Abstract
In response to the COVID-19 pandemic, scientists coordinated a complex immunization effort that developed and distributed vaccines by December 2020. This study aimed to explain COVID-19 vaccination decision-making process to inform vaccine communication with patients and the public. Building on quantitative research on COVID-19 vaccine hesitancy, we conducted a grounded theory study, collecting 30 qualitative interviews with employees at a U.S. university that provided vaccine eligibility in December 2020. Analysis followed the Sort and Sift, Think and Shift method. Participants who had chosen to receive the vaccine and those who had not both described five factors that impacted their decision-making: emotional response, understanding, personal values, culture, and social norms. Across these factors, we identified three cross-cutting themes: time, trust, and communication tactics. In a time of emerging science and changing answers, the constant introduction of new information created information overload for participants. COVID-19 vaccine development was a “grand experiment globally,” which required trust, not only knowledge, to overcome hesitancy. The complex information environment surrounding COVID-19 vaccination requires multi-level intervention that cannot rely on knowledge translation alone. We need to help patients build trusting relationships with experts that can create scaffolding for future information processing.
In response to the COVID-19 pandemic, scientists coordinated a complex immunization effort that developed and distributed vaccines using old and new technologies by multiple pharmaceutical manufacturers around the world (Baden et al., Citation2021; Livingston, Malani, & Creech, Citation2021; Polack et al., Citation2020). As scientists worked toward this goal, social scientists began investigating how to address vaccine hesitancy that could emerge alongside the vaccine. Even before COVID-19, the World Health Organization (WHO) named vaccine hesitancy as a top 10 threat to global health in 2019 (“Ten threats to global health in Citation2019,” 2019).
Vaccine hesitancy is a continuum that rests between full acceptance and full refusal of all recommended vaccines (MacDonald, Citation2015). The WHO described the “3 Cs” model, highlighting three dimensions of hesitancy: complacency, confidence, and convenience (MacDonald, Citation2015). Vaccine hesitancy arises when an individual perceives a low need for a vaccination (complacency), questions a vaccination’s efficacy or safety (low confidence), or faces obstacles to access (convenience) (Larson, Schulz, Tucker, & Smith, Citation2015; MacDonald, Citation2015; Quinn, Jamison, An, Hancock, & Freimuth, Citation2019). Confidence is an individual’s combined trust in vaccine effectiveness and safety, trust in the system that delivers the vaccine, and trust in the motivations of policymakers (Lee, Whetten, Omer, Pan, & Salmon, Citation2016). Individuals who are willing to get a vaccine trust that the vaccine is needed, that it will work, and that it is safe (Freeman et al., Citation2020).
Betsch and colleagues recommend constraints as an alternate to convenience, asserting that constraints is a more comprehensive concept, encompassing structural and psychological barriers, including physical availability, affordability, or geographical accessibility and limitations to the ability to understand vaccine information (Betsch et al., Citation2018). Researchers have also recommended two additional “Cs.” Collective responsibility describes an individual’s willingness to protect others by getting a vaccine (Fine, Eames, & Heymann, Citation2011), and calculation describes an individual’s enactment of extensive information searching that prompts evaluation of risks of infections and vaccination to derive a reasoned decision (Betsch et al., Citation2018). However, the quality of information inputs can negatively affect decisions. Extensive information seeking can expose individuals to repeated misinformation, after which they may miscalculate and remain vaccine hesitant. Hesitancy has been associated with poor ability to detect “fake news” (Montagni et al., Citation2021).
Early research about COVID-19 vaccine hesitancy suggests that the 3 Cs continue to impact vaccine decision-making. Complacency is especially salient as populations experience COVID-19 in different ways. Because the mortality rate for COVID-19 was initially low in some populations, including the young (Felsenstein & Hedrich, Citation2020), some individuals perceived COVID-19 as low severity, negatively influencing vaccine acceptance (Reiter, Pennell, & Katz, Citation2020; Ruiz & Bell, Citation2021). Rather than convenience, constraints was a more accurate concept to describe access to COVID-19 vaccination. For individuals without reliable internet access or with low computer literacy, initial COVID-19 vaccinations were inaccessible, despite being free (Iyasere et al., Citation2021). Confidence remains critical as the COVID-19 pandemic has been characterized by shifting information (Dib, Mayaud, Chauvin, & Launay, Citation2021) that seeds doubt in science and medicine.
