resident (fish-eating) killer whales, and the AT1 transient (marine mammal–eating) group, a very small, reproductively isolated population that last reproduced in 1984. In 1985–1986, several AB pod members were shot by fishermen defending their catch from depredation, which explains some of the deaths. Understanding the other deaths is complicated by the Exxon Valdez oil spill (March 1989) and uncertainties about the causes and times of the deaths. For AB pod, possible factors involved in the post-spill mortalities are delayed effects of bullet wounds, continued shooting, oil exposure, and consequences of being orphaned. For the AT1 group, possible factors are oil exposure, small population size, old age, and high-contaminant burdens. An analysis of possible effects of inhalation of volatile organic compounds, contact with the oil slick, and ingestion of oil with water or prey did not reveal route(s) of exposure that could explain the mortalities. The cause(s) of the killer whale deaths recorded following the oil spill remain uncertain.
INTRODUCTION
During the period 1985–1990 in Prince William Sound (PWS), Alaska, two groups of killer whales (Orcinus orca) experienced unusually high mortality rates, but others did not (Dahlheim and Matkin Citation1994; Matkin et al. Citation2008). Those affected were AB pod (35 members in 1984), part of the southern Alaska population of resident (fish-eating) killer whales, and the AT1 transient (marine mammal–eating) group, a very small (22 members in 1984), reproductively isolated population. AB pod is part of a rapidly growing, closely monitored group (AB, AI, AK, AE, AJ, AD05, AD16, and AN10 pods) of resident killer whales that numbered 117 in 1984, while, in contrast, the AT1 group last reproduced in 1984. During the period in question, AB pod lost 21 members, and the AT1 group lost 9. What caused the unexpected deaths? When did they occur? And why were just two groups affected? Unfortunately, dead killer whales infrequently strand, and even when they do, they rarely give much insight into the exact time or cause of death. One is left, then, to infer explanations from incomplete, imprecise, and often conflicting information.
Eight members of AB pod died in 1985–1988, when killer whales from that group regularly depredated the catches of longline fishermen. The fishermen, faced with severe economic losses, responded with high-power rifles and underwater explosives (MMC Citation1988; Matkin et al. Citation2008). Bullet wounds documented on some whales confirmed the shooting (Hall and Cornell Citation1986; Matkin Citation1988). Thirteen deaths in AB pod and nine in the AT1 group were discovered in 1989–1990, after the Exxon Valdez oil spill (EVOS) (Dahlheim and Matkin Citation1994; Matkin et al. Citation2008), which naturally sparked concerns that the spill had been responsible. However, 4 years after the spill, Dahlheim and Matkin (Citation1994) comprehensively reviewed the data and concluded that the evidence did not support a clear conclusion about the cause(s) of these deaths. But more recently, Matkin et al. (Citation2008) concluded that the deaths discovered in 1989–1990 were caused by the EVOS. Their new analysis, however, has left several important questions incompletely examined. For example, why were the deaths confined to just two of the six groups of killer whales observed in oiled waters in the weeks and months after the spill? Are there plausible routes of oil exposure that could have led to the whales’ deaths? Might more than a single factor have been involved?
KILLER WHALES' BIOLOGY
Two reproductively isolated ecotypes of killer whale are present in PWS, each with its own distinctive natural history. The resident ecotype eats only fish, while the transient ecotype eats marine mammals almost exclusively (Saulitis et al. Citation2000). The resident population comprises several highly stable social groups, termed “pods,” which in turn are made up of one or more matrilines (Matkin et al. Citation1999, 2008; Parsons et al. Citation2009). Each matriline is comprised of a matriarch and all of her surviving offspring of both sexes and all of the surviving offspring of her daughters and so on (Bigg et al. Citation1990). Males are generally thought to breed outside of their pod, which would promote out-breeding (Barrett-Lennard Citation2000), and consequently, their offspring would be linked to other pods. Within this breeding structure, the loss of females from a pod has significant consequences for the pod's future, while the loss of males does not. Recently, however, intra-pod matings have been confirmed in the southern resident population, which occurs mainly in Washington State and British Columbia waters (Ford et al. Citation2011); whether this pattern is more widespread is unknown.
Killer whale mothers routinely live well beyond reproductive sunset, often by several decades (Ward et al. Citation2009). This extended period of post-reproductive survival is longer than that of any other non-human mammal (Foster et al. Citation2012), and the possible adaptive significance of this has been debated for years (e.g.,Norris and Pryor Citation1991). Recently, Foster et al. (2012) analyzed the survivorship of killer whales from the SR and NR populations following the death of their mothers, and the results were surprising. This analysis showed that in the year following the death of a killer whale mother, the probability of death of her offspring generally increased significantly, especially for older males. For males ≤30 yr, the mortality rate increased 3.1-fold, but for males >30 yr, the risk of death increased 8.3-fold. In contrast, there was no increased mortality risk for females aged ≤30 yr, although the risk of death increased 2.7-fold for females >30 yr. If this relationship holds for resident killer whales in Prince William Sound as well, then the loss of killer whale mothers would have implications that extend beyond the mere loss of reproductive potential. No similar analysis exists for transient killer whales.
Of the resident pods that use PWS, eight are reliably present each year, which permits them to be censused annually (Matkin et al. Citation1999). In 1984, these pods numbered 117 individuals, 35 of which belonged to AB pod (Matkin et al. Citation2008). About 135 additional resident-type whales also used PWS, but not every year. Because of strong population growth, a total of roughly 500 resident killer whales are believed to comprise the entire population using PWS in 2009 (Allen and Angliss Citation2010).
The size and structure of transient populations are less well defined than are those of residents (Ford and Ellis Citation1999). AT1 transient social groups are usually composed of 2–4 individuals, often a female and 1–3 offspring (Saulitis et al. Citation2000, Baird and Whitehead Citation2000). Most transients in PWS belong to the Gulf of Alaska transient population, which currently numbers more than 300 (Allen and Angliss Citation2010). Transients are not seen as reliably as are residents, so that gaps of several years in the record are common (Ford and Ellis Citation1999). The AT1 transient group, however, is exceptional. Most members of this very small, reproductively isolated population, which numbered just 22 when first described in 1984 (Leatherwood et al. Citation1990), have been seen each year, allowing its composition to be followed more closely than that of other transient populations, although gaps of a year are common in the record (Matkin et al. Citation1994, 2008). For example, three AT1 members that were not observed in 1989, proved to be alive in 1990 (Matkin et al. Citation1994). In addition to being very small, the AT1 population also stands out in being genetically distinct and reproductively isolated from all other transient populations in the eastern North Pacific (Barrett-Lennard Citation2000) and in not having reproduced since 1984 (Matkin et al. Citation2008).
Annual photographic censuses of resident and AT1 transient killer whales in the eastern North Pacific rely on unique marks and color patterns to identify individuals (Bigg et al. Citation1990; Matkin et al. Citation1999; Parsons et al. Citation2009). Killer whales are assumed to have died if unobserved for a period of time prescribed by convention, which differs between ecotypes (Matkin et al. Citation2008). Residents are assumed dead if unobserved after several encounters with the pod spanning a period of ≥1 yr; the assigned year of death is the first year in which the whale was unobserved. In contrast, an AT1 transient is declared dead only after it has been unrecorded for four consecutive years, but as with the residents, the assigned year of death is the year in which it first was missing.
In many respects, the killer whale databases developed in Alaska, British Columbia, and Washington State are extraordinary. The status of nearly every member of certain resident pods and the AT1 transient group is documented in most years—a level of detail and accuracy rarely achieved in population studies. Notwithstanding the strengths of the databases, however, there are significant limitations to the accuracy of the dates of birth and death (Bigg et al. Citation1990; Parsons et al. Citation2009). For PWS residents that die, the actual time of death lies somewhere between the date when an individual was last seen in 1 year and the date when its pod was well censused in the next, a period that could span 9–14 months. The limitations become especially apparent when evaluating the potential link between mortality and a particular event, such as the EVOS. Greater uncertainty attends the actual date of death of AT1 transients, where there is an approximately 20% chance that a permanently missing whale died more than a year after it was last seen (Matkin et al. Citation2008). Although the conventions for tallying killer whale deaths offer a practical way of handling data, the uncertainty about cause, time, and place of death put limits on what can be reliably concluded.
THE MISSING WHALES
Between 1985 and 1990, 21 members of AB pod died (). Of these, 17 were maturing or adult animals (7–37 yr) with high probabilities (>0.99) of survival, based on a 24-year study of the northern resident (NR) killer whale population in British Columbia (Olesiuk et al. Citation2005). The remaining four mortalities were calves, aged 2–4 yr at the time that death was assigned; two had been orphaned. Life expectancies were also high (0.97–0.98) for calves accompanied by their mothers (Olesiuk et al. Citation2005), but comparable information about orphaned calves does not exist. Because killer whale calves depend on their mothers for nutrition and social development (Ford et al. Citation2000; Newsome et al. Citation2009) orphans would be expected to have reduced survival prospects. Thus, except possibly for the two orphaned calves, the other members of AB pod that died during 1985–1990 all had excellent survival prospects ().
