ABSTRACT
Decisions regarding the use of building materials are being made based solely on the hazards of chemicals, without conducting risk assessments that account for realistic potential exposures and effects. We present copper as an example of a versatile, sustainable building material for which hazard classification has been misapplied. As a result, copper has been “blacklisted” for use as an exterior building material. However, its purported human health effects are not relevant for exposure to exterior building materials; furthermore, the potential environmental effects to aquatic life are not considered in appropriate contexts. We recommend evaluating risks of copper in runoff water at the point in temporal, chemical, and physical spaces at which organisms of concern will be exposed, instead of evaluating copper concentrations at the point of runoff from copper roofs, gutters, etc. Instead of banning a building material, appropriate institutional controls and/or best management practices should be required to control the release of related substances, if needed. In the absence of risk and/or life cycle assessments, architects and builders might choose regrettable substitutions in which materials posing unknown but potentially higher risks will replace more completely characterized materials that have lower risk in a given application.
Introduction
It is well accepted that hazard identification is a necessary early step in the evaluation of potential human health and environmental effects of chemicals. For example, various classification schemes (EEC Citation1967; ATSDR Citation2014; NIH Citation2015; OECD Citation2016a) have been used for several decades to categorize the hazards of chemicals (defined herein as the potential for adverse effects of a given chemical in any conceivable situation). Those classifications are being used to generate massive lists of substances that then become of concern to some regulatory agencies and the general public (State of California Citation2013; Perkins and Will Citation2014). Inclusion of some chemicals in a list often forces to search for an alternative material, effectively “blacklisting” or banning products made from these chemicals.
But taken alone, hazard identification is not sufficient for a valid evaluation of the potential for adverse effects associated with specific use scenarios of a chemical (Boobis et al. Citation2016). To risk-analysis and risk-management practitioners, that statement is obvious and might even seem unnecessary. However, the reality is that important decisions based solely on hazards of a variety of materials are currently being made at some regulatory and design levels. Therefore, the important distinction between the analysis of chemical hazards and the analysis of chemical risks is wholly ignored in some sectors. Herein, we define risk as the probability of adverse effects occurring in a potential receptor organism if exposed at a specified time in its life cycle to a specified physical–chemical form of the chemical. The lack of consideration of risks, assessing both the hazard and the exposure in the form of the chemical associated with its specific uses in building materials, is the topic of this paper.
We present copper as an example of misapplication of generic hazard-classification schemes for versatile, sustainable building materials, and we also suggest solutions for architects and construction-specification experts in order to provide an alternative path forward. Because copper has numerous forms and uses in building materials, an evaluation of generic hazards cannot possibly capture the range of realistic exposure scenarios. Other materials that are “blacklisted” for their worst-case properties could also have been used as examples, and interestingly, some of those materials sometimes are even suggested as substitutes for copper in exterior building uses (e.g., galvanized roofing in Perkins and Will (Citation2014)). The main point is the more that is known and publicized about the constituent chemistry of a material and the toxicological properties of those constituents, the more likely it is to be “blacklisted.” That in turn can lead to the pitfalls of a “regrettable substitution” (Howard Citation2014) in which the material having the least well-known information about its chemistry and toxicological properties can become the most-favored option, even if the actual risks associated with the substitute material are similar to or greater than the risks of using the “blacklisted” material (Atlee Citation2011).
Background
The problem
Copper has been erroneously misclassified as a chemical of concern in exterior building materials based on generic concerns about potential human health and environmental effects, instead of being based on use-specific physical, chemical, and toxicological properties. Additionally, some of the misclassification is the result of information that is inaccurate or has been taken out of context.
Cautionary lists prepared for architects and builders are secondary or tertiary lists that are ultimately based on primary lists of known or suspected effects of copper or copper-containing compounds. As such, the secondary and tertiary lists can become intricately cross-referenced, and they are not vetted for applicability to specific materials and their use in environments. An often-cited, non-primary list is the State of California's Safer Consumer Products Regulations Informational List of Candidate Chemicals and Chemical Groups, which states that “Chemicals that exhibit a hazard trait and/or environmental or toxicological endpoint and are listed on one or more of the lists identified in Section 69502.2(a) of Title 22 of the California Code of Regulations are Candidate Chemicals” (State of California Citation2013). Twenty-three authoritative lists from jurisdictions around the world were used to compile this “list of lists” that contains approximately 2300 chemicals. Although the website for the California list cautions users to “refer to the organization(s) that identified the chemical on the authoritative list for specific information relating to this chemical and/or the hazard trait (or traits) that were the basis for listing it,” the reality is that the California list, or subsets thereof, could be used to make decisions that do not heed that caution.
It can be easy to misapply hazard lists if context-specific reasons for the listing of chemicals and materials are not taken into consideration. For example, the California List of Candidate Chemicals and Chemical Groups (State of California Citation2013) includes common, beneficial food and public health ingredients such as “chloride,” “fluorides,” “nitrate,” “phosphorus,” “sodium,” and “sulfate” without clarification, in addition to essential trace elements like copper, iron, selenium, and zinc (). Even more exhaustive, the National Institutes of Health Haz-Map list (NIH Citation2015) includes “birds,” “caffeine,” “cellulose,” “corn oil,” the aquatic zooplankter “Daphnia,” “leather dust,” “magnesium,” “oxygen,” “potassium,” “starch,” “sucrose,” “tea,” “vegetable oil,” “water,” and “wood dust” (). The listing of Daphnia illustrates this point quite well. Daphnia are a taxonomic group of small (characteristically a few millimeters long) freshwater invertebrates that eat algae and in turn are eaten by some predaceous invertebrates and fish. Despite their beneficial uses in aquaculture, Daphnia are included in the Haz-Map list because “occupational asthma [was] reported in a fish storage worker.” Although it is important to convey such information to the public, it is not likely that Daphnia pose a major human health or environmental risk. Analogously, building materials and their constituent chemicals should not be indiscriminately transferred from hazard lists to blacklists.
Table 1. Example listings of copper and other elements, ions, non-organic materials, and selected other materials of interest for their potential adverse human health and/or environmental effects.
Despite the need for such caution, lists being promoted in the architectural and building arenas rely on indiscriminate uses of hazard lists. For example, the GreenScreen List Translator is generated by the non-governmental organization Clean Production Action (CPA Citation2016) and was compiled by consulting 43 specified lists of substances that have been classified as persistent, bioaccumulative, and toxic (PBT), very persistent and very bioaccumulative (vPvB), carcinogens, mutagens, reproductive/developmental toxicants, neurotoxicants, skin sensitizers, respiratory sensitizers, and physical hazards, as well as substances shown to elicit endocrine activity and toxic effects on ecological receptors. Those lists included the California Proposition 65 list of chemicals known to the State to cause cancer or reproductive toxicity (State of California Citation2016). None of the referenced lists is specific to risks associated with the use of copper or other elements/compounds in building materials. Very importantly, the listings are not vetted for applicability to specific materials and their use in environments, even though the GreenScreen List Translator is relied upon extensively in the building trades [i.e., as an input to several other product transparency and hazard identification tools including Health Product Declarations (HPDs; HPDC Citation2016) and the Pharos Building Product Library (HBN Citation2016)].
The architectural firm Perkins and Will maintains a website to guide its architects and the architects in other firms about, among other things, appropriate materials to specify for buildings they design. Part of that website is their Precautionary List, which includes “copper (for exterior material)” because “When copper is used as an exterior material it is a known toxic [sic] to aquatic life and a suspected toxicant to humans” (Perkins and Will Citation2014). That website statement ignores the implausibility of exposure pathways in an exterior building-material context that would be needed to lead to the suspected human health effects. Finally, it relies on unsubstantiated suspicions about adverse effects of copper to humans.
Human health concerns
Even a partial inspection of lists that include suspected and documented human health effects of copper () might suggest to a naïve reader that extreme caution should be exercised for the incorporation of copper into building materials. However, the investigation of the reasons for including copper in those lists indicates that less concern is warranted, as discussed later. The same reasoning also applies to many other metals and inorganic compounds in those lists. To conserve space, many hundreds of organic chemicals that are in the cautionary lists (some of which are commonly used in the manufacturing of building materials) are not included in .
As an example, the following five “suspected health effects” of copper are listed on the Perkins and Will (Citation2014) website: 1) cardiovascular or blood toxicant, 2) developmental toxicant, 3) gastrointestinal or liver toxicant, 4) reproductive toxicant, and 5) respiratory toxicant. To cause these suspected health problems, the first four effects would require ingestion of the building material and the last would require inhalation of copper fumes, mists, and dusts. In each scenario, exposure would also have to occur at levels known to elicit the related health effects. The probability of ingestion of copper-containing sheet roofing, flashing, downspouts, etc., by fabricators, construction workers, building inhabitants, and the general public is effectively zero. Additionally, the probability of inhalation of copper fumes by construction workers, building inhabitants, and the general public is also effectively zero, although some copper dust might be inhaled if grinding of the copper-containing material occurs without the use of appropriate personal protective equipment (PPE) during installation.
Starting early in the chain of supply and use of copper, concerns have been expressed that fabricators of copper materials can be exposed to copper fumes from the metal at high temperature (e.g., from molten copper or during brazing), thus justifying not using exterior copper building materials because of health implications for fabrication workers. However, metal fume exposure is limited by institutional controls that require protection against exposure to elevated concentrations in the workplace (Ahsan et al. Citation2009). Moreover, no data conclusively demonstrate a link between metal fume fever and exposure to copper fumes (Borak et al. Citation2000). Instead, zinc oxide is a known cause of metal fume fever (Borak et al. Citation2000), and copper inhalation exposure is often merely concurrent with zinc oxide inhalation exposure. Borak et al. (Citation2000) concluded that if copper causes metal fume fever, it must be a rare event.