This study aimed to explain COVID-19 vaccination decision-making process to inform vaccine communication with patients and the public. This qualitative inquiry builds on quantitative research on COVID-19 vaccine acceptance and hesitancy, including studies that linked the Health Belief Model to intentions to vaccinate (Calvillo, Ross, Garcia, Smelter, & Rutchick, Citation2020; Zampetakis & Melas, Citation2021). Quantitative studies have established that individuals without a college degree, individuals with conservative political beliefs, Black Americans, and females are more likely to be undecided or unwilling to vaccinate (Calvillo et al., Citation2020; Daly & Robinson, Citation2021; Ma & Ma, Citation2021; Moore et al., Citation2021; Reiter et al., Citation2020).
Materials and Methods
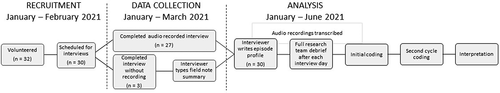
This grounded theory study was designed to develop a model of vaccine decision-making grounded in data from the rich descriptions of participant perspectives and experiences. Two female researchers (CJWL, LC), who are extensively trained in qualitative methods, designed and implemented the study. See for the study timeline.
Data was collected via interviews with employees at Augusta University (AU), a large academic institution in the Southeastern U.S. AU employees were offered the COVID-19 vaccine beginning in December 2020 (“Augusta University health frontline caregivers get COVID-19 vaccine,” Citation2020). As one of the first large institutions in Georgia to provide eligibility for employees (regardless of individual characteristics such as age or comorbidities), AU created a systematic, accessible opportunity for vaccination. With minimal constraints, this population was well suited for studying vaccine decision-making.
The semi-structured interview guide was developed to capture overall perception of COVID-19 and participant’s vaccine decision-making. Using the Health Belief Model (Rosenstock, Citation1974) as an organizing framework, the initial interview guide included questions about participant perceptions of COVID-19 susceptibility and severity (Calvillo et al., Citation2020; Chu & Liu, Citation2021) and perceptions of the benefits of and barriers to vaccination.
Theoretical and purposeful sampling, by risk exposure and vaccine status, provided representation of various perspectives. provides descriptions of four levels of risk exposure. In January 2021, following Institutional Review Board approval, university staff forwarded a recruitment e-mail from researchers to the same university-wide set of employees who had received e-mail notification of vaccine availability. As interviews proceeded, we continued to purposively sample on risk exposure and vaccine status by following up with work units that represented different risk levels. Of 48 employees who responded to the recruitment e-mail, 32 volunteered for interviews. After completing 30 interviews, recruitment ended when saturation of responses emerged within each additional interview.
Table 1. Sample characteristics (n = 30)
We recognized that COVID-19 vaccination was a highly charged, potentially threatening or stigmatizing topic (Lyu, Han, & Luli, Citation2021). To ensure participants could freely share their perspective, we scheduled private interviews with interviewers who did not have a previous relationship with the participant, and we asked participants for permission to audio record. During the consent process, interviewers described the purpose of the study and explained confidentiality procedures. Throughout this discussion and interview, the interviewer maintained equipoise as to the evidence for vaccination, in line with evidence available at time of data collection (Epstein, Alper, & Quill, Citation2004). After the participant provided verbal consent, the audio recorder was activated (or remained off), and the interview began. Four trained researchers (CJWL, LC, CR, AG) conducted the interviews January through March 2021 in-person and virtually during normal working hours. The average length of an interview was 27 minutes, with a range of 13 minutes to 52 minutes. When audio recording was not used (n = 3), the interviewer recorded detailed field notes throughout the conversation and prepared a full summary after the interview to be used in analysis.
After interviews were transcribed, the first and second author analyzed data using the Sort and Sift, Think and Shift method (Maietta, Mihas, Swartout, Petruzzelli, & Hamilton, Citation2021), which allowed researchers to look deeply into each case, gaining a sense of content, dimensions, and properties. The codebook was established using an iterative process, connecting themes, and establishing main concepts. Analysis was informed by, but not limited to, the Cs framework (complacency, confidence, constraints, collective responsibility, and calculation). A second coding of the data finalized the categories. Stepping back, we diagrammed each case together to elucidate important categories and identify recurring topics. Throughout this process, we monitored for larger themes that threaded throughout the data.
Results
The sample consisted of 30 full-time vaccine-eligible employees, representing diverse workplace roles. See for sample characteristics.
Participants affirmed previously identified health decision-making factors in the context of vaccine decision-making (). Vaccine hesitancy, acceptance, or resistance was a complex decision of multiple factors. Participants who had received the vaccine and those who had not received the vaccine both described five factors that impacted their decision-making: (1) emotional response, (2) understanding, (3) personal values, (4) culture, and (5) social norms.
Table 2. Decision-making factors associated with vaccine decisions
Across these factors, we identified three cross-cutting themes. First, in the context of a novel disease that required the development of new treatments and new vaccines, participants repeatedly described the role of time in vaccine decisions. Second, participants described the impact of trust on the five factors. Third, participants described communication tactics they enacted to make sense of the dynamic information environment.