Table 1 Characteristics of resident killer whales of AB pod that died, 1985–1990.
Of the 21 killer whales that died during 1985–1990, 8 of the 9 adult females were also mothers (). Half of these were discovered to have been missing before the EVOS; half afterwards, although only one, which was discovered in 1990, is known with certainty to have died after the spill. Three of the mothers (AB9, AB21, and AB23) that died also had offspring that died (AB1, AB19, and AB37). Of course, we cannot know the degree to which the mother's absence may have contributed to the mortalities of their offspring, but given the apparent significance of killer whale mothers that was reported by Foster et al. (Citation2012), this may have been a contributing factor in some of the deaths. (Note that AB1 also carried documented bullet wounds.)
Whatever the cause(s) of the statistically unexpected deaths in AB pod, the other seven closely monitored PWS resident pods were not similarly affected (Matkin et al. Citation1994, 2008). It is important to remind ourselves that the timing of the mortalities is known with an accuracy of only several months, and although Matkin et al. (Citation2008) presumed that the deaths recorded in 1989 occurred after the EVOS, there is no evidence that this was in fact true. Nor is there any evidence that the whales all died at the same place, at the same time, or from the same cause.
The sex composition of the dead AB whales is only partially known, but this information is important because the reproductive value of males and females to their pods differ radically. Of the 12 animals of known sex, nine were female (). In my analysis (below), whales of unknown sex were assumed to have been split equally between males and females.
Table 2 Maximum estimated concentrations of BTEX compounds and light aliphatics directly over the Exxon Valdez oil spill compared to recommended human exposure limits.
Nine of the 22 members of the AT1 group of marine mammal–eating transients went missing sometime between fall 1989 and spring 1990 (Matkin et al. Citation1994, Citation2008). These marine mammal–eating whales have never been implicated in depredating fisheries, and none was documented to have been shot, so it seems unlikely that the deaths resulted from shooting. Two of the whales that were missing in 1990 were males estimated to have been 33 yr old, which is beyond the mean life span of male PWS resident killer whales (Matkin et al. Citation2008), and suggests age-related mortality. In 1990, two AT1 whales were discovered stranded on a beach, but the cause of death could not be determined (Matkin et al. Citation1994).
KILLER WHALES' INTERACTIONS WITH FISHERIES
In 1984, killer whales of AB pod began taking sablefish (Anoplopoma fimbria) and halibut (Hippoglossus hippoglossoides) off the longlines of commercial fishermen in PWS (Matkin and Saulitis Citation1994; Matkin et al. Citation2008). They took an estimated one-quarter of the sablefish catch, which constituted a major economic loss for the fishermen (Dahlheim Citation1988). Fishermen responded by shooting the whales with high-power rifles and detonating underwater explosives near them. In 1985–1986, 13 AB whales were documented photographically to have been shot (Matkin et al. Citation2008). Four of these and two others died at this time (). Although no bodies were ever recovered, it is generally accepted by regulators, fishermen, and other close observers that these deaths likely resulted from the fishermen's actions (MMC Citation1988; Matkin et al. Citation2008). Two additional whales were recorded missing in 1987–1988, one of which was known to have been shot. Because they had apparently good survival prospects (), their deaths were unexpected and may also have been due to the fishery interactions. Until 1987, fishermen were not restricted in the actions that they could take to protect their catch and gear, but in that year, the National Marine Fisheries Service developed regulations to prevent fishermen from using lethal countermeasures (MMC Citation1988). In 1988, the U.S. Congress amended the Marine Mammal Protection Act (MMPA) to prohibit the intentional killing of any cetacean.
Nevertheless, some shooting has continued. Dahlheim and Waite (Citation1993) reported that seven of 12 resident pods that they photographed in Alaskan waters (outside of PWS) in 1992 contained individuals with recent bullet wounds. As Matkin et al. (Citation2008) point out, the sablefish fishery was closed in September 1988 and did not re-open until June 1989, limiting the potential for interactions during that period. But the halibut fishery was open for 2-day periods in September and October 1988, and again in September and October 1989 (IPHC Citation1989, Citation1990), presenting both potential opportunity and motive for additional shooting. In October and November 1989, the sablefish fishery was also open, and fishermen reported damage to both gear and catch (Matkin et al. Citation1989). Matkin and Ellis (Citation1990) reported that AB27 had acquired a recent bullet wound, which they suggested had come from a halibut fisherman.
The number of killer whales shot in PWS is probably larger than the number photo-documented for three reasons. First, some may have died soon after being shot and before their wounds could be photo-documented: AB9 and AB15 may have been such animals (). Second, only wounds on the dorsal fin or high on the left side of the body, which was preferentially photographed during the studies, would usually have been photo-documented. The documented wounds on the dorsal fins of AB1, AB7, and AB34 (Hall and Cornell Citation1986) do not appear to have been life threatening (Matkin et al. Citation2008); the whales’ deaths in 1985–1986, however, suggest that they had been shot elsewhere on their bodies as well. Third, cetacean skin repairs itself so quickly that entry wounds can heal over and become undetectable in just a few months (Brown et al. Citation1983; Bruce-Allen and Geraci Citation1985; Matkin Citation1986; Matkin et al. Citation1987), while the status of deep-tissue damage would not be apparent. For these reasons, therefore, in addition to the whales that were documented to have been shot, others were likely wounded as well.
What are the potential fates of whales that are shot? Matkin et al. (Citation2008) worked with the assumption that, if death is the outcome, it occurs soon after the event and bullets that are not immediately fatal sit harmlessly in the animal's body for the rest of its life. They based this belief on the fact that many of the killer whales that entered captivity survived well, even though they carried bullets that had been acquired before the capture. Yet, observations of captive animals show that another outcome is also possible. Cornell (Citation2010) necropsied two captive killer whales that had died of complications from abscesses caused by bullets acquired prior to capture. One of these died 6 years after capture (Ridgway Citation1979); the history of the other whale is unknown. In a third case involving a captive killer whale, Cornell (Citation2010) removed a bullet and bone fragments from a draining lesion that had developed in captivity. This whale was treated and survived. These observations underscore what may seem obvious. First, not all bullet wounds are immediately fatal. Second, some killer whales that are shot can survive for years, only to die later of their wounds. The latter outcome may explain at least some of the deaths in AB pod following the increased shooting in 1985 and 1986 ().
THE EXXON VALDEZ OIL SPILL AND KILLER WHALES
The spike in killer whales' deaths reported in the 2 years following the EVOS naturally sparked concern that the two events were linked. However, whether the seven AB pod members that were discovered to be missing 1 wk after the spill, died before or after the event is uncertain. No observations were made between September 1988, when fieldwork concluded for that year, and March 31, 1989, when AB pod was first encountered in that year. Aerial and vessel surveys for marine mammals began the day after the spill (Harvey and Dahlheim Citation1994; Zimmerman et al. Citation1994), but no AB whales were reported until the end of the first week, although some AT1 transients were photographed near the tanker on day 2 (see below). The six AB and nine AT1 whales that were recorded as missing in the year following the spill died over the winter of 1989–1990 (Matkin et al. Citation2008). Thus, these whales must have died 5–14 months after the spill.
When Matkin et al. (Citation1994) and Dahlheim and Matkin (Citation1994) reviewed the matter, they found the evidence linking the spill and the deaths to be inconclusive; however, Matkin et al. (Citation2008) revised the earlier finding. They based their new conclusion of causation related to the EVOS on what they considered to have been a “synchronous” decline in AB pod and the AT1 group and on the observation of some of the whales in oiled waters, although no AT1 whales were recorded as missing until 1990. They argued that the likelihood that two separate groups of killer whales would suffer a large number of deaths in a 2-year time frame was so low as to establish cause-and-effect. Such a large number of deaths in 2 years certainly is unusual, but can the deaths that are spread out over 2 years really be considered to be “synchronous”? And how might the oil spill have acted to cause the deaths?
The Oil Spill
To most people, the idea of an oil spill conjures up an image of water covered in a thick layer of sticky, black coagulum. It seems plausible that whales in such a hypothetical situation might have physical difficulty in breathing, or that they might be injured through contact with oil. The reality of an oil spill, however, is very different from this preconception. In their discussion of the use of dispersants to minimize the effects of oil spills, the National Research Council (NRC Citation2005) first considered the baseline behavior of oil in the absence of dispersants. The report's authors state that oil on open water and under calm conditions, such as prevailed in the first days of the EVOS, rapidly spreads out into a thin slick until it reaches a thickness of ∼0.1 mm, whereas the thick accumulations that feature prominently in the media represent a shoreline phenomenon that develops when oil is driven onshore, primarily by wind (Galt Citation1978). The characteristics of the EVOS slick over the first few days of the spill are outlined below. This description is based on (1) spill mass of 35,500 t (Wolfe et al. Citation1994), (2) daily slick maps prepared by Galt et al. (Citation1991), (3) evaporation and dissolution losses estimated by Galt et al. (Citation1991), and (4) an estimated water area of PWS of 9,000 km2 (Okey and Pauly Citation1999). (The area of PWS was used to estimate the proportion of the sound that was affected by oil.)