Regarding copper-caused cardiovascular or blood toxicosis, hemolysis/hemolytic anemia can occur in people that have Wilson Disease (a rare genetic disorder that affects approximately 1 in 30,000 people in which excess copper accumulates and cannot be excreted; Balkema et al. Citation2008; Wang et al. Citation2010; NIH NIDDK Citation2014) and after toxic exposure to massive amounts of copper during suicide attempts [e.g., after drinking 8 g of copper sulfate (Takeda et al. Citation2000) or ingesting a fungicide containing copper-8-hydroxyquinolate (Yang et al. Citation2004)]. Additionally, methemoglobinemia resulted from the massive intake of copper during suicide attempts (Yang et al. Citation2004; Sinkovič et al. Citation2008) and an “accidental ingestion of a cup of copper sulfate (powdered form)” (Hassan et al. Citation2010, p. 490), and in an infant who drank water containing elevated concentrations of nitrate and copper (CDC Citation1993). Methemoglobinemia is a disorder in which the hemoglobin contains ferric (oxidized) iron instead of ferrous (reduced) iron, thus decreasing its ability to deliver oxygen to the tissues of the body.
In the only report related to copper-related developmental toxicosis of which we are aware, copper possibly caused liver damage in the fetus and newborn child of a woman with untreated Wilson Disease during pregnancy (Oga et al. Citation1993). Regarding gastrointestinal and liver toxicosis, copper can cause gastrointestinal upset [e.g., in suicide attempts (Takeda et al. Citation2000), accidental ingestion (Hassan et al. Citation2010), or drinking of water containing copper concentrations that exceed 3 mg/L (Pizarro et al. Citation1999)]; see also Landner and Lindström (Citation1999, pp 297–299). Chronic exposure to copper [e.g., resulting from Wilson Disease (Balkema et al. Citation2008) or long-term drinking of water in excess of the U.S. Environmental Protection Agency (USEPA) Maximum Contaminant Level (USEPA Citation2016a)] can cause liver damage (ATSDR Citation2004). Regarding reproductive toxicosis, “copper is toxic to sperm when in direct contact” (Sokas Citation1998, p. 136). In fact, an intentional direct contact with devices designed to maximize contact with copper is the posited mode of action of some Intra-Uterine Devices (IUDs).
This information is the basis for Perkins and Will (Citation2014) listing “suspected health effects” of copper. However, for all these types of toxicosis (with the exception of IUDs), either copper appears not to be a causative factor or no plausible pathway is expected from the exposure to copper-containing exterior building materials.
Environmental concerns
Environmental concerns about copper in exterior building materials are not related to direct contact with or ingestion of copper roofing, gutters, downspouts, etc., by humans. Instead, the environmental concerns stem from the mobilization of copper into water that contacts the copper surfaces, and the consequent transport of the mobilized copper through pipes, across paved surfaces, into soils, and eventually mixing with natural surface waters. Bioavailability then determines whether the transported copper will cause adverse effects. Herein, we refer to a given form of a chemical as “bioavailable” if it can pass through biological membranes and thus be assimilated by an organism (Skeaff et al. Citation2002), thereby becoming available inside the organism to potentially cause toxicity.
As the website of Perkins and Will (Citation2014) correctly states, copper can be toxic to aquatic organisms, and it also can be toxic to terrestrial organisms. However, the physical and chemical contexts of an exposure to copper are extremely important in determining if toxicity will occur. In fact, the USEPA has established national freshwater aquatic life criteria for copper and has proposed national estuarine–marine aquatic life criteria for copper that are based on bioavailability, as determined by several key water quality parameters including pH, alkalinity, water hardness, and dissolved organic carbon (DOC) concentration (USEPA Citation2007a, Citation2016b). As long as those nationally recommended copper criteria are not exceeded, survival, growth, and reproduction of at least 95% of the aquatic species are not impaired. Additionally, Meyer and Adams (Citation2010) and DeForest et al. (Citation2011) have demonstrated that despite recent concerns, olfaction (the sense of smell) in salmon is not impaired by copper if the USEPA's bioavailability-based criteria are not exceeded. The following discussion focuses on the exposures to copper in aquatic environments, but the same bioavailability concepts also apply to terrestrial environments.
Copper can leach from exterior building materials when they are in contact with water, most notably rain water and melted snow. “Pure” rainwater has a pH of approximately 5.6, “unpolluted” rain commonly has a pH of approximately 5.0, and acidic precipitation caused by anthropogenic activities can have even lower pH values. Furthermore, rain water usually contains only low concentrations of major inorganic ions (e.g., Ca2+, Mg2+, Na+, K+, HCO3−, CO32−, Cl−, and SO42−) and DOC (Drever Citation1997). At low pH, water can dissolve copper from the surface of roofing materials, gutters, downspouts, etc., because, like most other metals, the solubility of copper increases considerably as pH decreases below approximately 7.0 (i.e., into the acidic range; Stumm and Morgan Citation1996). As a result, copper concentrations in water running off a copper roof can range from hundreds to thousands of micrograms per liter, depending on many factors including the degree of weathering of the copper materials, the water chemistry, and the length and angle of the roof (He et al. Citation2001; Boller and Steiner Citation2002; Boulanger and Nikolaidis Citation2003; Landner and Reuther Citation2004; Arnold Citation2005; Athanasiadis et al. Citation2006; Bielmyer et al. Citation2012). Hedberg et al. (Citation2014) reviewed physical and chemical factors affecting the mobilization and runoff rates of copper from roofing materials and also found that, in general, copper concentrations in roof runoff have decreased in recent decades, as acid precipitation has decreased.
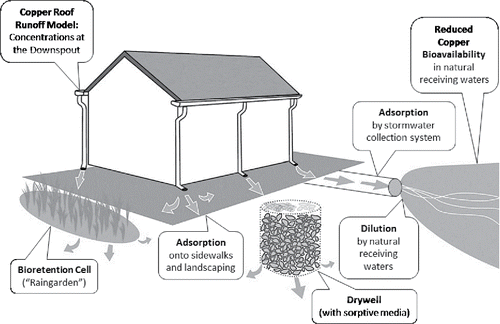
The high concentrations of copper in water flowing off copper roofing could cause acute or chronic toxicity to sensitive aquatic organisms if exposed to copper in the low-ion-concentration water within the rain gutter. However, those sensitive aquatic organisms (e.g., cladocerans, insects, and fish) do not live in rain gutters and downspouts; instead, they live in surface waters that are separated from the gutters and downspouts by stormwater-conveyance systems, paved surfaces, stormwater control features, landscaping materials, and/or soils. The runoff must flow through or across some or all of those physical features before reaching a receiving water body.
The intervening materials between a roof and a receiving water body can interact with copper in the runoff to sorb copper to or precipitate it onto surfaces. Concrete conduit and possibly cast-iron pipe can remove copper from water, whereas polyvinylchloride pipes do not remove copper (Perkins et al. Citation2005). For example, 45% of the copper in a roof runoff water was removed as it flowed through a 57.9-m cast-iron-and-concrete internal piping network (Boulanger and Nikolaidis Citation2003). Likewise, copper can precipitate onto concrete slabs (e.g., sidewalks) and other surfaces as copper carbonate, copper hydroxide, or copper sulfate minerals, thus removing considerable percentages of the total copper released from copper roofing during runoff events (Liu et al. Citation2005; Bahar et al. Citation2008; Sondhi Citation2010). Additionally, soils and retention-basin sediments can retain copper as runoff water flows through them (Nightingale Citation1987b; Wilde Citation1994; Bertling et al. Citation2006), making commonly installed stormwater management features such as retention ponds, bioretention planter boxes, biofiltration swales, and lawns effective sinks for copper (>90%) and other metals (Davis et al. Citation2001, Citation2003; LaBarre Citation2014; LaBarre et al. Citation2016).
In addition to removing copper, the concrete and soils can alter the chemistry of runoff waters. For example, pH, water hardness (an index of the concentrations of Ca2+ and Mg2+ ions), and alkalinity (an index of the acid-buffering capacity of the water, dominated by HCO3− and CO32− ions in most fresh waters) generally increase as runoff passes over/through concrete and some soils (Nightingale Citation1987a; Boulanger and Nikolaidis Citation2003; Athanasiadis et al. Citation2006, Citation2007; Kwiatkowski et al. Citation2007; Bahar et al. Citation2008; Sondhi Citation2010; LaBarre Citation2014). Furthermore, DOC concentrations usually increase as runoff passes through soil (Bertling et al. Citation2002; Boulanger and Nikolaidis Citation2003; Li and Davis Citation2009; LaBarre Citation2014). In fact, the increased pH and HCO3− and CO32− concentrations cause carbonate and hydroxide minerals of copper and other metals to precipitate from the water onto concrete surfaces and soil particles (Athanasiadis et al. Citation2007; Sondhi Citation2010).
Changes in water chemistry are also important because they alter the bioavailability of copper to organisms even before the water reaches a stream, river, pond, lake, estuary, or ocean (Bertling et al. Citation2002; Boulanger and Nikolaidis Citation2003; Bertling et al. Citation2006; Bahar et al. Citation2008; Li and Davis Citation2009; LaBarre Citation2014). The receiving water dilutes and further alters the chemistry (and thus the bioavailability of copper) in the roof runoff. In general, the toxicity of copper and other metals to aquatic organisms decreases as pH, water hardness, alkalinity, and DOC concentration increase (Meyer et al. Citation2007).