Theme 1: Time
The cross-cutting theme of time compounded the emotional responses of alternately excitement/relief and guilt. When sharing positive emotional responses, participants described watching months of worldwide effects and the building anticipation for a vaccine. Excitement was a release in response to the time waiting for vaccine availability. Conversely, participants described guilt in receiving the vaccine was a factor of timing. The limited resourcing/availability of the vaccine and the perception that they received it early manifested feelings of guilt. For both responses, time played a critical role in the emotional response. Participant 25 (risk level 3, vaccinated) described intentionally waiting for the second vaccine delivery, “ … I waited for the second [vaccine availability announcement]. I was like all right, you [clinicians] had your chance, it’s my turn. Because I don’t want to take it from somebody that is more involved with the public than I import to be, so I waited.”
After initial emotional responses, participants described their cognitive processes to understanding the vaccine. Participants situated this understanding in terms of comparative speed, describing the timeline relative to their perception of how long other treatments take to develop. Participants expressed hesitancy when scientists have not discovered a cure for other diseases. Participant 21 (risk level 1, non-vaccinated) reasoned, “I am not a physician or scientist by a long shot. I just know it can take almost 3 years for them to put a Tylenol out, so for them to have a vaccine as early as that, it just raises a few red flags with me.”
Participants who avowed a previous understanding of the process of vaccine development and distribution described the timeline being “expedited” but not too fast. “Expedited” was a positive term, referencing the removal of bureaucratic barriers. Participant 14 (risk level 3, vaccinated) explained, “It was just expedited, cutting some of the red tape and fixing some of those issues that were able to fast track the study was not a problem … I’ve worked in clinical trials and research for years now. I understand the time that it takes and how you can remove those kind of things that take so much time and don’t spare any science.”
Time was also connected to the values of protecting the vulnerable, leading by example, and public service. For these three values, it was the participant’s ability to receive the vaccination immediately upon availability that motivated them to vaccinate. Participant 18 (risk level 3, vaccinated) commented, “I think in a collective mindset it was fairly unanimous that this was something that we needed to jump on as quickly as possible. On a family level, my spouse felt that if you have the opportunity you should take advantage of it.”
Participants also describe how the newness of the disease and of the vaccine limited how much can be known about the vaccine, including safety concerns. Participant 29 (risk level 2, non-vaccinated) specified, “[I’m] very frustrated because I just wanted an answer [as a pregnant woman] … I wasn’t sure because when it comes to me, I’m okay with myself being a Guinea pig. I am not okay with my unborn child being a Guinea pig.” When participants talked about “we don’t know yet,” it did not explicitly prevent vaccination; rather, it was a source of hesitancy. Some participants specified that if science could identify an answer they would get the vaccine.
The dense information environment surrounding COVID-19 made it harder for participants who were not trained in science to make sense of daily emerging evidence. Participants described the vaccine approval and distribution process as science in action. Seeing the scientific process advance in real time increased participant uncertainty and created mixed messages from public health and medicine as evidence was discovered. Participants recognized they were part of a natural experiment, which prompted questions that would normally be answered prior to vaccination. Participant 30 (risk level 2, non-vaccinated) observed, “I consider this like a grand experiment globally as they go through the countries that are choosing to vaccinate right now.” This perception of participation in an experiment was especially visceral for individuals from groups who have historically suffered research abuses (e.g., Black Americans in the American South), which set a foundation of mistrust. Participant 17 (risk level 2, vaccinated) explained, “Being an African American and hearing about the syphilis trials there is always you know a thought lingering in the back of your head is it safe to take at this point? Is everyone getting it? You always think twice.”
Theme 2: Trust
Trust was also a cross-cutting theme. Participant 2 (risk level 3, vaccinated) explained how her African-American community connected trust and cultural experience, “They look at things that happened back in the past about syphilis and how they gave a vaccine to the Blacks and they look at the color of Henrietta Lacks, and how they used her cells without her consent. They made money off of it and stuff like that, so I think that had a lot of do with people of color so afraid of the vaccine.”
Participant 28 (risk level 1, vaccinated), a White American, described how his recognition of distrust in the community influenced his decision to get the vaccine. “Many of my friends, gentlemen and women of color, were very hesitant, and I wanted them to see that I would get it. I wanted them to see that I would go with them to get it, but some of them said, I want to get it when you get it and I want it from the same vial that you get.” His informal leadership reduced hesitancy stemming from cultural distrust.