When the oil started to flow from the ruptured tanker in the early hours of 24 March, it immediately began to spread in all directions and continued to do so under relatively calm conditions until late on day 3 (; Galt et al. Citation1991). By the end of day 1, the slick had spread over a circular area around the vessel (radius = ∼5 km), covering roughly 79 km2 (∼0.9%) of PWS with oil averaging 0.37 mm thick. The slick continued to spread on day 2, doubling in radius and quadrupling in area, thereby reducing the average thickness to 0.092 mm. Spreading continued under calm conditions until the afternoon of day 3, when a storm developed. By this time, the slick had spread to about 491 km2 (5.5%) of PWS with an average thickness of 0.058 mm.
Figure 1 The area affected by the Exxon Valdez oil spill. The oil spread under calm conditions by gravity until late on day 3, when a storm carried the oil to the southwest (Galt et al. Citation1991). The storm also increased the amount of oil that evaporated and that was forced into solution, while converting much of the surface oil to an oil-in-water emulsion (mousse). Following the storm, most of the oil on open water was in a thin layer that was a few microns thick.
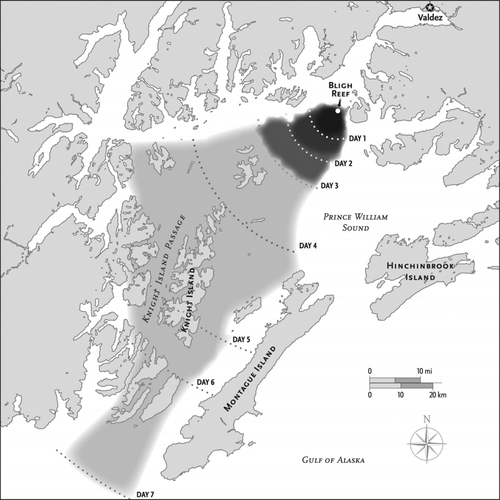
The storm not only carried the spill to the southwest, into the Knight Island area and beyond (), but it also changed the physical properties of the oil in three important ways (Galt et al. Citation1991). First, the more-or-less contiguous slick was broken-up into bands and streaks, which meant that the oil became less uniform in thickness. Roughly 90% of the slick was just a few microns thick, with thicker oil concentrated in convergence zones. Second, the amount of surface oil changed, because evaporative loss increased, accounting for 15–20% of the spill volume, while another 15–20% was dispersed into the water column. Third, much of the remaining surface oil was agitated by storm-generated wave action to form a water-in-oil emulsion (mousse) that was stickier and slower to weather than was the original oil. With a water content of about 70%, the mousse more than tripled the volume of contaminated material, much of which was driven onto islands.
By late April, floating oil in PWS was reduced mainly to surface sheens, except close to heavily oiled shorelines, where winds and tides continued to move and redistribute the oil (Wolfe et al. Citation1994). Over the course of the summer of 1989, the amount of oil floating on open water decreased to near zero, while the amount that evaporated, dispersed, or was beached and recovered increased. Although some EVOS oil found its way into all compartments of PWS, most of it was lost to evaporation, dispersion, and biodegradation. Some oil ended up on PWS beaches and some of this found its way to adjacent compartments.
Killer Whales in Oiled Waters
There were no reported killer whale observations during the first day of the spill, but four members of the AT1 group were photographed near the Exxon Valdez by a Los Angeles Times photographer, apparently on day 2. Although Matkin et al. (Citation2008) state that these photographs were taken on the day of the spill, the photographer (Kaul Citation2010) believes that they were taken on March 25, the day after the spill and the day before they appeared in the Los Angeles Times, which was March 26—unfortunately, except for the publication date, the written record has been lost, but Kaul (Citation2010) is clear that the images would have been published the day after they had been taken. As discussed below, when Ms Kaul took the photos matters because maximum exposure to potentially toxic vapors could have occurred only in the first hours of the spill. The next killer whale observations were on March 31, one week after the spill, when resident AB and AJ pods were encountered in lower Knight Island Passage, about 100 km from the spill site, in water with a sheen of oil (Harvey and Dahlheim Citation1994). Other observations of resident killer whales in oiled waters were made on April 2 (AK pod), but then nothing further until September 2–3 (AB, AI, and AN pods). Thus, whales belonging to five resident pods were observed in waters with an oil sheen on just 4 days in 1989 (Matkin et al. Citation1994), and the AT1 transients just once. The failure to obtain many observations of killer whales in oiled waters, despite an extensive survey effort, suggests that the whales did not spend much time in the spill area during the summer of 1989, possibly in response to the noise and disturbance that accompanied the cleanup. In particular, AB pod was missing for nearly 4 months (March 31–July 27). On July 27, AB pod was photographed in an unoiled part of PWS, but was seen 3 days later within the oiled part (but in water without an oil sheen)(Matkin et al. Citation1994).
Inhalation of Volatile Organic Compounds (VOCs)
To mammals, the most toxic VOCs are the BTEX (benzene, toluene, ethylbenzene, xylenes) compounds and light-weight alkanes (Neff Citation1990). Therefore, it is important to know the VOC concentrations in the air above the spill, and what threshold exposures are necessary to cause harm.
Virtually all of the oil escaped in the first 5 h after the tanker struck Bligh Reef, just 4 min after midnight on March 24, releasing VOCs at the same time (Hanna and Drivas Citation1993; Wolfe et al. Citation1994). To estimate the in-air VOC concentrations above the EVOS, Hanna and Drivas (Citation1993) considered the composition and volatility of the compounds in Prudhoe Bay crude oil, the volume and area of the spill, and the prevailing environmental conditions, particularly wind and temperature. They estimated that the highest concentrations of BTEX compounds and light aliphatics occurred 1–3 h into the spill, after which these chemicals rapidly dispersed into the air (); virtually all of the lighter-weight compounds (through nonane) had evaporated by the end of day 1. Some of the heavier VOCs, dodecane to pentadecane, persisted at very low concentrations (<1 × 10−4–6.4 × 10−4 ppm) for a few days to <1 week (Hanna and Drivas Citation1993, their Table IV). To have been exposed to the highest VOC concentrations, then, the killer whales would have to have been within the slick before 0300 h on day 1. If it is assumed, based on the overall rate of dispersion, that the spill area at this time (i.e., after ∼3 h) was an eighth of its extent at the end of day 1, it would have had a radius of 0.625 km. To have been exposed to the highest VOC concentrations, whales would have to have been inside this relatively small area very early in the morning of March 24. The spill zone would have been shrouded in darkness at this time, so had any killer whales been present, they probably would not have been seen.
Although there are no data on the threshold concentrations and exposure times for effects of VOCs that apply specifically to cetaceans, this information does exist for some terrestrial mammals. Using this information, Geraci and St. Aubin (Citation1987) estimated that the threshold for neurologic effects (e.g., narcosis) lies at air concentrations of ∼300 ppm and an exposure time of ≥3 h. They further estimated that the exposure thresholds that may cause death are approximately ≥2000 ppm for 3 h or 600 ppm for ≥12 h. The sum of the VOC maxima above the EVOS slick was <30 ppm (), an order of magnitude below the 3-h exposure threshold for the onset of neurologic effects and far less than the concentrations likely to cause death, even in the first hours of the spill.
Another approach to assessing the potential for harm from inhalation is to compare concentrations of particular VOCs above the spill against the Recommended Exposure Limits (RELs) set for humans by the U.S. National Institute for Occupational Safety and Health (NIOSH Citation2007), which are summarized in . Except for benzene, none of the estimated concentrations approached the RELs—but note that the REL for benzene is set very low to protect against long-term carcinogenic effects, not acute toxicity. After reaching a maximum at 1 h into the spill, benzene dissipated to 0.015 ppm, well below the REL, by noon of the first day (Hanna and Drivas Citation1993). Thus, the VOC maxima over the slick were transient, never exceeded acute danger levels, and after day 1, concentrations had fallen nearly to zero. Although the RELs set by NIOSH (Citation2007) were intended to be applied to humans in a workplace situation, the physiological basics should be similar (Geraci Citation1990) and, with the exception of benzene, most of the VOC concentrations did not exceed 5% of the RELs (). In the weeks and months that followed, the whales observed in oil sheens would not have been exposed to significant VOC concentrations. Therefore, given our understanding of the VOCs in the spill area and their potential effects, inhalation of VOCs as a cause of injury or death of killer whales does not seem plausible.