Therefore, much occurs between the time and the location at which copper-laden water leaves a copper roof and the time and location at which it enters surface water that contains aquatic organisms of concern. The ensuing losses of copper, the changes in bioavailability, and the dilution by the receiving water determine the ecological risks associated with copper leached from exterior building materials (Sundberg and Odnevall Wallinder Citation2001; Landner and Reuther Citation2004). Considering only the concentration of copper in water as it flows off a roof can lead to overly conservative predictions of the potential toxic effects of copper to aquatic organisms. Even a high copper concentration of several thousand micrograms per liter in rain water as it flows off a roof might not adversely affect aquatic organisms in a receiving water body, depending on (1) the changes in water chemistry in transit to the receiving water body, (2) the amount of copper removed by the sorption onto surfaces and by precipitation of copper-containing minerals from the runoff water, and (3) the chemistry of and amount of dilution by the receiving water.
Consequently, although it is correct that copper can be toxic to aquatic organisms, not all runoff from copper surfaces on exterior building materials pose a significant risk to aquatic organisms. When significant risks are plausible, physical–chemical treatment can be used to further decrease the copper concentrations (see “Management of runoff” section).
Copper's attributes
Copper naturally occurs in water, soil, sediment, rocks, and even in the air (ATSDR Citation2004). It is essential for all life (Olivares and Uauy Citation1996; Eisler Citation1998), being a key component of numerous enzymes and other proteins involved in electron transfers in all organisms and in the transport of oxygen in some arthropods (Fraústo da Silva and Williams Citation1991). However, copper can also be toxic in exposures ranging from relatively low concentrations (e.g., for aquatic organisms exposed to dissolved copper; USEPA Citation2007a) to relatively high concentrations (e.g., for aquatic organisms exposed via diet, and for birds, humans, and other mammals exposed via drinking water and diet; Eisler Citation1998; ATSDR Citation2004; DeForest and Meyer Citation2015). In humans, high copper concentrations generally cause short-term digestive problems like acute nausea; in fact, the World Health Organization (WHO) and United States drinking water guidelines/standards for copper (2.0 and 1.3 mg/L, respectively; WHO Citation2011; USEPA Citation2016a) are set to protect against acute nausea. Over the long term, “intentionally high intakes of copper can cause liver and kidney damage and even death” (ATSDR Citation2004, p. 7). However, copper has not been demonstrated as a cause of cancer in humans (ATSDR Citation2004).
Copper is present in sulfide- and carbonate-containing ores that are mined and processed into a variety of solids, ranging from crystalline salts that generally have high solubility (e.g., CuCl2) to copper sheeting (i.e., more than 99% pure copper in its elemental form) that is less-readily dissolved (a characteristic of the elemental form of all metals; Skeaff et al. Citation2008). Herein, we refer to the crystalline salts and related forms of copper as “dispersive” because of the relative ease with which they can disperse through the environment in solid or liquid forms, and we refer to the more consolidated forms such as copper sheeting as “durable” because of their rigidity and cohesiveness, thus leading to a tendency to maintain their original form and not disperse through the environment. Furthermore, at the end of useful life, almost all the durable copper is still in the product and can be recycled (Goonan Citation2009). Approximately one-third to one-half of the amount of copper that is put into use worldwide is eventually recycled at the end of life – often decades or centuries later (Landner and Lindeström Citation1999; USGS Citation2009).
Because copper sheeting is malleable yet rigid and lasts for a long time, it is a versatile building material used in many durable forms. For example, copper roofing material can last for many centuries (Gowen Citation1955; Schade Citation2007) and is appreciated for the decorative and protective green patina finish that develops as it weathers (Landner and Lindeström Citation1999; Swift Citation2011). In the interior of buildings, copper has long been used in electrical and plumbing systems, and it is even a key heat-transfer material in heating, ventilation, air conditioning, and refrigeration equipment used in buildings (Hepbasli and Kalinci Citation2009). Moreover, unlike some synthetic substitutes, copper in durable products is fully recyclable with a minimal carbon footprint (Schade Citation2007). Therefore, its use can contribute in many ways to the long-term sustainability of a building.
Inappropriate consideration of bioavailability
Despite its essentiality, copper at elevated concentrations can be toxic when it is in a bioavailable form as the dissolved, “free” cupric ion (Cu2+). But if copper is not in the “free” form in a dissolved phase (e.g., if Cu2+ forms a chemical complex with an inorganic or organic ligand such as a bicarbonate ion or a DOC form like humic acid), its bioavailability and potential toxicity decrease considerably (Meyer et al. Citation2007). The key is that an organism must be exposed to toxic concentrations of “free” copper while in water (for aquatic organisms that exchange electrolytes, O2, and CO2 across membranes exposed to water), or the organism must ingest copper via drinking or eating and then convert the bound copper to a chemical form (e.g., “free” copper) that is assimilated across the digestive tract. Illustrating the importance of the chemical form of copper to which an organism is exposed, it is recommended that cupric oxide (CuO) should not be used as a copper supplement for humans and other animals because of its low bioavailability (especially relative to the highly soluble copper sulfate [CuSO4] salt; Baker Citation1999).
Even if copper is directly or indirectly bioavailable to an organism, the internal pool of copper must exceed the organism's ability to sequester, detoxify, and/or excrete it before toxic effects will be manifested (Luoma and Rainbow Citation2008, pp. 153–159, 206–208). Therefore, not all forms and amounts of copper are detrimental (Landner and Reuther Citation2004); instead, copper is beneficial in small amounts. This important conclusion is lost in simplistic considerations of hazard-classification lists.
Physical–chemical forms of copper used in most exterior building materials are not the forms of copper that demonstrate toxicity in the laboratory. Menzie et al. (Citation2008) emphasized that when conducting environmental assessments, it is important to understand the chemical form of a metal to which an organism is exposed, and to that point, we add that it is also important to understand the physical form of the material in which the chemical is being used. Physical properties of metal-containing building materials, especially their physical phase (i.e., solid, liquid, or gas), solubility, and vapor pressure, are extremely important in determining potential exposure of human and ecological receptors to the metal.
For example, instead of using durable sheets of copper roofing or downspouts to dose waterborne- or dietborne-exposure toxicity tests, researchers usually use highly soluble copper salts (e.g., CuCl2 and CuSO4) in laboratory toxicity studies conducted with aquatic and terrestrial organisms (some of which are also used to infer toxicity to humans); see LaBarre (Citation2014) for an exception. Copper in those salts is highly bioavailable relative to copper in roofing sheets and downspouts, thus representing worst-case exposures. In feeding studies, copper salts are more readily dissolved and assimilated in the consumer's digestive tract than elemental copper, copper oxides, copper alloys with other metals, and copper incorporated into low-digestibility food items (e.g., fibrous material). In aquatic toxicity tests, the exposure waters often contain relatively low concentrations of copper-complexing inorganic ions and organic matter, thus maximizing the bioavailability and toxicity of copper in those waterborne exposures (Meyer et al. Citation2007). Results of these worst-case toxicity tests are then often used as the basis for thresholds in hazard-classification schemes. The user of such thresholds then makes the incorrect inference that this level of hazard applies to all applications of copper, and the threshold determines whether the use of copper in building materials should be voluntarily limited, restricted by regulations, or even banned. Yet the highly bioavailable copper salts that are routinely used in toxicity tests (the basis for hazard classifications of copper) represent only a small percentage of worldwide copper production (e.g., less than or equal to approximately 1% of the annual supply of copper products in Europe and worldwide; Landner and Lindeström Citation1999; Tables 2–4 in ECI (Citation2008)) and are generally not used in building materials, which are constructed from solid-phase copper.
Table 2. Incidence of metals, polycyclic aromatic hydrocarbons (PAH), and phthalates (Phthal) that leached from 16 metal-based, organic-chemical-based, tile, and wood roofing materials at detectable concentrations more than those reported for the runoff from control glass sheets (summarized from the results in Winters and Graunke (Citation2014) and Table ES-2 in Winters et al. (Citation2014)).
Recognizing the importance of physical form, the Pharos project (HBN Citation2016) recently began to distinguish hazards based on the chemical form in their Chemical and Material Library (CML). For example, Pharos now has a copper dust/powder/fume profile which is separate from the solid copper profile. This is a positive development, and it is likely that HPDs, which make use of the Pharos CML (HPDC Citation2016), will also move in this direction.
Discussion
By definition, hazard classifications consider worst-case examples of the potential effects of highly bioavailable forms of a chemical, whether or not a potential human or ecological receptor would be exposed to a given form of the chemical under conditions that would result in the adverse effect (Skeaff et al. Citation2002; CCOHS Citation2015). A hazard-based approach can lead to situations in which a chemical's hazard can be ranked high based on worst-case scenarios but the risk of using it in a given situation is low. Evaluating hazards for a chemical that has numerous forms and uses is overly simplistic because it ignores the wide range of possible exposures (or a lack thereof).
Such disconnects between hazards and risks often occur for versatile, sustainable building materials. Some of those materials are being misclassified as health and environmental concerns despite the fact that they do not present their constituent chemicals in a highly bioavailable form to which humans or ecological receptors will be directly or indirectly exposed. Therefore, these types of generic hazard-classification schemes can be risky, because they can lead to inappropriate and sometimes even non-sensical decisions when taken out of context.