For individuals who chose to vaccinate, trust in medicine or science was connected to their personal values or culturally normative practices. Family stories and relationships taught participants who they can trust, but family stories and norms could also cultivate mistrust. Participant 21 (risk level 1, non-vaccinated) shared, “looking at things that have happened to my family that didn’t make the history books or didn’t make the news. There is a lot of skepticism in immunizations with things that have happened to us. And as much as we try to educate ourselves and just try to push through, I can’t sit here and tell you that some of that still makes me a little skeptical.” For individuals who chose to not vaccinate, they described mistrust and suspicion about coercion by the government, institutions, or pharmaceutical companies. Participant 30 (risk level 2, non-vaccinated) explained, “I am suspicious that the vaccine – they’re probably paid off. I don’t know if each organization that gets dosages, if they are given an incentive as an organization for how many people they can vaccinate, so it becomes not a matter of the science but it becomes money-driven.”
Theme 3: Communication
Participants made sense of the information environment with a variety of communicative behaviors to figure out what is known and what is unknown. Participant 9 (risk level 3, non-vaccinated) explained, “I’m getting some solid information, not just some made up, something that I feel like people are jumping on this. I feel like it’s become such a hot topic that people enjoy talking about it and it’s almost becoming this way of life kind of thing. So when all that dies down (slight laugh) and nobody thinks about it anymore, then I’ll probably be like, okay, I’m ready to get vaccinated.”
The sensationalism surrounding the COVID-19 vaccine created rumors that disrupted individual understanding. Participant 6 (risk level 4, vaccinated) recounted, “I have heard the stories about microchipping and actually putting the coronavirus in the vaccine. Those are two of the biggest ones … and a lot of people that don’t know how to distinguish and filter through this stuff, it can be very misleading.” Participant 10 (risk level 3, vaccinated) acknowledged the effect of rumors even when he did not believe them. “I obviously was concerned about what’s in the vaccine. You hear the rumors of, ‘oh they’re going to put trackers in your body’ or whatever, which I don’t believe obviously, but there is a concern.”
Vaccine conversations, previously private in nature, entered into routine conversations in the family and workplace. These conversations helped participants identify social norms. Participants described talking to other people who had received the vaccine and observing others receive vaccinations. Seeing other people get the vaccine made them think it was safe. Participant 10 (risk level 3, vaccinated) said, “I know a lot of my friends got it and then when I actually volunteered and I saw all these doctors and nurses and researchers lining up to get the vaccine I’m like well if all these people are getting it there should be no fear for me.” Participants also encountered people in their community choosing not to vaccinate. Participant 2 (risk level 3, vaccinated) shared, “I know a lot of people of my color that had not taken the vaccine and a lot of them saying they not going to take the vaccine because they afraid, and I feel like maybe me being around them and they not vaccinated, that could be a chance where they spread.” Sometimes, seeing these similar others reinforced a norm that people like them were not getting the vaccine.
Trust provided a foundation for communicative processes. When participants did not trust information sources, they questioned the information presented. Participants described how trust was critical in how they interpreted messages about availability and efficacy of the vaccine. “Seeing the way that stuff is reported I don’t take the time to dig into it to see if I can find the flaw and the lie in everything …. I have known people who have sent me things here and there that they have been doing, but I just don’t have the time. I feel like if I get [COVID-19], then whatever” (Participant 9, risk level 3, non-vaccinated).
Time intersected the nexus of trust and communication. In the absence of an individual’s ability to sort through emerging original research, critically appraise studies, and personally apply findings, individuals relied on communication with trusted others to make vaccine decisions. Participant 28 (risk level 1, vaccinated), described this connection, “I looked and said there is [hospital leader 1], there’s [hospital leader 2], there’s [hospital leader 3]. Those three people would never hurt me. Those are three people I trust. So, as soon as they tell me I can get this I’m going to get this … I get to go right to people who know me and ask very specific questions and get the latest best information and that’s privilege and I understand that.”
Discussion
COVID-19 presents a unique, but likely to be repeated, scenario of a disease, treatment, and vaccine that were all discovered within 12 months. This expedited timeline was in contrast to other vaccines in which hesitancy manifested after vaccine development rather than alongside vaccine development. For example, research on vaccine hesitancy in response to the human papilloma virus (HPV), a well understood disease (de Sanjosé et al., Citation2007), vaccine describes gaps in understanding about the vaccine itself and emergent post-marketing risks and side effects of a new vaccine (Briones, Nan, Madden, & Waks, Citation2012; Calloway, Jorgensen, Saraiya, & Tsui, Citation2006; Hendry, Lewis, Clements, Damery, & Wilkinson, Citation2013; Karafillakis et al., Citation2019; Rendle & Leskinen, Citation2017). Public attention to COVID-19 vaccine development is also in contrast to Middle East Respiratory Syndrome and Severe Acute Respiratory Syndrome, which were novel diseases that scientists investigated in real time (Berry, Wharf-Higgins, & Naylor, Citation2007; Stockman, Bellamy, Garner, & Low, Citation2006), but that were still limited by regulatory processes of vaccine development (Mubarak, Alturaiki, & Hemida, Citation2019; Roper & Rehm, Citation2009). The speed of vaccine production will likely never be the same again. However, the public may be slow to accept rapid development given the historical norms such as decades-long searches for polio, malaria, and HIV vaccines (Allen, Citation2007).