Table 3 Lost reproductive potential (calves/yr) due to deaths in AB pod, 1985–1990. See text for explanation of values.
Oil Contact
The first record of killer whales (AT1 transients) in oiled waters was on day 2 of the spill, when the mean thickness of the slick would have been approximately 0.23 mm (the mean of the average thicknesses at the ends of days 1 and 2). The effect of this exposure is unknown, although no AT1 whale was reported missing in the year of the spill (Matkin et al. Citation2008, 1994). The first sightings of residents in oiled waters were on days 7 and 9, when AB, AJ, and AK pods were seen in lower Knight Island Passage, about 100 km from the spill site (Harvey and Dahlheim Citation1994). These whales were reported to have been swimming in water with an oil sheen (Matkin et al. Citation1994), which would have ranged in thickness from near zero to a few microns, and this would have characterized most of the open water in the spill area (Galt et al. Citation1991). By this time, much of the oil had been carried out of PWS by winds and currents or had been deposited on beaches; some mousse patches would also have been present. Matkin et al. (Citation1994) reported no other sightings of killer whales in oiled waters until August 5, 1989 (some non-AT1 transients) and September 2–3, 1989 (AB, AI, AN residents). After July, whales would have encountered only light sheens associated with shoreline oil concentrations and cleanup operations (Lowry et al. Citation1994; Gundlach Citation2011).
Observations from other spills show that cetaceans often do not avoid oil and thus may be exposed to slicks. Geraci (Citation1990) compiled the published records of whales and dolphins swimming in oiled waters, ranging in type from crude oil to diesel, none of which appeared to indicate an adverse effect. However, these ad hoc observations did not offer opportunities to closely examine the animals. To probe the possible effects of contact with spilled oil in more detail and under controlled conditions, Geraci and St. Aubin (1982, Citation1985) conducted a series of experiments on captive bottlenose dolphins (Tursiops truncatus). Unlike sea otters, in which the deposition of oil in the fur results in loss of insulation and direct ingestion of oil during grooming, in cetaceans the primary concern associated with skin contact is damage to the integument itself. To test for effects, the investigators soaked small sponges with crude oil or gasoline and then held these against the skin for up to 75 min—an exposure, because of the rinsing effect of sea water, that far exceeded any that would potentially occur under natural conditions. Even this extreme exposure failed to elicit more than a mild, reversible reaction, thus demonstrating that cetacean skin is a highly effective barrier that tends to prevent the harmful components of crude oil from entering the body (Geraci Citation1990).
In addition to inhaling VOCs and contacting skin, the possibility of inhaling liquid oil itself should also be considered. When whales break the water's surface to breath, there is an “explosive” exhalation, followed immediately by an inhalation (Hansen Citation1992; Geraci and St. Aubin Citation1980). The forceful blow, which clears the blowhole area to avoid breathing in water, probably would also serve to clear oil in most circumstances. The slick thickness at which whales might experience breathing problems is not known, but some indication may be found in the experiments summarized by Geraci (Citation1990). In one set of experiments, bottlenose dolphins surfaced without consequence in an experimental pen that was covered with a 1-cm layer of mineral oil, although after surfacing in it just once, they showed a strong tendency to avoid the oiled pen. In other experiments, dolphins surfaced repeatedly in a pen with a 0.1 mm layer of motor oil, again without consequence. The investigators concluded that the dolphins were able to “feel the oil,” and that they preferred to avoid surface oil. It is clear, then, that bottlenose dolphins, and presumably killer whales as well, can surface and breath in the presence of thin layers of oil, but the limits of this ability are uncertain. (Note that these experiments were intended to test the response to surface oil and that both mineral oil and motor oil lack the toxic components of crude oil.)
Oil Ingestion
Could killer whales have ingested toxic amounts of oil, either with their prey or with seawater? Again drawing on data from terrestrial species, Geraci and St. Aubin (Citation1987) estimated that a 3000-kg killer whale would have to consume the equivalent of 15–75 L of fuel oil, depending on oil type, to be at risk. Because refined products such as fuel oil contain higher concentrations of toxic constituents than does weathered crude, more crude oil would have to be ingested to have the same effect. Cetaceans normally drink little seawater, and a 3000-kg killer whale might drink only 3–9 L/d (Geraci and St. Aubin Citation1987). The seawater concentrations of total polycyclic aromatic hydrocarbons (PAHs), the most toxic petroleum constituents, in the spill area in early April 1989 averaged about 0.2 ppb (= μg/L) (Neff and Stubblefield Citation1995), which equates to an estimated potential daily consumption of 0.6–1.8 μg, several orders of magnitude below the quantities at which toxic effects would be expected.
Killer whales might also consume oil through contaminated prey―fish for residents and marine mammals for transients. Hom et al. (Citation1996) measured the PAHs in fish in the spill area in 1989 and 1990, including coho salmon (Oncorhynchus kisutch), a primary prey of PWS residents (Saulitis et al. Citation2000). They found that most fish sampled contained <3.0 ppb (= μg/kg) hydrocarbon residues, which is within the range found in salmon at the reference site in southeast Alaska. As Hom et al. (Citation1996) noted, salmon migrate across vast stretches of the north Pacific, so that a contaminant found in PWS salmon did not necessarily originate there. Matkin et al. (Citation2008) also noted that AB pod presumably fed on the same stocks of salmon prey as did the pods that did not suffer unusual mortalities. The concentrations of petroleum residues found in salmon bile by Hom et al. (Citation1996) also indicated that the liver played a large role in metabolizing the petroleum compounds into compounds that can be excreted.
Regardless of source, how much PAH might a salmon-eating resident whale have consumed in PWS? Williams et al. (Citation2011) estimated the amount of Chinook salmon (Oncorhynchus tshawytscha) that an average southern resident killer whale might consume each day to be about 57 kg. Assuming that killer whales in PWS eat a similar amount of coho, they would consume about 171.0 μg PAH/d (= 3.0 μg PAH/kg × 57 kg). This is orders of magnitude less than the 15–75 L of refined fuel oils that Geraci and St. Aubin (Citation1987) estimated could be tolerated by a 3000-kg killer whale, and it is also similar to background levels (Hom et al. Citation1996).
The AT1 transients, which prey to a large extent on harbor seals (Saulitis et al. Citation2000), might have consumed seals that had been injured by oil or that had weathered oil on their skin, or both (Matkin et al. Citation2008). Although there are no records of killer whales having fed on seals from oiled haulouts, haulouts in the path of the spill () were oiled, and many of the seals that used them also became oiled (Lowry et al. Citation1994). Of the common prey of transients, harbor seals were the most exposed because of their use of shoreline haulout sites, where oil tends to accumulate. The amount of oil on the seals was greatest in the first few months of the spill, that is, April–July, when substantial amounts of oil were present in the shoreline environment. During this period cleanup operations and natural wave and tidal action progressively removed most of the shoreline oil. Major haulouts were given priority for cleanup before May 10, in advance of the pupping season, although not all of them were cleaned (Zimmerman et al. Citation1994). In addition to acquiring oil on their skin, the seals’ behavior may also have been affected. Lowry et al. (Citation1994) reported that, during April–June 1989, some oiled harbor seals at heavily oiled sites appeared “… sick, lethargic, or unusually tame.” (Whether such a change in behavior might have made the seals more or less attractive to the AT1s is unknown.) In September 1989 and May–July 1990, seals at the same sites behaved normally (Lowry et al. Citation1994). These observations agree with the pathologic findings of reversible lesions consistent with hydrocarbon toxicity in the brains of some oiled seals (Spraker et al. Citation1994). Because any oil that was still adhering to the pelage (skin + fur) of adults would have been shed during the molt in late August to mid-September (Frost et al. Citation1994a; Hoover-Miller et al. Citation2001), much of the remaining oil would have been lost at that time; pups do not molt in their first year. Thus, seals would have been most heavily oiled in the first few weeks following the spill, after which time, the haulouts would have become progressively cleaner (Lowry et al. Citation1994). By 1990, little oil remained on the major haulouts, although the amount is unknown. However, no seals collected in the spill area in 1990 had elevated levels of hydrocarbons (Frost et al. Citation1994b).
Marine mammals, in common with other mammals, have multi-function oxidase systems (cytochrome P-450) that can degrade oil into compounds that can be excreted (Engehhardt et al. 1977; Lee and Anderson 2005; Ortiz de Montellano Citation2005). The relevant research was summarized by St. Aubin (1990) in his review of the effects of oil on seals and by Geraci (Citation1990) in his parallel review on cetaceans. The studies on seals in PWS yielded results that were consistent with exposure and excretion of petroleum hydrocarbons following the EVOS (Frost et al. Citation1994b). It is noteworthy that harbor seals do not normally groom themselves, so that oil ingestion during that activity is not a potential route of exposure (St. Aubin 1990). However, Frost et al. (Citation1994b) suggested that the levels of contamination seen in harbor seal pups reflected ingestion of both external and internal hydrocarbons from their mothers while nursing. Geraci (Citation1990) reported that cytochrome P-450 enzymes have been found in several species of cetaceans and suspected that these multi-function oxidases were widespread among cetaceans. No killer whale carcasses examined during the EVOS were fresh enough to yield direct information about their ability to metabolize hydrocarbons.