Architects and the general public are justifiably concerned about sustainability and potential human health and environmental effects of a wide variety of building materials (USGBC Citation2010; Atlee Citation2011; Gannon Citation2011; Perkins and Will Citation2014). However, as a result of increasing application of the Precautionary Principle (Collegium Ramazzini Citation2005; Richter and Laster Citation2005), that concern has led to well-intentioned but not fully informed “blacklists” (Bardelline Citation2009) that have been prepared based on worst-case hazards of substances instead of being based on risks associated with specific applications (Perkins and Will Citation2014). These lists of chemicals of concern are then consulted by architects and builders, and sometimes by local government agencies, who avoid or recommend avoiding the listed substances without considering use-specific risks and without also considering risks associated with non-listed substances that might be in substitute building materials. That approach ignores the fact that risk is the intersection of hazard and exposure, and that no (or very little) exposure results in no risk regardless of how hazardous the substance is.
Consequently, although lists of chemicals of concern might initially have been intended merely as guides to help architects and builders consider more benign options, they have become akin to “thou shalt not use” commandments. The current general misclassification of building materials begs for a change to a risk-oriented approach in which the physical–chemical form of the substance, the potential exposures of human and environmental receptors, and recycling/post-disposal challenges are considered in addition to the hazards posed by the most bioavailable form of each individual chemical constituent in the building materials. More appropriate approaches that consider risk instead of hazard are available (USEPA Citation2007b).
Appropriate approaches
It is important to list major human health and environmental hazards, so regulators and the general public can make informed decisions about potential uses of chemicals and materials. However, listing every chemical and material that has ever been suspected of causing an adverse effect, no matter how minor, site-specific, and/or rare, can lead to questionable applicability for many uses [e.g., see listings of cellulose, Daphnia, leather dust, and wood dust among others in the Haz-Map list (NIH Citation2015)]. For example, if wood dust were treated the same as copper (which is also in the Haz-Map list), wood would be blacklisted as a building material because it poses a potential hazard at least during the construction phase of buildings. Obviously, institutional controls can be used to minimize the potential effects of (and thus present a low risk for) the use of wood in building materials, but a strict hazard-based approach would not take into account factors that ameliorate exposure to wood dust. Therefore, it is more reasonable to rely on risk-based decisions instead of hazard-based decisions when classifying chemicals and materials of concern for use in buildings.
Regulatory bodies that deal with both risks and hazards (instead of dealing only with hazards) adopt more appropriate approaches to classify chemicals and materials. For example, in contrast to most of the generalized hazard lists discussed earlier, copper is not included in the USEPA list of 31 priority chemicals for waste minimization (USEPA Citation2015a). Copper is also not in the USEPA Resource Conservation and Recovery Act (RCRA) list of 39 toxicity characteristic chemicals (USEPA Citation2005). Copper cyanide (an industrial process chemical which is not incorporated into solid-phase building or other products) is the only copper-containing compound in the list of chemicals that are considered a hazardous waste under RCRA when discarded (USEPA Citation2015b).
In place of the hazard-based approach to classify copper and other metals that are used in building materials, we recommend a risk-based approach such as the one outlined in the USEPA (Citation2007b) Framework for Metals Risk Assessment. Additionally, the European Union currently uses a risk-ranking method for existing chemicals (Hansen et al. Citation1999), and it might be possible to adapt a recently proposed approach for the risk-based classification of nanomaterials (Tervonen et al. Citation2009) to an analogous classification of building materials. The USEPA's risk-based approach recognizes that some metals like copper are essential for survival, growth, and reproduction of organisms, and it takes into account local background concentrations and factors that affect the bioavailability of metals when evaluating potential impacts. Very importantly, USEPA (Citation2007b) clarified that metals should not be evaluated like organic chemicals. Therefore, the PBT concept does not apply to metals like copper. As a result, copper is not included in the USEPA list of 31 priority chemicals (USEPA Citation2015a); however, it is still erroneously being considered a PBT in some green building schemes (USGBC Citation2010).
Several industry organizations have also recognized the need to provide frameworks for evaluating risks of chemicals and materials. For example, the International Council on Mining and Metals provides guidance for human health and environmental risk assessments of metals (ICMM Citation2016a, b). The Resilient Floor Covering Institute (RFCI) has developed a process that takes a step away from the simplistic approach used for HPDs, which list all the chemicals in a flooring product and screen them against 32 specified hazard lists. Instead, RFCI (Citation2013) has progressed to recommend Product Transparency Declarations (PTDs), which indicate whether the chemicals in a finished product would be present in a form that would pose the hazard for which the chemical is included in one or more of a large number of hazard lists. In that way, all relevant chemicals are still identified, but potential exposure to users and installers is placed in a more appropriate perspective. A similar approach for exterior building materials could help to differentiate among potential risks.
Life cycle assessments (LCAs) and/or life cycle impact assessments (LCIAs) (Bare Citation2006; Udo de Haes et al. Citation2006) could complement risk assessments of building materials, as in the Green Globes (Citation2016) building-certification program and in Environmental Product Declarations (EPDs) used for the Leadership in Energy and Environmental Design (LEED) certification (USGBC Citation2016). In fact, Socolof and Geibig (Citation2006) suggested that an LCA could be used to determine whether a focused risk assessment might be needed. Important considerations that are not captured in risk assessments include the longevity, recycling potential, and energy/carbon footprint of the materials. Additionally, historical preservation can be an important consideration (Swift Citation2011). A combination of a risk assessment and an LCA/LCIA could be especially important when evaluating potential substitute materials (see “Regrettable substitutions” section). However, caution must be used in comparing the results of LCA/LCIA, given the potential for differences in underlying assumptions (i.e., the same Product Category Rules [PCRs] should be used if EPDs are compared).
Detailed risk assessments for building materials would not have to be conducted on a building-by-building, site-specific basis and thus would not have to lead to a prohibitively expensive overall effort. Instead, a generalized risk assessment approach would probably eliminate copper as a human health concern for common uses in exterior building materials, and it would leave only exposure of aquatic organisms to copper in the runoff as a possible concern.
The key to any risk assessment would be the estimation of the potential exposure of humans and other organisms to copper and the associated potential effects at the predicted concentrations under realistic environmental conditions. The first types of considerations in such an analysis should include whether a pathway exists in a water body containing aquatic organisms of concern and whether sufficient rainfall occurs to be of concern. Then, if those considerations are met, the analysis should consider not only the concentration of copper at the point at which water runs off a roof or other copper-containing exterior building material, but it should also consider all the processes that can decrease the copper concentration along the flow path of the runoff water before it enters a receiving water body (; Sundberg and Odnevall Wallinder Citation2001; Bertling et al. Citation2006; Hedberg and Odnevall Wallinder Citation2011). Additionally, the receiving water quality and potentially exposed aquatic organisms should be incorporated into the analysis (Hedberg and Odnevall Wallinder Citation2011). Water chemistry and dilution by the receiving water determine the bioavailability of copper, and the physiological characteristics of the aquatic species determine their sensitivity to the bioavailable copper (Meyer et al. Citation2007). A toolbox to conduct such an evaluation is discussed in “Solutions for design-team practitioners” section. If unacceptable risks remain after an assessment of water flow off exposed copper surfaces, through conveyance systems and soils, and across paved surfaces, the need for additional management of the water might have to be explored.
Management of runoff
Several options are available to manage the runoff from exterior copper surfaces. Some exterior surfaces can be protected from contact with rain and snow to minimize copper release. However, most roofing material by design is in direct contact with precipitation. In situations when high concentrations are expected to reach natural aquatic systems, leaching of copper from exposed surfaces can be decreased/prevented by using pre-patinated surfaces, clear-coat finishes, or sacrificial coatings (Barron Citation2006). In chemical terminology, a patina is “a thin usually green layer that forms naturally on the metals like copper and bronze when they are exposed to the air for a long time” (Merriam-Webster Citation2015) and consists of “compounds with low solubility in water containing hydroxyl ions as well as sulfate or chloride ions” (Landner and Lindeström Citation1999, p. 17; see also Krätschmer et al. (Citation2002)). A patina can also be produced by the chemical treatment in industrial processes (Bertling et al. Citation2006). Although copper in a patina can still be soluble in water to varying extents depending on environmental conditions (Bertling et al. Citation2006), the patina can decrease further corrosion and subsequent copper-leaching rates (Landner and Lindeström Citation1999; Sundberg and Jonsson Citation2005; Hedberg and Odnevall Wallinder Citation2011; Hedberg et al. Citation2014). Sacrificial coatings are a layer of, for example, lead or zinc between the copper and the air that prevent corrosion of the underlying copper (Barron Citation2006). However, corrosion and subsequent leaching of metals such as lead and zinc merely substitute one metal for another in the runoff, analogous to the problem engendered by the use of substitute roofing materials (see “Regrettable substitutions” section).
If the runoff from exterior copper-containing surfaces contains elevated copper concentrations that pose an unacceptable risk to organisms in a receiving water, best management practices (BMPs) that include stormwater control measures (SCMs; e.g., treatment of the runoff water) might need to be implemented. Barron (Citation2006) listed metallic-exchange and ion-exchange systems as physical–chemical treatment options, and effective filter systems containing granulated iron oxyhydroxide and calcium carbonate have been developed in Switzerland (Boller and Steiner Citation2002; Steiner and Boller Citation2006; Steiner et al. Citation2006), but those options are relatively expensive and require maintenance that, although being feasible for large commercial and public buildings, might exceed routine residential capabilities and habits. Athanasiadis et al. (Citation2006) demonstrated more than 90% removal of copper by passing the copper roof runoff through filtration-media systems containing various types of zeoliths, with much of the removal probably due to the precipitation of copper-containing minerals at the elevated pH values in the concrete tanks (Athanasiadis et al. Citation2007).