COVID-19 presented a fast-moving context that made emerging data and preliminary findings more accessible to the lay public (Anderson & Ledford, Citation2021). This increasingly open scientific environment (Besançon et al., Citation2021) will challenge researchers and communicators. As we tailor messages to target audiences, we must be ready to adapt as emerging science repeatedly changes the content of the message and moves the target audience.
The cross-cutting theme of time intersected with trust. As participants in a “grand experiment globally,” individuals were faced with making a vaccine decision amidst “science in action.” More information is not what motivated vaccine acceptance. Rather, individuals needed trusting relationships with family physicians and even scientists to overcome hesitancy or resistance. For COVID-19, family physicians have continually not had a single, clear answer at the time of clinical decision-making. In this epoch, when family physicians and the public have not had time to digest the fast-moving science, trust is more important than ever. This time-limited decision-making is similar to childbirth when family physicians and patients have to make immediate, fast care decisions for the health of the mother and the baby. Mothers must trust the clinician to make the best choice, relying on the relationship that was built throughout prenatal care (Ledford, Canzona, Womack, & Hodge, Citation2016). In the case of COVID-19 vaccination, individuals need an established relationship with family physicians to help them make decisions in a time- and information-limited environment.
Trust as a cross-cutting theme aligns with the previous conceptualization of confidence. If individuals do not trust the sources of vaccine information, whether it is physicians who recommend a vaccine, scientists or companies who developed the vaccines, government agencies who approved the vaccines, or institutions that mandate vaccines, they are likely to develop vaccine hesitancy. Previous research also connected the important role of trust with vaccine novelty. Confidence is especially important when a vaccine is new (Karafillakis et al., Citation2019). As science emerged regarding COVID-19 so did disinformation, particularly in social media, including false claims that COVID-19 is a hoax (Grimes, Citation2021). When people lack personal knowledge (Zingg & Siegrist, Citation2012) regarding a vaccine, they experience information insufficiency and are motivated to seek additional information (Lu, Winneg, Jamieson, & Albarracín, Citation2020). During information seeking, they may encounter disinformation, misinformation, or conspiracies, and if their confidence is low, information seekers are more susceptible to believe false information (Viswanath, Lee, & Pinnamaneni, Citation2020). This finding provides a framework for segmenting the audience based on levels of trust. Communicators can identify information sources and tailor messages appropriate for each segment.
As qualitative work is interpreted within its sampling approach, results are limited to the sample described here. The sample was diverse in race/ethnicity, age, and education. In the data that addressed cultural and personal beliefs, data only included references to Christian faith. We do not know if other faith traditions have relevant beliefs or if they did not emerge here. Results should be interpreted within the context of the contemporary setting within which the data was collected. Here, it is notable that interviews were conducted in the American South, beginning in January 2021. Participant 2 (risk level 3, vaccinated) summarized the current environment, “within this last year, all this racism that came out, lost a lot of trust with African Americans. The killing of George Floyd, Breonna Taylor, Aubrey, so they are scared, they don’t know if they can trust, even some of the people, white Caucasians, that they know. They still have in the back of their head some discomfort about trust and lack of trust.” The interviewer followed up, asking, “Is it trust in the system or trust in people?” To which the participant answered, “I think it’s both.”
Our study reveals that the complex information environment surrounding COVID-19 vaccination requires multi-level intervention that cannot rely on knowledge translation alone. In a time of emerging science and changing answers, information boluses created information overload as the information environment became more and more complex. Instead, we need to focus on building trusting relationships with experts that will create the scaffolding for future information processing.
Having a sufficient percentage of the general population vaccinated will substantially reduce the risk of transmission (Mellet & Pepper, Citation2021). In the face of an environment in which COVID vaccination is viewed with increasing levels of suspicion, achieving a high rate of vaccination among the general public requires a sustained, consistent, and coordinated message. Clinicians can be a key influence for vaccine uptake. Previous research shows that clinician recommendation predicts vaccine uptake (Callahan, Coleman-Cowger, Schulkin, & Power, Citation2021; Walker, Owens, & Zimet, Citation2020). However, clinicians cannot ignore individuals’ emotional response (Slovic & Peters, Citation2006) or the historical context of medical research when discussing emerging diseases and novel treatments with patients. By using empathetic listening (Comer & Drollinger, Citation1999) and patient-centered communication, clinicians can build trusting relationships with patients. Only then can patient education or public communication campaigns make a difference.