To estimate how long it would take for an oiled seal to become clean, Lowry et al. (Citation1994) soaked a piece of heavily oiled seal skin in clean seawater. After 7 d, most the oil had been removed. Similarly, two seals harvested by subsistence hunters in an unoiled area in PWS in the month after the spill were determined to have been oiled only on close inspection (Hoover-Miller et al. Citation2001). As oil was removed from haulouts or as some seals moved out of the spill area, oiled seals would soon have lost most of the oil from their skins. Harbor seals also ingested oil, and this was reflected in elevated residues in the bile (Frost et al. Citation1994b). Petroleum residues were elevated in 1989, but had fallen to within the normal range in 1990. The degree to which the AT1s may have fed on oiled harbor seals is not clear, and it should be borne in mind that transients feed on a number of other marine mammal species as well, particularly Dall's porpoise (Saulitis et al. Citation2000).
Some oiled seals moved away from oiled haulouts in PWS and into unoiled areas (Lowry et al. Citation1994; Hoover-Miller et al. Citation2001), where they comprised just part of those seal populations and would have quickly shed most of the external oil within a few days. Thus, it appears that the greatest potential for AT1 transient killer whales to have fed on oiled seals would have been in oil-affected areas from about March 27, 1989, when the oil was first blown onto the islands of southwestern PWS, until late July 1989, by which time most of the oil had been cleaned up (Lowry et al. Citation1994). These observations are consistent with those made by Geraci and Smith (1976) during their experiments with oiling ringed seals.
Perhaps the very large cleanup effort created enough disturbance that the AT1s spent less time in the spill area than they might have otherwise. Note in this regard that in 1989, a massive cleanup operation focused on oiled shorelines in PWS (Carpenter et al. Citation1991). This involved hundreds of vessels and thousands of workers, along with aircraft support. The resulting noise and disturbance might have caused killer whales to avoid oiled areas. A number of observations of killer whales being disturbed by cleanup operations were also reported (Dahlheim and Loughlin Citation1989).
Between AB pod and AT1 group of killer whales in PWS, it is the AT1 transients that appear to have had the greatest potential to ingest oil, particularly as a consequence of preying on harbor seals. The amount of oil that a transient might have ingested would depend on the number of seals consumed, the proportion of the seals that were oiled, and whether the skin was eaten. The potential for consuming oiled seals was greatest in the weeks immediately after the spill, and then it diminished as the cleanup progressed, with most of the oil having been removed from the environment by the end of July. Although Matkin et al. (Citation1999, Citation2008) suggest that the AT1s fed to a significant degree on oiled seals after the spill, there are no direct observations that this occurred. Cetacean prey, such as Dall's porpoise, would have had little, if any, external oil (Harvey and Dahlheim Citation1994). The potential for AB pod members to have consumed damaging amounts of oil in their food appears to be virtually nil, as the calculations earlier in this section show; and, as Matkin et al. (Citation2008) noted, AB pod presumably ate salmon from the same migratory stocks as did the other resident whales, which did not suffer any unusual mortality. The fact that the fish-eating AB residents and the marine mammal–eating AT1 transients feed at different trophic levels also suggests that whatever caused the killer whale mortalities did not act through the food chain, or at least not in the same manner.
Changes in Prey Abundance
Could the EVOS have adversely affected the availability killer whale prey? This question has been addressed to some degree in the preceding section, where changes in the distribution of harbor seals were considered. Matkin et al. (Citation2008) also noted that AB pod probably fed on the same salmon stocks as did the other resident killer whales, whose pods have continued to grow. Thus, at our present level of understanding, there are no indications that changes in the prey base of AB pod has affected population growth. Harbor seals, which are important prey of the AT1 transients, present a more complicated picture. First, the harbor seal population in PWS was in the midst of an ongoing decline at the time of the EVOS, so separating the effects of that decline from those of the EVOS would have been inherently difficult. Second, Hoover-Miller et al. (Citation2001) challenged several key assumptions that had led to the estimate that 302 seals had been killed (Frost et al. Citation1994a) and suggested that the apparent decline in the number of seals in the spill area reflected a shift in distribution, perhaps in response to disturbance from cleanup operations. No information exists about changes in the populations of other PWS marine mammal prey, such as harbor porpoise and Dall's porpoise, although none is suspected.
Other Observations of Cetaceans in Oil
The observations of killer whales and other cetaceans in PWS after the spill add little to the overall picture. None of the killer whales observed in oiled waters showed signs of distress (Harvey and Dahlheim Citation1994; Matkin et al. Citation1994). From April 1–9, 1989, Harvey and Dahlheim (Citation1994) conducted boat-based surveys, focusing on the most heavily oiled part of PWS. They saw groups of Dall's porpoise on 28 occasions and one group of harbor porpoise (Phocoena phocoena) in oiled waters. All behaved normally, except for one Dall's porpoise observed 10 d after the spill. This animal “… was blowing every 10 to 12 s and remaining at the surface for extended periods of time (1–2 minutes),” but the reasons for this behavior are not known.
Thirty-seven dead cetaceans (26 gray whales (Eschrichtius robustus), five harbor porpoises, two minke whales (Balaenoptera acutorostrata), and three unidentified) were found stranded in the PWS-northern Gulf of Alaska region between late March and October 1989 (Loughlin Citation1994). Some of the carcasses were outside of the oil-affected area. Most were given a gross examination in the field, but only seven also yielded tissues that were fresh enough for meaningful analysis. The number of dead cetaceans found in 1989 was higher than usual, but this was attributed to the unusually high level of search effort. One gray whale had superficial deposits of petroleum hydrocarbons, but the source was uncertain and circumstances indicated that the oil had been acquired postmortem. Cause of death could not be determined for any of the cetaceans.
In their review of the literature, Matkin et al. (Citation2008) cite only one reference that indicated an adverse effect of oil exposure on cetaceans. They state (p. 277) that “Griffiths et al. (Citation1987) described the deaths of seven dolphins caused by respiratory stress due to oil inhalation after a spill in the Arabian Sea.” However, a careful reading of Griffiths et al. (Citation1987) shows that they simply repeated the contents of a two-sentence account in the Oil Spill Intelligence Report, a weekly newsletter (Anonymous Citation1983). In essence, the newsletter account tells of dead dolphins (7), sea snakes, and sea turtles found on the Saudi Arabian coast in the aftermath of an Iranian oil platform spill following an attack by Iraqi armed forces. There is no documented trail of evidence on how or by whom the carcasses were examined, nor was the basis for the diagnosis given. In other words, there is nothing to substantiate the newsletter account, and therefore, the information must be considered scientifically unreliable.
Other Factors
Certain other details of the 1989–1990 killer whale mortalities add to the puzzle. Young killer whales, because of their smaller size, might be expected to be more vulnerable to oil exposure than older, larger animals, yet two calves survived their mothers in the year of the spill (). Yearling calf AB38 lived after losing its mother, AB31, which had been previously documented as having bullet wounds, and AB23 was survived by its 3-year-old calf, AB36, which was missing in 1990.
Three other aspects of the mortality pattern relate to the possible effects of the EVOS. First, what sort of exposure effects could have operated that would have delayed the deaths recorded in 1990 until 5–14 mo after the spill? Note that none of the AT1 transients that were seen close to the Exxon Valdez on day 2 of the spill was missing until the next year. Second, of the five resident pods observed in oiled waters in the year of the spill, why was AB pod the only one to have suffered unusual losses? Third, as mentioned earlier, the presence of killer whale mothers appears to significantly improve the survival prospects of their offspring, particularly adult males. No answer to the first question is apparent, although this gap in the timing of the deaths would seem to point to multiple causes. With respect to the second question, AB pod was unique among resident pods in that it had been involved in depredating fisheries and that several of its members had been shot by fishermen (). Perhaps recent wounds or the delayed effects of bullet wounds acquired before the spill contributed to the mortalities. Finally, the 3 females that died in 1985–1990 left behind offspring that also died during the period in question.
After thoroughly reviewing the literature on oil effects on marine mammals, Geraci (Citation1990) concluded that “… there is no gripping evidence that oil contamination has been responsible for the death of a cetacean.” This is not to say that oil cannot harm cetaceans, nor that the EVOS did not somehow contribute to the deaths of PWS killer whales recorded in 1989–1990. However, a critical examination of the facts and suggested explanations does not reveal a clear and plausible causal connection.