In contrast, bioretention systems incorporated into the overall stormwater management of a building site offer a lower cost, lower maintenance, and lower impact option. Previous research has demonstrated that more than 90% of the copper in the simulated runoff can be removed from biofiltration boxes and cells that contain plants and soil (Davis et al. Citation2003), and recent research has demonstrated high removal (>90%) of copper in runoff from an outdoor copper roof (LaBarre Citation2014; LaBarre et al. Citation2016). The latter system tested biofiltration boxes (which contained plants, soil, gravel, and stone) and biofiltration swales (which contained grass, compost, soil, gravel, and crushed limestone). Limestone and crushed concrete help to neutralize the pH of the runoff water and thus help to enhance the precipitation of copper-containing minerals as the water percolates through the soil–limestone mixtures (Bertling et al. Citation2002, Citation2006; Athanasiadis et al. Citation2007; Kwiatkowski et al. Citation2007; Sondhi Citation2010), and soils alone can retain considerable copper (Bertling et al. Citation2002). In addition to decreasing the copper concentrations, biofiltration boxes and swales decrease the bioavailability and toxicity of copper in roof runoff by several orders of magnitude (LaBarre Citation2014). Even in bioretention cells that removed smaller percentages of copper from highway and parking lot runoffs (average removals of 62–72%), the increased DOC content of the effluent water from the bioretention cells decreased the bioavailability of the remaining copper (Li and Davis Citation2009). Therefore, bioretention systems can be a key component of low-impact development (LID) systems (Davis Citation2005; Dietz Citation2007). Göbel et al. (Citation2008) provided recommendations for uses of swales and trenches with sand, gravel, and lime when treating runoff from copper and other types of roofs.
Regrettable substitutions
Substitutes are sometimes sought when a chemical or material is identified as a hazard or has an unacceptable risk associated with its use. In fact, “The theory behind hazard-based chemical assessment is that by eliminating hazard, exposure of any sort (and any associated potential risk) is no longer a concern” (Reid Citation2016, p. 1). However, replacement of one chemical or material without careful scrutiny of the replacement can lead to a “regrettable substitution” (Howard Citation2014) that merely shifts from a known hazard (with or without a high context-specific risk) to a lesser known or unrecognized hazard. For example, the Precautionary List of Perkins and Will (Citation2014) suggests stainless steel, galvanized steel, and paint finishes for flashing as alternative materials to replace exterior copper materials. However, the two suggested metal replacements also have potential human health or environmental concerns associated with them. Paint finishes are a management option for decreasing the runoff from copper roofing material (see “Management of runoff” section), but they also have potential environmental issues such as leaching of organic chemicals and/or pigments from paints. Therefore, alternative chemicals and materials that are not well vetted can possibly lead to regrettable substitutions in which the alternative has less desirable consequences than the original.
For example, alternatives such as organic-polymer materials, tiles, and wood are sometimes suggested to replace metals for use as roofing materials. However, a recent comparison of 16 metal-based, organic-polymer, tile, and wood roofing materials demonstrated that metals, polycyclic aromatic hydrocarbons, and/or phthalates leached out of all the tested materials, and metals commonly leached from the tile, wood, and organic-polymer materials (; Winters and Graunke Citation2014; Winters et al. Citation2014, Citation2015). In addition to potential environmental effects of leached chemicals in runoff water, the production of the organic polymers raises sustainability concerns about hydrocarbon use and human health and environmental safety (Thornton Citation2002; Stern and Lagos Citation2008). Therefore, the more one investigates, the more one discovers that no perfect solution exists.
This discussion of regrettable substitutions is not meant to disparage any of the available exterior building materials. Instead, this simply implies that institutional controls, barrier coatings, and/or SCMs/BMPs might have to be considered for all exterior building materials, depending on their context-specific uses. Additionally, this discussion demonstrates a problem that hazard-based analyses can lead to, if building materials and their chemical components are evaluated based on limited knowledge or current fads.
In concept, alternatives-assessment approaches could help to avoid regrettable substitutions. “The goal of alternatives assessment is to inform choices of alternative chemical and non-chemical options when a decision has been made for scientific, market, or regulatory reasons to avoid a substance of concern. It differs from risk or safety assessments, which aim to identify a safe level of exposure or characterize the impacts associated with a particular chemical.” (Chemical Watch Citation2015). Thereby, alternatives assessments are intended to avoid regrettable substitutions by screening out unfavorable alternatives (Cohen and Lewandowski Citation2016), as practiced by several organizations (CPA Citation2014; OECD Citation2016b; SciVera Citation2016). Very importantly, alternatives assessments can include consideration of exposure and other factors (e.g., life cycle attributes and socioeconomics; NRC Citation2014). However, a wide variety of approaches exist (e.g., see reviews by OECD (2013), Gauthier et al. (Citation2014), and NRC (Citation2014)). Many alternatives' assessment procedures assign primary and high priorities to the hazard-assessment component (Chemical Watch Citation2015), and they generally refer to the same “authoritative” lists. Few chemical assessment tools characterize risk (Gauthier et al. Citation2014). We favor the approach recommended by NRC (Citation2014), which places human health, ecotoxicity, and exposure considerations at an equal level and concurrently analyzes potential alternatives.
It is also especially important to consider the availability (or lack) of hazard data when identifying potential alternatives, because absence of evidence of effects is not necessarily evidence of absence of effects (SETAC Citation1999). To that end, it is encouraging that the USEPA Safer Choice Program (formerly the Design for the Environment Safer Product Labeling Program) requires ingredient data for every chemical in a product and effectively penalizes a lack of data (USEPA Citation2015c). However, the emphasis of that program on hazards still only addresses half of the important considerations for product safety, leaving exposure as a secondary consideration. In concept, the absence of evidence about the safety of a chemical/product could be penalized by assigning default assumptions about the hazards associated with it when no information is available or by assigning conservative safety factors when minimal information is available. Then data-rich chemicals like copper, nickel, and zinc would be placed on a more equal footing with data-poor alternatives that might appear to be benign merely because of a lack of evidence to the contrary.
In summary, increasing the amount of knowledge about a given chemical or material increases the probability of identifying a possible human health or environmental hazard. Consequently, the chemical or material is currently more likely to be blacklisted if risk is ignored and only hazard is considered, thus leading to a potential regrettable substitution in which the chemical or material for which the least information is known becomes the recommended alternative regardless of the risks associated with its use.
Solutions for design-team practitioners
As a fundamental basis of their profession, “architects are familiar with risk management” (AIA Citation2016, p. 1). Incorporating human health and environmental risk assessment and management into the selection of building materials will merely require architects to expand the fundamental concept of risk = hazard × exposure into the realm of concerns related to fabrication, installation, use, and disposal/recycling of building materials. Despite the temptation to apply simplistic concepts like the Precautionary Principle, architectural practitioners who faced real-world tradeoffs will need practical solutions. Therefore, “traditional risk assessments will always have their place” (Reid Citation2016, p. 2) despite the allure of one-size-fits-all hazard lists.
Hazard lists should not be the endpoint for decisions about the selection of building materials; instead, they should contribute just one of the several considerations. Other considerations should include documented and anticipated exposures of human and ecological receptors to the components in the building materials and the incorporation of BMPs and SCMs into building designs. The American Institute of Architects (2016, p. 24) stated that “product content is one factor among many,” and “an architect may select a product that contains a hazardous substance in order to meet some other project requirement, as long as it is in line with the expressed goals of the project.” Those goals should include the protection of human health and the environment in realistic scenarios.
Architects need tools for evaluating human health and environmental risk that are simple to use and interpret. To this end, prototype risk assessment “toolboxes” are being developed to predict the fate and effects of copper in the roof runoff. For example, a currently available tool allows the calculation of the allowable copper roof area in a drainage basin, based on specified surface water hydrology and receiving water chemistry (to account for bioavailability-modifying factors) (personal communication, Kevin Rader, Mutch Associates, LLC, Ramsey, NJ, USA, November 10, 2016). Alternatively, that tool can be used in reverse to determine whether a specified amount of roof area will cause exceedances of the allowable copper concentration in the receiving water. If the default rate of copper leaching from a roof is not considered appropriate in a given situation, a site-specific attenuated rate (to account for differences in weathering of the roofing and/or differences in removal of copper in runoff-management/treatment systems) can be inputted into the model. Additionally, Göbel et al. (Citation2008) provided a decision matrix for the treatment of runoff from a variety of surfaces including copper roofs, based on soil and hydrogeological conditions. The development of more tools such as these that are easy to use and interpret could avoid the temptation for architectural practitioners to rely on simplistic hazard lists.
Conclusions
Ad hoc, sometimes proprietary hazard-based procedures that lack a sound science-based approach are being used to classify building materials and force substitutions that might have comparable or greater risks than the substituted materials. These hazard-based approaches generally list chemicals and materials for which an adverse human health or environmental effect has been demonstrated, regardless of how small, isolated, and individual- or site-specific it is. The hazard-based approaches often rely on subjective, inconsistent decisions that are based on anecdotes about worst-case potential hazards instead of realistic risks, without consideration of expected exposure (or lack thereof) and expected effects (or lack thereof). Although risk-analysis and risk-management practitioners use hazard information only as a starting point in a balanced evaluation of the potential for harm to human health and/or the environment, the acknowledged limitations of strict hazard-assessment approaches are usually lost in translation to architectural and construction-specification practitioners.