Note: I confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Presentations
An earlier version of this manuscript was presented as “COVID-19 vaccine decisions amidst fast-changing science: A qualitative study” at the 2021 virtual International Conference on Communication in Healthcare in October 2021.
Acknowledgments
We acknowledge Nicollette Lewis and Dan Spell for their assistance with analysis of the data. We also acknowledge Varsha Chiruvella, who provided an extensive review of the literature that informed this content.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ten threats to global health in 2019. (2019). Retrieved from https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
- Allen, A. (2007). Vaccine: The controversial story of medicine’s greatest lifesaver. New York, USA: W.W. Norton & Company.
- Anderson, L. N., & Ledford, C. (2021). Who's Guarding the Gate? The Reach of Prereviewed Emerging Science and Implications for Family Medicine Education. Family medicine, 53(8), 670–675. https://doi.org/https://doi.org/10.22454/FamMed.2021.246112
- Augusta University health frontline caregivers get COVID-19 vaccine. (2020). Augusta Chronicle. Retrieved from https://www.augustachronicle.com/story/news/local/2020/12/17/augusta-university-health-frontline-caregivers-get-covid-19-vaccine/3937700001/
- Baden, L. R., El Sahly, H. M., Essink, B., Kotloff, K., Frey, S., Novak, R., … Zaks, T. (2021). Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med, 384(5), 403–416. doi:https://doi.org/10.1056/NEJMoa2035389
- Berry, T. R., Wharf-Higgins, J., & Naylor, P. J. (2007). SARS Wars: An Examination of the Quantity and Construction of Health Information in the News Media. Health Communication, 21(1), 35–44. doi:https://doi.org/10.1080/10410230701283322
- Besançon, L., Peiffer-Smadja, N., Segalas, C., Jiang, H., Masuzzo, P., Smout, C., … Leyrat, C. (2021). Open science saves lives: Lessons from the COVID-19 pandemic. BMC Med Res Methodol, 21(1), 117. doi:https://doi.org/10.1186/s12874-021-01304-y
- Betsch, C., Schmid, P., Heinemeier, D., Korn, L., Holtmann, C., Böhm, R., & Angelillo, I. F. (2018). Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLoS One, 13(12), e0208601. doi:https://doi.org/10.1371/journal.pone.0208601
- Briones, R., Nan, X., Madden, K., & Waks, L. (2012). When vaccines go viral: An analysis of HPV vaccine coverage on YouTube. Health Communication, 27(5), 478–485. doi:https://doi.org/10.1080/10410236.2011.610258
- Callahan, A. G., Coleman-Cowger, V. H., Schulkin, J., & Power, M. L. (2021). Racial disparities in influenza immunization during pregnancy in the United States: A narrative review of the evidence for disparities and potential interventions. Vaccine, 39(35), 4938–4948. doi:https://doi.org/10.1016/j.vaccine.2021.07.028
- Calloway, C., Jorgensen, C. M., Saraiya, M., & Tsui, J. (2006). A content analysis of news coverage of the HPV vaccine by U.S. newspapers, January 2002-June 2005. Journal of Women’s Health, 15(7), 803–809. 15409996), Retrieved from Publisher URL. https://doi.org/10.1089/jwh.2006.15.803
- Calvillo, D. P., Ross, B. J., Garcia, R. J. B., Smelter, T. J., & Rutchick, A. M. (2020). Political Ideology Predicts Perceptions of the Threat of COVID-19 (and Susceptibility to Fake News About It). Social Psychological and Personality Science, 11(8), 1119–1128. doi:https://doi.org/10.1177/1948550620940539
- Chu, H., & Liu, S. (2021). Integrating health behavior theories to predict American’s intention to receive a COVID-19 vaccine. Patient Educ Couns, 104(8), 1878–1886. doi:https://doi.org/10.1016/j.pec.2021.02.031
- Comer, L. B., & Drollinger, T. (1999). Active empathetic listening and selling success: A conceptual framework. Journal of Personal Selling & Sales Management, 19(1), 15–29.