CONSEQUENCES OF THE EXCESS DEATHS
The consequences of the mortalities to the dynamics of the two affected killer whale groups differ. AB pod's reduced growth rate since 1990 can be explained by the loss of maturing and reproductive females (Matkin et al. Citation2008). A similar phase of slow growth in the southern resident killer whale population followed the removal, in the 1960s and 1970s, of reproductive female killer whales for public display, which is believed to have impaired subsequent reproduction (NMFS Citation2008). The AT1 group, in contrast, had not produced a calf, at least not a viable one, since 1984, which suggests that intrinsic factors were operating.
To assess the effect of the loss of females to AB pod, I calculated the reproductive potential of the whales that died from 1985–1990 (), based on four assumptions, which, in turn, are based on life history parameters of the Northern Resident killer whale population (Olesiuk et al. Citation1990, Citation2005). First, the reproductive span of a resident female extends from 15–40 yr of age. Second, the interval between calves is 5 yr, so that the probability that a female would produce a calf is 0.2 in each of her reproductive years. Third, for whales of unknown sex at the time of death, I assumed that half were female, so that each of these animals had an effective 0.1 probability of producing a calf in each year of the reproductive span. Fourth, I assumed that none of the females that went missing in 1985–1990 would have died before age 40, which is reasonable because the probability of survival of these individuals was generally >0.99.
The loss of the females that died during 1985–1990 had a significant impact on the reproductive potential of AB pod, with a calculated annual reduction of 1.7–1.8 calves for most years from 1990–2011 (). About 25% of this was attributable to losses recorded in 1985–1988, when four reproductive females died (two just 3 yr from reproductive sunset), along with one male and three immatures of unknown sex. The remaining 75% of the potential reduction was attributable to the deaths recorded in 1989–1990 of five reproductive females and six immatures of unknown sex. Whatever the reproductive performance of AB pod after 1990, the Southern Alaska Resident (SAR) population as a whole has exhibited robust growth. Although AB pod grew at an average annual rate of 1.6% from 1991–2005, the other closely monitored SAR pods in PWS grew twice as rapidly at 3.2% (Matkin et al. Citation2008).
The AT1 group presents an entirely different situation. These whales are reproductively isolated from all other transient-type killer whales (Barrett-Lennard Citation2000; Barrett-Lennard and Ellis Citation2001), and thus constitute a distinct and separate population. When first described in 1984, the group included just six reproductive females and 10 reproductive males (Leatherwood et al. Citation1990). Thus, the sex ratio was unfavorable and the population had by that time fallen below the “quasi-extinction threshold” of 10 reproductive pairs (Mills Citation2007). The AT1s have produced no calves since 1984 (Matkin et al. Citation2008). To have been viable as a population, the AT1 group must once have been much larger, but by 1984, it appears to have entered the “extinction vortex,” the point at which a number of mutually reinforcing factors (e.g., Allee effects, environmental stochasticity), lead very small populations inexorably to extinction (Gilpin and Soulé Citation1986; Fagan and Holmes Citation2006; Mills Citation2007; Courchamp et al. Citation2008). As apex predators that feed on other marine mammals, the AT1s are also heavily contaminated with persistent organochlorine pollutants (POPs) (e.g., polychlorinated biphenyls, DDT, polychlorinated dibenzodioxins) (Ylitalo et al. Citation2001), which can interfere with both reproduction and immune function (Ross and Birnbaum Citation2003). In a survey of organochlorine contaminants in PWS killer whales, Ylitalo et al. (Citation2001) identified 77,000 ppb as the threshold lipid concentration of total chlorobiphenyls (ΣCBs) that is linked to reproductive dysfunction in several species of marine mammals and to immune suppression in Rhesus monkeys (Macaca mulatta). All but 1 of the 13 AT1 transients sampled exceeded this threshold, while none of the 64 residents (AB, AD5, AD16, AE, AI, AJ, AK, AN10, AS, AX pods) did. Another possible indication of POP effects is the failure of some AT1 males to develop the larger dorsal fins that normally occurs at maturity (Matkin et al. Citation1999; Matkin Citation2010). Thus, in addition to the problems associated with small population size, the AT1s may also be particularly vulnerable to reproductive failure and disease due to POP burdens.
In summary, the consequences of the excess deaths during 1985–1990 differ radically between the two affected killer whale groups. For AB pod, most important are the changes to reproductive potential due to the loss of reproductive-age females. At its greatest, the reduced reproductive capacity approached two calves/yr. This effect has diminished over time and is calculated to disappear completely after 2028. By this time, all of the females that died during 1985–1990 would have passed the age of reproductive sunset (i.e., 40 yr). The AT1 deaths had no apparent effect on the reproductive potential of that group, because the AT1s appear to have reached a state of reproductive arrest several years before the spill.
CONCLUSIONS
Two groups of PWS killer whales suffered unusually high rates of mortality during the period 1985–1990, while the other seven closely monitored pods did not. Although a well-documented database accounts for the status of most individuals in most years, the lack of direct information on the causes and times of the deaths and the fact that the whales are not observed for most of the year, greatly limits the ability to explain the mortalities. In 1985–1986, a number of members of the resident AB pod were documented to have been shot by fishermen defending their catch, and the conclusion that at least some of the mortalities recorded in those years were a consequence is generally accepted by knowledgeable observers. The Exxon Valdez oil spill, which occurred in March 1989, has been proposed as the presumptive cause of the mortalities discovered after the spill. The deaths of seven AB whales were discovered 7 d after the spill, but whether the deaths actually occurred before or after the spill is uncertain–these whales had not been seen for >6 months. Some had been previously wounded, while others may have interacted with the halibut longline fishery in fall 1988. The 6 AB and 9 AT1 transient mortalities that were discovered in 1990 clearly occurred after the spill, but by 5–14 months. Both halibut and sablefish fisheries were open in fall 1989, and AB pod was reported to have depredated the latter. A review of available data on the spill (i.e., slick behavior, volatile organic compound concentrations, contamination of water and prey) and on the potential effects of oil exposure (i.e., inhalation, contact, and ingestion) yielded no clear explanatory mechanism(s)—the potential exposures appear insufficient by a large margin. Evidence of delayed effects of bullets in killer whales was uncovered, which may explain some of the AB deaths. Finally, for the resident AB whales, the loss of the mother at any age may reduce the probability of survival, particularly for males. The effects of old age and very high contaminant levels may have contributed to the AT1 deaths. However, in the absence of more complete data, the killer whale deaths recorded in 1989 and 1990 must remain unexplained.
The failure of AB pod to grow as rapidly as the other resident pods since 1990 is explained by the loss of reproductive females over the entire period in question. However, the situation presented by the AT1 group of transients is fundamentally different. This very small population, which carries high contaminant burdens and may be subject to inbreeding depression, has not reproduced since its discovery in 1984.
ACKNOWLEDGMENTS
I am grateful to Craig Matkin for sharing insights into the killer whale research program that he has led for nearly three decades. Lanny Cornell and Marilyn Dahlheim provided details about captive killer whales that had been wounded before their capture. Steven Hanna and Peter Drivas clarified aspects of their research on in-air concentrations of VOCs following the EVOS. Photographer Rosemary Kaul shared her recollections of the photographs that she took of killer whales after the spill. Erich Gundlach provided the insights into oil spill behavior during the first few days of the EVOS. This article was greatly improved by critical reviews by Peter Chapman, Russell Fraker, Joe Geraci, Erich Gundlach, Jerry Neff, and four anonymous reviewers.
ExxonMobil Corporation provided funding; however, the analyses, interpretations, and conclusions expressed are solely those of the author.
REFERENCES
- Allen , R B and Angliss , R P . 2010 . Alaska Marine Mammal Stock Assessments, 2009. NOAA Tech Memo NMFS-AFSC-206 . Available at http://www.nmfs.noaa.gov/pr/pdfs/sars
- Anonymous . 1983 . Iraqi forces strike 26-well platform south of Nowruz . Oil Spill Intell Rep , VI : 1 – 2 .
- Baird , R W and Whitehead , H . 2000 . Social organization of marine mammal-eating killer whales: Group stability and dispersal patterns . Can J Zool , 78 : 2096 – 105 .
- Barrett-Lennard , LG. 2000 . Population Structure and Mating Patterns of Killer Whales, Orcinus orca, As Revealed by DNA Analysis. PhD Dissertation , Vancouver , BC, Canada : University of British Columbia .