We recommend that instead of these automated, piecemeal approaches, integrated risk-based and LCA/LCIA-based analyses should be used to classify building materials in a transparent, science-based approach. Risk-based approaches consider the context and location of the use of chemicals and materials, and unlike many hazard lists, risk-based approaches do not lump all forms of a chemical such as copper into one category. Despite the allure of relying on simplistic hazard lists to broadly apply the Precautionary Principle, traditional risk assessment will always have its place in cost-effective, practical architectural solutions.
In that context, blanket bans of building materials should be avoided because some materials that contain nominally “hazardous” chemicals, but in durable forms, provide benefits compared to their alternatives and do not result in significant exposure. Risk management (including the use of BMPs and SCMs) should be encouraged for building materials if trace releases are a concern. Durable building products made from copper are sustainable and present little risk to humans and the environment, and similar conclusions might be reached regarding other durable building materials that contain chemicals that appear in a variety of hazard lists.
In the end, all chemicals and materials have a possible hazard associated with them. Risk-based and LCA/LCIA-based assessments are needed to decide which of the chemicals and materials would be the most desirable in a given use. Otherwise, architects, builders, and their clients run the risk of unintended adverse consequences to human health and/or the environment by defaulting to a risky building material they know little about.
Abbreviations
| ATSDR | = | Agency for Toxic Substances and Disease Registry |
| BMP | = | Best management practice |
| CCOHS | = | Canadian Centre for Occupational Health and Safety |
| CDC | = | Centers for Disease Control |
| CML | = | Chemical and Material Library |
| CMPBS | = | Center for Maximum Potential Building Systems |
| CPA | = | Clean Production Action |
| CuCl2 | = | Copper chloride |
| CuSO4 | = | Copper sulfate |
| DOC | = | Dissolved organic carbon |
| ECI | = | European Copper Institute |
| EEC | = | European Economic Community |
| EPD | = | Environmental product declaration |
| HAZ-MAP | = | Occupational health database maintained by U.S. National Library of Medicine |
| HBN | = | Healthy Building Network |
| HCWH | = | Health Care without Harm |
| HPD | = | Health product declaration |
| HPDC | = | Health Product Declaration Collaborative |
| ICMM | = | International Council on Mining and Metals |
| LCA | = | Life cycle analysis |
| LCIA | = | Life cycle impact analysis |
| LEED | = | Leadership in Energy and Environmental Design |
| LID | = | Low-impact development |
| NIH | = | National Institutes of Health |
| NRC | = | National Research Council |
| OECD | = | Organization for Economic Co-operation and Development |
| OEHHA | = | Office of Environmental Health Hazard Assessment (California) |
| PBT | = | Persistent, bioaccumulative, and toxic |
| PCR | = | Product category rule |
| PPE | = | Personal protective equipment |
| PTD | = | Product transparency declaration |
| RCRA | = | Resource Conservation and Recovery Act |
| RFCI | = | Resilient Floor Covering Institute |
| SciVera | = | SciVera LLC, Charlottesville, Virginia, USA |
| SCM | = | Stormwater control measure |
| SETAC | = | Society of Environmental Toxicology and Chemistry |
| USEPA | = | United States Environmental Protection Agency |
| USGBC | = | United States Green Building Council |
| USGS | = | United States Geological Survey |
| vPvB | = | Very persistent and very bioaccumulative |
| WHO | = | World Health Organization |
Acknowledgments
The authors thank an anonymous reviewer for helpful insights and suggestions.
Funding
This work was supported by the International Copper Association and the Copper Development Association.
References
- Ahsan SA, Lackovic M, Katner A, et al. 2009. Metal fume fever: A review of the literature and cases reported to the Louisiana Poison Control Center. J Louisiana State Med Soc 161:348–51
- AIA (American Institute of Architects). 2016. Materials Transparency and Risk for Architects: An Introduction to Advancing Professional Ethics while Managing Professional Liability Risks. The American Institute of Architects, Washington, DC, USA
- Arnold R. 2005. Estimations of copper roof runoff rates in the United States. Integr Environ Assess Manage 1:333–42
- Athanasiadis K, Helmreich B, and Horn H. 2007. On-site infiltration of a copper roof runoff: Role of clinoptilolite as an artificial barrier material. Water Res 41:3251–8
- Athanasiadis K, Helmreich B, and Wilderer PA. 2006. Infiltration of a copper roof runoff through artificial barriers. Water Sci Technol 54(6–7):281–9
- Atlee J. 2011. Selecting safer building products in practice. J Cleaner Prod 19:459–63
- ATSDR (Agency for Toxic Substances and Disease Registry). 2004. Toxicological Profile for Copper. U.S. Department of Health and Human Services, Washington, DC, USA
- ATSDR (Agency for Toxic Substances and Disease Registry). 2014. Minimal Risk Levels (MRLs). December 2014. Available at http://www.atsdr.cdc.gov/mrls/index.asp (accessed on February 28, 2015)
- Bahar B, Herting G, Odnevall Wallinder I, et al. 2008. The interaction between concrete pavement and corrosion-induced copper runoff from buildings. Environ Monit Assess 140:175–89
- Baker DH. 1999. Cupric oxide should not be used as a copper supplement for either animals or humans. J Nutr 129:2278–9
- Balkema S, Hamaker ME, Visser HPJ, et al. 2008. Haemolytic anemia as a first sign of Wilson's Disease. Netherlands J Med 66:344–7
- Bardelline J. 2009. Perkins+Will launches first chemical blacklist for building designers. Available at http://whatisgreenrealestate.blogspot.com/2009/11/perkins-will-precautionary-list.html (accessed on March 8, 2015)
- Bare JC. 2006. Risk assessment and life-cycle impact assessment for human health cancerous and noncancerous emissions: Integrated and complementary with consistency within the USEPA. Hum Ecol Risk Assess 12:493–509
- Barron TS. 2006. Architectural Uses of Copper: An Evaluation of Storm Water Pollution Loads and BMPs. November 2000; Revised 3, March 2006. Report prepared for Palo Alto Regional Water Quality Control Plant. Palo Alto, CA, USA
- Bertling S, Odnevall Wallinder I, Berggren Kleja D, et al. 2006. Long-term corrosion-induced copper runoff from natural and artificial patina and its environmental impact. Environ Toxicol Chem 25:891–8
- Bertling S, Odnevall Wallinder I, Leygraf C, et al. 2002. Immobilization of copper in runoff water from roofing materials by limestone, soil, and concrete. Proceedings of the 15th International Corrosion Congress, Granada, Spain, September 22–27, 2002, pp 719–26. International Corrosion Council, Madrid, Spain
- Bielmyer GK, Arnold WR, Tomasso JR, et al. 2012. Effects of roof and rainwater characteristics on copper concentrations in roof runoff. Environ Monit Assess 184:2797–804
- Boller MA and Steiner M. 2002. Diffuse emission and control of copper in urban surface runoff. Water Sci Technol 46(6–7):173–81
- Boobis AR, Cohen SM, Dellarco VL, et al. 2016. Classification schemes for carcinogenicity based on hazard-identification have become outmoded and serve neither science nor society. Regul Toxicol Pharmacol 82:158–66
- Boulanger B and Nikolaidis NP. 2003. Mobility and aquatic toxicity of copper in an urban watershed. J Am Water Res Assoc 39:325–36
- Borak J, Cohen H, and Hethmon TA. 2000. Copper exposure and metal fume fever: Lack of evidence for a causal relationship. Am Ind Hygiene Assoc J 61:832–6
- CCOHS (Canadian Centre for Occupational Health and Safety). 2015. Hazard and risk. Available at http://www.ccohs.ca/oshanswers/hsprograms/hazard_risk.html (accessed on March 8, 2015)
- CDC (Centers for Disease Control). 1993. Methemoglobinemia in an infant – Wisconsin, 1992. Morbid Mortal Wkly Rep 42:217–9
- Chang M, McBroom MW, and Beasley RS. 2004. Roofing as a source of nonpoint water pollution. J Environ Manage 73:307–15
- Chemical Watch. 2015. Business Guide to Safer Chemicals in the Supply Chain: Including Alternatives Assessment. CW Research Ltd, Shrewsbury, UK
- Cohen J and Lewandowski T. 2016. Alternatives assessment: On the hunt for safer chemicals. Gradient Trends: Risk Science and Application Spring 2016. 66:3–4
- Collegium Ramazzini. 2005. The Precautionary Principle: Implications for research and policy making. Hum Ecol Risk Assess 11:3–4
- CPA (Clean Production Action). 2014. GreenScreen® For Safer Chemicals. Clean Production Action, Somerville, Massachusetts, USA. Available at http://www.greenscreenchemicals.org/resources/entry/greenscreen-two-pager (accessed on October 12, 2016)
- CPA (Clean Production Action). 2016. GreenScreen® List Translator Version 1.3 (1e) Specified Lists. Clean Production Action, Somerville, Massachusetts, USA. Available at http://www.greenscreenchemicals.org/method/method-documents (accessed on November 30, 2016)