- Daly, M., & Robinson, E. (2021). Willingness to Vaccinate Against COVID-19 in the U.S.: Representative Longitudinal Evidence From April to October 2020. Am J Prev Med, 60(6), 766–773. doi:https://doi.org/10.1016/j.amepre.2021.01.008
- de Sanjosé, S., Diaz, M., Castellsagué, X., Clifford, G., Bruni, L., Muñoz, N., & Bosch, F. X. (2007). Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect Dis, 7(7), 453–459. doi:https://doi.org/10.1016/s1473-3099(07)70158-5
- Dib, F., Mayaud, P., Chauvin, P., & Launay, O. (2021). Online mis/disinformation and vaccine hesitancy in the era of COVID-19: Why we need an eHealth literacy revolution. Human Vaccines & Immunotherapeutics, 1–3. doi:https://doi.org/10.1080/21645515.2021.1874218
- Epstein, R. M., Alper, B. S., & Quill, T. E. (2004). Communicating evidence for participatory decision making. Jama, 291(19), 2359–2366. doi:https://doi.org/10.1001/jama.291.19.2359
- Felsenstein, S., & Hedrich, C. M. (2020). SARS-CoV-2 infections in children and young people. Clin Immunol, 220, 108588. doi:https://doi.org/10.1016/j.clim.2020.108588
- Fine, P., Eames, K., & Heymann, D. L. (2011). “Herd immunity”: A rough guide. Clin Infect Dis, 52(7), 911–916. doi:https://doi.org/10.1093/cid/cir007
- Freeman, D., Loe, B. S., Chadwick, A., Vaccari, C., Waite, F., Rosebrock, L., … Lambe, S. (2020). COVID-19 vaccine hesitancy in the UK: The Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol Med, 1–15. doi:https://doi.org/10.1017/s0033291720005188
- Grimes, D. R. (2021). Medical disinformation and the unviable nature of COVID-19 conspiracy theories. PLoS ONE, 16(3), e0245900. https://doi.org/https://doi.org/10.1371/journal.pone.0245900
- Hendry, M., Lewis, R., Clements, A., Damery, S., & Wilkinson, C. (2013). “HPV? Never heard of it!”: A systematic review of girls’ and parents’ information needs, views and preferences about human papillomavirus vaccination. Vaccine, 31(45), 5152–5167. doi:https://doi.org/10.1016/j.vaccine.2013.08.091
- Iyasere, J., Garcia, A., Prabhu, D. V., Procaccino, A., Spaziani, K. J., Smith, L., & Berchuck, C. M. (2021). Trustworthy and Trusted: Strategies to Improve Confidence in Covid-19 Vaccines. NEJM Catalyst Innovations in Care Delivery, 2(2). doi:https://doi.org/10.1056/cat.21.0158
- Karafillakis, E., Simas, C., Jarrett, C., Verger, P., Peretti-Watel, P., Dib, F., … Larson, H. (2019). HPV vaccination in a context of public mistrust and uncertainty: A systematic literature review of determinants of HPV vaccine hesitancy in Europe. Hum Vaccin Immunother, 15(7–8), 1615–1627. doi:https://doi.org/10.1080/21645515.2018.1564436
- Larson, H. J., Schulz, W. S., Tucker, J. D., & Smith, D. M. (2015). Measuring vaccine confidence: Introducing a global vaccine confidence index. PLoS Curr, 7. doi:https://doi.org/10.1371/currents.outbreaks.ce0f6177bc97332602a8e3fe7d7f7cc4
- Ledford, C. J., Canzona, M. R., Womack, J. J., & Hodge, J. A. (2016). Influence of Provider Communication on Women’s Delivery Expectations and Birth Experience Appraisal: A Qualitative Study. Family Medicine, 48(7), 523–531.