- Barrett-Lennard , L G and Ellis , G M . 2001 . Population Structure and Genetic Variability in Northeastern Pacific Killer Whales: Towards an Assessment of Population Viability. Canadian Science Advisory Secretariat Research Document 2001/065 . Available at http://www.dfo-mpo.gc.ca/csas/
- Bigg , M A , Olesiuk , P F Ellis , G M . 1990 . Social organization and genealogy of resident killer whales in the coastal waters of British Columbia and Washington state . : 386 – 406 . Report of the International Whaling Commission (Special Issue 12)
- Brown , W R , Geraci , J R Hicks , B D . 1983 . Epidermal cell proliferation in the bottlenose dolphin (Tursiops truncatus) . Can J Zool , 61 : 1587 – 90 .
- Bruce-Allen , L J and Geraci , J R . 1985 . Wound healing in the bottlenose dolphin (Tursiops truncatus) . Can J Fish Aquat Sci , 42 : 216 – 28 .
- Carpenter , A D , Dragnich , R G and Smith , M T . 1991 . Marine operations and logistics during the Exxon Valdez cleanup. Proc 1991 Intl Oil Spill Conf. Prevention, Behavior, Control, Cleanup , 205 – 11 . Washington , DC, USA : American Petroleum Institute .
- Cornell , L. 2010 . Personal communication, 13 October 2010 92038 La Jolla , CA, USA Address: PO Box 2950
- Courchamp , F , Berec , L and Gascoigne , J . 2008 . Allee Effects in Ecology and Conservation , Oxford , UK : Oxford University Press .
- Dahlheim , M E . 1988 . Killer Whale (Orcinus orca) Depredation on Longline Catches of Sablefish (Anoplopoma fimbria) in Alaskan Waters. Processed Report 88–14 , Seattle , WA, USA : US Department of Commerce, National Marine Fisheries Service, Northwest and Alaska Fisheries Center .
- Dahlheim , M E and Loughlin , T R . 1989 . Assessment of Injuries to Killer Whales in Prince William Sound, Kodiak Archipelago, and Southeast Alaska. Ann Rep, Marine Mammals Study Number 2 , Seattle , WA, USA : Department of Commerce, National Marine Fisheries Service, National Marine Mammal Laboratory .
- Dahlheim , M E and Matkin , C O . 1994 . “ Assessment of injuries to Prince William Sound killer whales ” . In Marine Mammals and the Exxon Valdez , Edited by: Loughlin , T R . 163 – 71 . San Diego , CA, USA : Academic Press .
- Dahlheim , M E and Waite , J M . 1993 . Abundance and Distribution of Killer Whales (Orcinus orca) in Alaska in 1992. 1992 Annual Report , Silver Spring , MD, USA : Marine Mammal Protection Act Assessment Program, Office of Protected Resources, National Marine Fisheries Service .
- Engelhardt , F R , Geraci , J R and Smith , T G . 1977 . Uptake and clearance of petroleum hydrocarbons in the ringed seal, Phoca hispida . J Fish Res Board Can , 34 : 1143 – 7 .
- Fagan , W F and Holmes , E E . 2006 . Quantifying the extinction vortex . Ecol Lett , 9 : 51 – 60 .
- Ford , J KB and Ellis , G M . 1999 . Transients: Mammal-hunting Killer Whales of British Columbia, Washington, and Southeastern Alaska , Vancouver , BC, Canada : University of British Columbia Press .
- Ford , J KB , Ellis , G M and Balcomb , K C III . 2000 . Killer Whales: The Natural History and Genealogy of Orcinus orca in British Columbia and Washington State, 2nd edit , Vancouver , BC, Canada : University of British Columbia Press .
- Ford , M J , Hanson , M B Hempelmann , J A . 2011 . Inferred paternity and male reproductive success in a killer whale (Orcinus orca) population . J Hered , 102 : 537 – 53 .
- Foster , E A , Franks , D W Mazzi , S . 2012 . Adaptive prolonged postreproductive life span in killer whales . Science , 337 : 1313
- Frost , K J , Lowry , L F Sinclair , E H . 1994a . “ Impacts on distribution, abundance, and productivity of harbor seals ” . In Marine Mammals and the Exxon Valdez , Edited by: Loughlin , T R . 97 – 118 . San Diego , CA, USA : Academic Press .
- Frost , K J , Manen , C-A and Wade , T L . 1994b . “ Petroleum hydrocarbons in tissues of harbor seals for Prince William Sound and the Gulf of Alaska ” . In Marine Mammals and the Exxon Valdez , Edited by: Loughlin , T R . 331 – 58 . San Diego , CA, USA : Academic Press .
- Galt , JA. 1978 . “ Investigations of physical processes ” . In The AMOCO CADIZ Oil Spill. NOAA/EPA Spec Rep Edited by: Hess , W N . 7 – 20 . Washington , DC, USA
- Galt , J A , Lehr , W J and Payton , D L . 1991 . Fate and transport of the Exxon Valdez oil spill . Environ Sci Technol , 25 : 202 – 9 .
- Geraci , JR. 1990 . “ Physiologic and toxic effects on cetaceans ” . In Sea Mammals and Oil: Confronting the Risks , Edited by: Geraci , J R and St Aubin , D J . 167 – 97 . San Diego , CA, USA : Academic Press .
- Geraci , J R and St Aubin , D J . 1980 . Offshore petroleum resources development and marine mammals: A review and research recommendations . Mar Fish Rev , 42 : 1 – 12 .
- Geraci , J R and St. Aubin , D J . 1982 . Study of the Effects of Oil on Cetaceans , Washington , DC, USA : US Department of the Interior, Bureau of Land Management .
- Geraci , J R and St Aubin , D J . 1985 . Expanded Studies of the Effects of Oil on Cetaceans. Final Rep , Washington , DC, USA : US Department of the Interior, Minerals Management Service .
- Geraci , J R and St Aubin , D J . 1987 . “ Effects of offshore oil and gas development on marine mammals and turtles ” . In Long-Term Environmental Effects of Offshore Oil and Gas Development , Edited by: Boesch , D F and Rabalais , N N . 587 – 617 . New York , NY, USA : Elsevier Applied Science .
- Gilpin , M E and Soulé , M E . 1986 . “ Minimum viable populations: Processes of species extinction ” . In Conservation Biology: The Science of Scarcity and Diversity , Edited by: Soulé , M E . 18 – 35 . Sunderland , MA, USA : Sinauer Associates .
- Griffiths , D J , Øritsland , N A and Øritsland , T . 1987 . Marine mammals and petroleum activities in Norwegian waters . Fisken og Havet , : 1 – 179 .
- Gundlach , E. 2011 . Personal communication, 26 September 2011 12561 New Paltz , NY, USA Address: 15 River Park Drive
- Hall , J D and Cornell , L H . 1986 . Killer whales of Prince William Sound, Alaska: Results of 1985 field research , Bournemouth , UK : Paper SC/38/SM2 presented to the Scientific Committee, International Whaling Commission .
- Hanna , S R and Drivas , P J . 1993 . Modeling VOC emissions and air concentrations from the Exxon Valdez oil spill . Air Waste , 43 : 298 – 309 .
- Hansen , DJ. 1992 . Potential Effects of Oil Spills on Marine Mammals that Occur in Alaskan waters , 92 – 0012 . Anchorage , AK, USA : US Department of the Interior, Minerals Management Service, Alaska OCS Region . Report MMS
- Harvey , J T and Dahlheim , M E . 1994 . “ Cetaceans in oil ” . In Marine Mammals and the Exxon Valdez , Edited by: Loughlin , T R . 257 – 64 . San Diego , CA, USA : Academic Press .
- Hom , T , Varanasi , U Stein , J E . 1996 . Assessment of the exposure of subsistence fish to aromatic compounds after the Exxon Valdez oil spill . Am Fish Soc Symp , 18 : 856 – 66 .
- Hoover-Miller , A , Parker , K R and Burns , J J . 2001 . A reassessment of the impact of the Exxon Valdez oil spill on harbor seals (Phoca vitulina richardsi) in Prince William Sound, Alaska . Mar Mammal Sci , 17 : 111 – 35 .
- IPHC (International Pacific Halibut Commission) . 1989 . Annual Report 1988 Seattle , WA, USA
- IPHC . 1990 . Annual Report 1989 Seattle , WA, USA
- Kaul , R. 2010 . Personal communication, 15 October 2010 87060 Tome , NM, USA Address: PO Box 408
- Leatherwood , S , Matkin , C O Hall , J D . 1990 . Killer whales, Orcinus orca, photo-identified in Prince William Sound, Alaska, 1976–1987 . Can Field-Natur , 104 : 362 – 71 .
- Lee , R F and Anderson , J W . 2005 . Significance of cytochrome P450 system responses and levels of bile fluorescent aromatic compounds in marine wildlife following oil spills . Mar Pollut Bull , 50 : 705 – 23 .
- Loughlin , TR. 1994 . “ Tissue hydrocarbon levels and the number of cetaceans found dead after the spill ” . In Marine Mammals and the Exxon Valdez , Edited by: Loughlin , T R . 359 – 70 . San Diego , CA, USA : Academic Press .