- Davis AP. 2005. Green engineering principles promote low-impact development. Environ Sci Technol 39:339A–344A
- Davis AP, Shokouhian M, Sharma H, et al. 2001. Laboratory study of biological retention for urban stormwater management. Water Environ Res 73:5–14
- Davis AP, Shokouhian M, Sharma H, et al. 2003. Water quality improvement through bioretention: Lead, copper, and zinc removal. Water Environ Res 75:73–82
- DeForest DK, Gensemer RW, Van Genderen EJ, et al. 2011. Protectiveness of water quality criteria for copper in western United States waters relative to predicted olfactory responses in juvenile Pacific salmon. Integr Environ Assess Manage 7:336–47
- DeForest DK and Meyer JS. 2015. Critical review: Toxicity of dietborne metals to aquatic organisms. Crit Rev Environ Sci Technol 45:1176–241
- Dietz ME. 2007. Low impact development practices: A review of current research and recommendations for future directions. Water Air Soil Pollut 186:351–63
- Døssing M and Skinhøj P. 1985. Occupational liver injury: Present state of knowledge and future perspective. Int Arch Occup Environ Health 56:1–21
- Drever JI. 1997. The Geochemistry of Natural Waters: Surface and Groundwater Environments. 3rd ed. Prentice-Hall, Upper Saddle River, NJ, USA
- Eisler R. 1998. Copper Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review. USGS/BRD/BSR–1997–0002. U. S. Department of the Interior, U. S. Geological Survey, Washington, DC, USA
- ECI (European Copper Institute). 2008. Copper Voluntary Risk Assessment. European Copper Institute, Brussels, Belgium
- EEC (European Economic Community). 1967. Council directive of 27 June 1967 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. Directive 67/548/EEC. Official Journal of the European Communities 16.8.67 (No. 196/1):234–56
- Fraústo da Silva JJR and Williams RJP. 1991. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. Clarendon Press, Oxford, UK
- Frazier LM. 1998. Disinfectants. In: Frazier LM and Hage ML (eds), Reproductive Hazards of the Workplace, pp 257–75. Van Nostrand Reinhold, New York, NY, USA
- Gannon J. 2011. Choice of materials seen as key to healthier indoor spaces. Environmental Compliance Bulletin 18 ECB 32, January 24
- Gauthier AM, Fung M, Panko J, et al. 2014. Chemical assessment state of the science: Evaluation of 32 decision-support tools used to screen and prioritize chemicals. Integr Environ Assess Manage 11:242–55
- Göbel P, Zimmermann J, Klinger C, et al. 2008. Recommended urban storm water infiltration devices for different types of run-off under varying hydrogeological conditions. J Soils Sedim 8:231–8
- Goonan TG. 2009. Copper Recycling in the United States in 2004. U.S. Geological Survey Circular 1196-X. U.S. Geological Survey, Reston, VA, USA
- Gowen JF. 1955. Modern Application of Sheet Copper in Building Construction. Copper & Brass Research Association, New York, NY, USA
- Green Globes. 2016. The practical building rating system. ECD Energy and Environment, Toronto, ON, Canada; and The Green Building Initiative. Portland, OR, USA. Available at http://www.greenglobes.com/home.asp (accessed on October 24, 2016)
- Hansen BG, van Haelst AG, van Leeuwen K, et al. 1999. Priority setting for existing chemicals: European Union risk ranking method. Environ Toxicol Chem 18:772–9
- Hassan S, Shaikh MU, Ali N, et al. 2010. Copper sulphate toxicity in a young male complicated by methemoglobinemia, rhabdomyolosis and renal failure. J College Physicians Surgeons Pakistan 20:490–1
- HBN (Healthy Building Network). 2016. The Pharos Project. Available at http://www.pharosproject.net (accessed on November 18, 2016)
- He W, Odnevall Wallinder I, and Leygraf C. 2001. A laboratory study of copper and zinc runoff during first flush and steady-state conditions. Corrosion Sci 43:127–46
- Hedberg YS, Hedberg JF, Herting G, et al. 2014. Critical review: Copper runoff from outdoor copper surfaces at atmospheric conditions. Environ Sci Technol 48:1372–81
- Hedberg Y and Odnevall Wallinder I. 2011. Protective green patinas on copper in outdoor constructions. J Environ Prot 2:956–9
- Hepbasli A and Kalinci Y. 2009. A review of heat pump water heating systems. Renew Sustain Energy Syst 13:1211–29
- Howard GJ. 2014. Chemical alternatives assessment: The case of flame retardants. Chemosphere 116:112–7
- HPDC (Health Product Declaration Collaborative). 2016. Health Product Declaration 2.0 Open Standard. Available at http://HPD-collaborative.org (accessed on November 30, 2016)
- ICMM (International Council on Mining and Metals). 2016a. Health Risk Assessment Guidance for Metals (HERAG). Available at https://www.icmm.com/website/publications/pdfs/chemicals-management/herag/herag-background-2007.pdf (accessed on October 19, 2016)
- ICMM (International Council on Mining and Metals). 2016b. Metals Environmental Risk Assessment Guidance (MERAG). Available at https://www.icmm.com/website/publications/pdfs/chemicals-management/merag/merag-background-2007.pdf (accessed on October 19, 2016)
- Klaassen CD, Amdur MO, and Doull J. 1996. Casarett and Doull's Toxicology: The Basic Science of Poisons. 5th ed. McGraw-Hill, New York, NY, USA
- Krätschmer A, Odnevall Wallinder I, et al. 2002. The evolution of outdoor copper patina. Corrosion Sci 44:425–50
- Kwiatkowski M, Welker AL, Traver RG, et al. 2007. Evaluation of an infiltration best management practice utilizing pervious concrete. J Am Water Res Assoc 43:1208–22
- LaBarre WJ. 2014. The Effectiveness of Bioretention Structures for Metal Retention and Toxicity Reduction of Copper Roof Runoff. Master of Science Thesis, Towson University, Towson, MD, USA
- LaBarre WJ, Ownby DR, Lev SM, et al. 2016. Attenuation of copper in runoff from copper roofing materials by two stormwater control measures. Water Res 88:207–15
- Landner L and Lindeström D. 1999. Copper in Society and the Environment: An Account of the Facts on Fluxes, Amounts and Effects of Copper in Sweden. 2nd revised ed. Swedish Environmental Research Group. Centraltryckeriet AB, Borås, Sweden
- Landner L and Reuther R. 2004. Metals in Society and the Environment: A Critical Review of Current Knowledge on Fluxes, Speciation, Bioavailability and Risk for Adverse Effects of Copper, Chromium, Nickel and Zinc. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Li H and Davis AP. 2009. Water quality improvement through reductions of pollutant loads using bioretention. J Environ Eng 135:567–76
- Liu D, Sansalone JJ, and Cartledge FK. 2005. Comparison of sorptive filter media for treatment of metals in runoff. J Environ Eng 131:1178–86
- Luoma SN and Rainbow PS. 2008. Metal Contamination in Aquatic Environments: Science and Lateral Management. Cambridge University Press, Cambridge, UK
- Menzie CA, Southworth B, Stephenson G, et al. 2008. The importance of understanding the chemical form of a metal in the environment: The case of barium sulfate (barite). Hum Ecol Risk Assess 14:974–91
- Merriam-Webster. 2015. Merriam-Webster dictionary. Available at http://www.merriam-webster.com/dictionary/patina (accessed on March 23, 2015)
- Meyer JS and Adams WJ. 2010. Relationship between biotic ligand model-based water quality criteria and avoidance and olfactory responses to copper by fish. Environ Toxicol Chem 29:2096–103
- Meyer JS, Clearwater SJ, Doser TA, et al. 2007. Effects of Water Chemistry on the Bioavailability and Toxicity of Waterborne Cadmium, Copper, Nickel, Lead, and Zinc to Freshwater Organisms. SETAC Press, Pensacola, FL, USA
- Mitchell CS, Schwartz BS, Kubnen AE, et al. 1998. Other chemicals. In: Frazier LM and Hage ML (eds), Reproductive Hazards of the Workplace, pp 333–66. Van Nostrand Reinhold, New York, NY, USA
- Nemery B. 1990. Metal toxicity and the respiratory tract. Eur Respir J 3:202–19
- Nightingale HI. 1987a. Water quality beneath urban runoff water management basins. Water Res Bull 23:197–205
- Nightingale HI. 1987b. Accumulation of As, Ni, Cu, and Pb in retention and recharge basins soils from urban runoff. Water Res Bull 23:663–72
- NIH (National Institutes of Health). 2015. Haz-Map listings. Available at http://hazmap.nlm.nih.gov/index.php (accessed on January 14, 2015)
- NIH NIDDK (National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases). 2014. Wilson Disease. NIH Publication No. 14–4684. National Institutes of Health, Bethesda, MD, USA
- NRC (National Research Council). 2014. A Framework to Guide Selection of Chemical Alternatives. The National Academies Press, Washington, DC, USA
- OECD (Organization for Economic Co-operation and Development). 2014. Current Landscape of the Alternatives Assessment Practice: A Meta-Review. OECD Environment, Health and Safety Publications, Series on Risk Management No. 26. ENV/JM/MONO(2013)24. Organization for Economic Co-operation and Development, Paris, France
- OECD (Organization for Economic Co-operation and Development). 2016a. Classification and Labelling of Chemicals. Available at http://www.oecd.org/env/ehs/risk-management/classificationandlabellingofchemicals.htm (accessed on October 19, 2016)