- Lowry , L F , Frost , K J and Pitcher , K W . 1994 . “ Observations of oiling of harbor seals in Prince William Sound ” . In Marine Mammals and the Exxon Valdez , Edited by: Loughlin , T R . 209 – 25 . San Diego , CA, USA : Academic Press .
- Matkin , CO. 1986 . Killer Whale Interactions with the Sablefish Longline Fishery in Prince William Sound, Alaska 1985, with Comments on the Bering Sea Juneau , AK, USA Unpublished report by North Gulf Oceanic Society, Cordova, Alaska, for National Marine Fisheries Service
- Matkin , CO. 1988 . Status of Prince William Sound Killer Whales and the Sablefish Fishery in Late 1987 Cordova , AK, USA Unpublished report to the Marine Advisory Program, University of Alaska
- Matkin , CO. 2010 . Monitoring, Tagging, Feeding Studies, and Restoration of Killer Whales in Prince William Sound/Kenai Fjords. Annual Progress Report , Anchorage , AK, USA : Exxon Valdez Trustee Council . Available from http://www.evostc.state.ak.us
- Matkin , C O and Ellis , G . 1990 . The Status of Killer Whales in Prince William Sound in 1990 Seattle , WA, USA Unpublished report by North Gulf Oceanic Society, Cordova, AK, for National Marine Mammal Laboratory, Alaska Fisheries Science Center, National Marine Fisheries Service
- Matkin , C O and Saulitis , E L . 1994 . Killer Whale (Orcinus orca) Biology and Management in Alaska. Final report for Marine Mammal Commission contract T75135023 . NTIS PB95–166203. Available at http://www.ntis.gov/pdf/price200805.pdf
- Matkin , C O , Steiner , R and Ellis , G . 1987 . “ Killer Whale Photoidentification and Sablefish Testfishing in Prince William Sound, Alaska, 1986 ” . Fairbanks , AK, USA Unpublished report by North Gulf Oceanic Society, Cordova, Alaska, for Sea Grant Marine Advisory Program, University of Alaska
- Matkin , C O , Ellis , G and Saulitis , E . 1989 . Killer Whales in Prince William Sound in 1989 after the Exxon Valdez Oil Spill Seattle , WA, USA Unpublished report by North Gulf Oceanic Society, Homer, AK, for National Marine Mammal Laboratory, Alaska Fisheries Science Center, National Marine Fisheries Service
- Matkin , C O , Ellis , G M Dahlheim , M E . 1994 . “ Status of killer whales in Prince William Sound, 1985–1992 ” . In Marine Mammals and the Exxon Valdez , Edited by: Loughlin , T R . 141 – 62 . San Diego , CA, USA : Academic Press .
- Matkin , C O , Ellis , G Saulitis , E . 1999 . Killer Whales of Southern Alaska , Homer , AK, USA : North Gulf Oceanic Society .
- Matkin , C O , Saulitis , E L Ellis , G M . 2008 . Ongoing population-level impacts on killer whales Orcinus orca following the “Exxon Valdez” oil spill in Prince William sound, Alaska . Mar Ecol Progr Ser , 256 : 269 – 81 .
- Mills , LS. 2007 . Conservation of Wildlife Populations: Demography, Genetics, and Management , Malden , MA, USA : Blackwell Publishing .
- MMC (Marine Mammal Commission) . 1988 . Annual Report for Calendar Year 1987 Washington , DC, USA
- Neff , JM. 1990 . “ Composition and fate of petroleum and spill-treating agents in the marine environment ” . In Sea Mammals and Oil: Confronting the Risks , Edited by: Geraci , J R and St Aubin , D J . 1 – 33 . San Diego , CA, USA : Academic Press .
- Neff , J M and Stubblefield , W A . 1995 . “ Chemical and toxicological evaluation of water quality following the Exxon Valdez oil spill ” . In Exxon Valdez Oil Spill: Fate and Effects in Alaskan Waters, ASTM STP 1219 , Edited by: Wells , P G , Butler , J N and Hughes , J S . 141 – 77 . Philadelphia , PA, USA : American Society for Testing and Materials .
- Newsome , S D , Etnier , M A Monson , D H . 2009 . Retrospective characterization of ontogenetic shifts in killer whale diets via δ13C and δ15N analysis of teeth . Mar Ecol Progr Ser , 374 : 229 – 42 .
- NIOSH (National Institute for Occupational Safety and Health) . 2007 . NIOSH Pocket Guide to Chemical Hazards. Publication No. 2005–149 , Washington , DC, USA : US Department of Health and Human Services . Available at http://www.cdc.gov/niosh
- NMFS (National Marine Fisheries Service) . 2008 . Proposed Recovery Plan for Southern Resident Killer Whales , Seattle , WA, USA : Northwest Regional Office, National Marine Fisheries Service . Available at http://www.nwr.noaa.gov/Marine-Mammals/Whales-Dolphins-Porpoises/Killer-Whales/ESA-Status/Orca-Recovery-Plan.cfm
- Norris , K S and Pryor , K . 1991 . “ Some thoughts on grandmothers ” . In Dolphin Societies, Discoveries and Puzzles , Edited by: Pryor , K and Norris , K S . 287 – 89 . Berkeley , CA, USA : University of California Press .
- NRC (National Research Council) . 2005 . Oil Spill Dispersants, Efficacy and Effects , Washington , DC, USA : National Academies Press .
- Okey , T A and Pauly , D . 1999 . Trophic Mass-Balance Model of Alaska's Prince William Sound Ecosystem, for the Post-Spill Period 1994–1996 Vancouver , BC, Canada Fisheries Centre Research Reports 7:4, Fisheries Centre, University of British Columbia
- Olesiuk , P F , Bigg , M A and Ellis , G M . 1990 . Life history and population dynamics of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington state . Rep Intern Whaling Comm , 12 : 209 – 43 .
- Olesiuk , P F , Ellis , G M and Ford , J KB . 2005 . Life History and Population Dynamics of Northern Resident Killer Whales (Orcinus orca) in British Columbia. Canadian Science Advisory Secretariat Research Document 2005/04. Canadian Science Advisory Secretariat Research Document 2001/065 . Available at http://www.dfo-mpo.gc.ca/csas/
- Ortiz de Montellano , P R . 2005 . Cytochrome P450: Structure, Mechanism, and Biochemistry , New York , NY, USA : Kluwer Academic/ Plenum Publishers .
- Parsons , K M , Balcomb , K C III Ford , J KB . 2009 . The social dynamics of southern resident killer whales and conservation implications for this endangered population . Anim Behav , 77 : 963 – 71 .
- Ridgway , SH. 1979 . Reported causes of death of captive killer whales (Orcinus orca) . J Wildl Dis , 15 : 99 – 104 .
- Ross , P S and Birnbaum , L S . 2003 . Integrated human and ecological risk assessment: A case study of persistent organic pollutants (POPs) in humans and wildlife . Hum Ecol Risk Assess , 9 : 303 – 24 .
- St. Aubin , D J . 1990 . “ Physiologic and toxic effects on pinnipeds ” . In Sea Mammals and Oil: Confronting the Risks , Edited by: Geraci , J R and St Aubin , D J . 103 – 27 . San Diego , CA, USA : Academic Press .
- Saulitis , E L , Matkin , C O Heise , K . 2000 . Foraging strategies of sympatric killer whale (Orcinus orca) populations in Prince William Sound, Alaska . Mar Mamm Sci , 16 : 94 – 109 .
- Spraker , T R , Lowry , L F and Frost , K J . 1994 . “ Gross necropsy and histopathological lesions found in harbor seals ” . In Marine Mammals and the Exxon Valdez , Edited by: Loughlin , T R . 281 – 311 . San Diego , CA, USA : Academic Press .
- Ward , E J , Holmes , E E and Balcomb , K C . 2009 . Quantifying effects of prey abundance on killer whale reproduction . J Appl Ecol , 46 : 632 – 640 .
- Williams , R , Krkošek , M Ashe , E . 2011 . Competing conservation objectives for predators and prey: Estimating killer whale prey requirements for Chinook salmon . PLoS ONE , 6 ( 11 ) : e26738 doi: 10.1371/journal.pone.0026738
- Wolfe , D A , Hameedi , M J Galt , J A . 1994 . The fate of the oil spilled from the Exxon Valdez . Environ Sci Technol , 28 : 561A – 8A .
- Ylitalo , G M , Matkin , C O Buzitis , J . 2001 . Influence of life-history parameters on organochlorine concentrations in free-ranging killer whales (Orcinus orca) from Prince William Sound, AK . Sci Total Environ , 281 : 183 – 203 .
- Zimmerman , S T , Gorbics , C S and Lowry , L F . 1994 . “ Response activities ” . In Marine Mammals and the Exxon Valdez , Edited by: Loughlin , T R . 23 – 46 . San Diego , CA, USA : Academic Press .