- OECD (Organization for Economic Co-operation and Development). 2016b. OECD Substitution and Alternatives Assessment Toolbox. Available at http://www.oecdsaatoolbox.org/Home/Index (accessed on March 30, 2016)
- OEHHA (Office of Environmental Health Hazard Assessment). 2014. Table of All Acute, 8-hour, and Chronic Reference Exposure Levels (chRELs) as of June 2014. Available at http://www.oehha.ca.gov/air/allrels.html (accessed on January 24, 2015)
- Oga M, Matsui N, Anai T, et al. 1993. Copper disposition of the fetus and placenta in a patient with untreated Wilson's disease. Am J Obstetrics Gynecol 169:196–8
- Olivares M and Uauy R. 1996. Copper as an essential nutrient. Am J Clin Nutr 63:791S–796S
- Perkins C, Nadim F, and Arnold WR. 2005. Effects of PVC, cast iron and concrete conduit on concentrations of copper in stormwater. Urban Water J 2:183–91
- Perkins and Will. 2014. Precautionary List – Copper (for Exterior Material). Available at http://transparency.perkinswill.com/Home/PrecautionaryListChemical/71 (accessed on December 31, 2014)
- Pizarro F, Loivares M, Gidi V, et al. 1999. The gastrointestinal tract and acute effects of copper in drinking water and beverages. Rev Environ Health 14:231–8
- Reid K. 2016. Gaining an edge with GreenScreen®. Gradient Trends: Science and Application 66:1–2
- RFCI (Resilient Floor Covering Institute). 2013. Product Transparency Declarations. Available at http://continuingeducation.construction.com/article.php?L=243&C=1128 (accessed on March 21, 2015)
- Richter ED and Laster R. 2005. The precautionary principle, epidemiology and the ethics of delay. Hum Ecol Risk Assess 11:17–27
- Schade AL. 2007. Copper in a sustainable context. Construct Specifier 60(11):68–74
- SciVera. 2016. Products: SciVera Lens™. SciVera LLC, Charlottesville, VA, USA. Available at http://www.scivera.com/products.php (accessed on March 30, 2016)
- SETAC (Society of Environmental Toxicology and Chemistry). 1999. Sound Science. Technical Issue Paper. Society of Environmental Toxicology and Chemistry, Pensacola, FL, USA
- Sinkovič A, Strdin A, and Svenšek F. 2008. Severe acute copper sulphate poisoning: A case report. Arh Hig Rada Toksikol 59:31–5
- Skeaff JM, Dubreuil AA, and Brigham SI. 2002. The concept of persistence as applied to metals for aquatic hazard identification. Environ Toxicol Chem 21:251–2590
- Skeaff JM, Hardy DJ, and King P. 2008. A new approach to the hazard classification of alloys based on transformation/dissolution. Integr Environ Assess Manage 4:75–93
- Socolof ML and Geibig JR. 2006. Evaluating human and ecological impacts of a product life cycle: The complementary roles of life-cycle assessment and risk assessment. Hum Ecol Risk Assess 12:510–27
- Sokas RK. 1998. Metals. In: Frazier LM and Hage ML (eds), Reproductive Hazards of the Workplace, pp 123–61. Van Nostrand Reinhold, New York, NY, USA
- Sondhi A. 2010. Release and uptake of copper from composite materials in the environment. PhD thesis, University of Delaware, Newark, DE, USA
- State of California. 2013. Safer consumer products regulations – Informational list of candidate chemicals and chemical groups. September 26, 2013. State of California Environmental Protection Agency, Department of Toxic Substances Control. Available at https://www.dtsc.ca.gov/SCP/ChemList.cfm (accessed on January 14, 2015)
- State of California. 2016. Chemicals known to the state to cause cancer or reproductive toxicity. October 21, 2016. State of California Environmental Protection Agency, Office of Environmental Health Hazard Assessment, Safe Drinking Water and Toxic Enforcement Act of 1986. Available at http://oehha.ca.gov/media/downloads/proposition-65//p65single10212016.pdf (accessed on November 17, 2016)
- Steiner M and Boller M. 2006. Copper and zinc removal from roof runoff: From research to full-scale adsorber systems. Water Sci Technol 53(3):199–207
- Steiner M, Pronk W, and Boller MA. 2006. Modeling of copper sorption onto GFH and design of full-scale GFH adsorbers. Environ Sci Technol 40:1629–35
- Stern BR and Lagos G. 2008. Are there health risks from the migration of chemical substances from plastic pipes into drinking water? A review. Hum Ecol Risk Assess 14:753–79
- Stumm W and Morgan JJ. 1996. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. 3rd ed. John Wiley & Sons, New York, NY, USA
- Sundberg R and Jonsson L. 2005. Runoff from copper roofs—Comparison between a new roof and a 40 year old artificially patinated roof. Metall 59:634–8
- Sundberg R and Odnevall Wallinder I. 2001. Can metal run off from roofs have an environmental effect? Metall 55:439–41
- Swift A. 2011. Inherited toxicity: An expanded concept of sustainability for preservation. Docomomo 44:56–67
- Takeda T, Yukioka T, and Shimazaki S. 2000. Cupric sulfate intoxication with rhabdomyolysis, treated with chelating agents and blood purification. Inter Med 39:253–5
- Tervonen T, Linkov I, Figueira JR, et al. 2009. Risk-based classification system of nanomaterials. J Nanopart Res 11:757–66
- Thornton J. 2002. Environmental Impacts of Polyvinyl Chloride Building Materials. Healthy Building Network, Washington, DC, USA
- Udo de Haes HA, Sleeswijk AW, and Heijungs R. 2006. Similarities, differences and synergisms between HERA and LCA—An analysis at three levels. Hum Ecol Risk Assess 12:431–49
- USEPA (U.S. Environmental Protection Agency). 2005. Introduction to United States Environmental Protection Agency Hazardous Waste Identification (40 CFR Parts 261). EPA530-K-05–012. USEPA, Washington, DC, USA
- USEPA (U.S. Environmental Protection Agency). 2007a. Aquatic Life Ambient Freshwater Quality Criteria—Copper: 2007 Revision. EPA-822-R-07–001. USEPA, Washington, DC, USA
- USEPA (U.S. Environmental Protection Agency). 2007b. Framework for Metals Risk Assessment. EPA 120/R-07/001. USEPA, Washington, DC, USA
- USEPA (U.S. Environmental Protection Agency). 2015a. National Waste Minimization Program Priority Chemicals. Available at http://www.epa.gov/osw/hazard/wastemin/priority.htm (accessed on March 19, 2015)
- USEPA (U.S. Environmental Protection Agency). 2015b. Resource Conservation and Recovery Act Chemicals That Are Considered a Hazardous Waste When Discarded. Available at http://www.gpo.gov/fdsys/pkg/CFR-2012-title40-vol27/xml/CFR-2012-title40-vol27-sec261-33.xml (accessed on March 19, 2015)
- USEPA (U.S. Environmental Protection Agency). 2015c. EPA's Safer Choice Standard. Available at https://www.epa.gov/sites/production/files/2013-12/documents/standard-for-safer-products.pdf (accessed on April 2, 2016)
- USEPA (U.S. Environmental Protection Agency). 2016a. Table of Regulated Drinking Water Contaminants. Available at https://www.epa.gov/ground-water-and-drinking-water/table-regulated-drinking-water-contaminants (accessed on October 12, 2016)
- USEPA (U.S. Environmental Protection Agency). 2016b. Aquatic Life Ambient Estuarine-Marine Quality Criteria—Copper: Draft, July 29, 2016. U.S. Environmental Protection Agency, Washington, DC, USA
- USGBC (U.S. Green Building Council). 2010. LEED 2009 for Healthcare. Leadership in Energy & Environmental Design—Healthcare. Available at http://www.usgbc.org/resources/leed-healthcare-v2009-current-version (accessed on April 14, 2014)
- USGBC (U.S. Green Building Council). 2016. LEED v4. Building Design and Construction Guide. Available at http://www.usgbc.org/guide/bdc (accessed on October 12, 2016)
- USGS (U.S. Geological Survey). 2009. Copper—A Metal for All Ages. Fact Sheet 2009–3031. U.S. Geological Survey, Reston, VA, USA
- Wang L-C, Wang J-D, Tsai C-R, et al. 2010. Clinical features and therapeutic response in Taiwanese children with Wilson's Disease: 12 years of experience in a single center. Pediatr Neonatol 51:124–9
- WHO (World Health Organization). 2011. Guidelines for Drinking-water Quality. 4th ed. World Health Organization, Geneva, Switzerland
- Wilde FD. 1994. Geochemistry and factors affecting ground-water quality at three storm-water-management sites in Maryland. Report of Investigations No. 59. Maryland Geological Survey. Baltimore, MD, USA
- Winters NL and Graunke K. 2014. Roofing Materials Assessment: Investigation of Toxic Chemicals in Roof Runoff. Publication No. 14-03-003. State of Washington Department of Ecology, Olympia, WA, USA
- Winters NL, Graunke K, and McCall M. 2015. Roofing materials assessment: Investigation of five metals in runoff from roofing materials. Water Environ Res 87:835–44
- Winters NL, McCall M, and Kingfisher A. 2014. Roofing Materials Assessment: Investigation of Toxic Chemicals in Roof Runoff from Constructed Panels in 2013 and 2014. Publication No. 14-03-033. State of Washington Department of Ecology, Olympia, WA, USA
- Yang CC, Wu ML, and Deng JF. 2004. Prolonged hemolysis and methemoglobinemia following organic copper fungicide ingestion. Veterinary Hum Toxicol 46:321–3
- Zimmerman HJ and Lewis JH. 1995. Chemical- and toxic-induced hepatotoxicity. Gastroenterol Clin North Am 24:1027–45