- Lee, C., Whetten, K., Omer, S., Pan, W., & Salmon, D. (2016). Hurdles to herd immunity: Distrust of government and vaccine refusal in the US, 2002-2003. Vaccine, 34(34), 3972–3978. doi:https://doi.org/10.1016/j.vaccine.2016.06.048
- Livingston, E. H., Malani, P. N., & Creech, C. B. (2021). The Johnson & Johnson Vaccine for COVID-19. Jama, 325(15), 1575. doi:https://doi.org/10.1001/jama.2021.2927
- Lu, H., Winneg, K., Jamieson, K. H., & Albarracín, D. (2020). Intentions to Seek Information About the Influenza Vaccine: The Role of Informational Subjective Norms, Anticipated and Experienced Affect, and Information Insufficiency Among Vaccinated and Unvaccinated People. Risk Anal, 40(10), 2040–2056. doi:https://doi.org/10.1111/risa.13459
- Lyu, J. C., Han, E. L., & Luli, G. K. (2021). Topics and Sentiments in COVID-19 Vaccine-related Discussion on Twitter. J Med Internet Res, 23(6), e24435. doi:https://doi.org/10.2196/24435
- Ma, Z., & Ma, R. (2021). Predicting Intentions to Vaccinate against COVID-19 and Seasonal Flu: The Role of Consideration of Future and Immediate Consequences. Health Commun, 1–10. doi:https://doi.org/10.1080/10410236.2021.1877913
- MacDonald, N. E. (2015). Vaccine hesitancy: Definition, scope and determinants. Vaccine, 33(34), 4161–4164. doi:https://doi.org/10.1016/j.vaccine.2015.04.036
- Maietta, R., Mihas, P., Swartout, K., Petruzzelli, J., & Hamilton, A. B. (2021). Sort and Sift, Think and Shift: Let the Data Be Your Guide An Applied Approach to Working With, Learning From, and Privileging Qualitative Data. The Qualitative Report, 26(6), 2045–2060. https://doi.org/https://doi.org/10.46743/2160-3715/2021.5013
- Mellet, J., & Pepper, M. S. (2021). A COVID-19 Vaccine: Big Strides Come with Big Challenges. Vaccines (Basel), 9(1). doi:https://doi.org/10.3390/vaccines9010039
- Montagni, I., Ouazzani-Touhami, K., Mebarki, A., Texier, N., Schück, S., Tzourio, C., & Group, C. (2021). Acceptance of a Covid-19 vaccine is associated with ability to detect fake news and health literacy. Journal of Public Health (Oxford, England), fdab028. doi:https://doi.org/10.1093/pubmed/fdab028
- Moore, J. X., Gilbert, K. L., Lively, K. L., Laurent, C., Chawla, R., Li, C., … Ledford, C. J. W. (2021). Correlates of COVID-19 Vaccine Hesitancy among a Community Sample of African Americans Living in the Southern United States. Vaccines (Basel), 9(8). doi:https://doi.org/10.3390/vaccines9080879
- Mubarak, A., Alturaiki, W., & Hemida, M. G. (2019). Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development. J Immunol Res, 2019, 6491738. 2019 https://doi.org/10.1155/2019/6491738
- Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., … Gruber, W. C. (2020). Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med, 383(27), 2603–2615. doi:https://doi.org/10.1056/NEJMoa2034577
- Quinn, S. C., Jamison, A. M., An, J., Hancock, G. R., & Freimuth, V. S. (2019). Measuring vaccine hesitancy, confidence, trust and flu vaccine uptake: Results of a national survey of White and African American adults. Vaccine, 37(9), 1168–1173. doi:https://doi.org/10.1016/j.vaccine.2019.01.033
- Reiter, P. L., Pennell, M. L., & Katz, M. L. (2020). Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine, 38(42), 6500–6507. doi:https://doi.org/10.1016/j.vaccine.2020.08.043
- Rendle, K. A., & Leskinen, E. A. (2017). Timing Is Everything: Exploring Parental Decisions to Delay HPV Vaccination. Qual Health Res, 27(9), 1380–1390. doi:https://doi.org/10.1177/1049732316664499
- Roper, R. L., & Rehm, K. E. (2009). SARS vaccines: Where are we? Expert Rev Vaccines, 8(7), 887–898. doi:https://doi.org/10.1586/erv.09.43
- Rosenstock, I. M. (1974). Historical origins of the health belief model. Health Education Monographs, 2(4), 328–335. doi:https://doi.org/10.1177/109019817400200403
- Ruiz, J. B., & Bell, R. A. (2021). Predictors of intention to vaccinate against COVID-19: Results of a nationwide survey. Vaccine, 39(7), 1080–1086. doi:https://doi.org/10.1016/j.vaccine.2021.01.010
- Slovic, P., & Peters, E. (2006). Risk Perception and Affect. Current Directions in Psychological Science, 15(6), 322–325. doi:https://doi.org/10.1111/j.1467-8721.2006.00461.x
- Stockman, L. J., Bellamy, R., Garner, P., & Low, D. (2006). SARS: Systematic review of treatment effects. PLoS Med, 3(9), e343. doi:https://doi.org/10.1371/journal.pmed.0030343
- Viswanath, K., Lee, E. W. J., & Pinnamaneni, R. (2020). We Need the Lens of Equity in COVID-19 Communication. Health Commun, 1–4. doi:https://doi.org/10.1080/10410236.2020.1837445
- Walker, K. K., Owens, H., & Zimet, G. (2020). “We fear the unknown”: Emergence, route and transfer of hesitancy and misinformation among HPV vaccine accepting mothers. Prev Med Rep, 20, 101240. doi:https://doi.org/10.1016/j.pmedr.2020.101240
- Zampetakis, L. A., & Melas, C. (2021). The health belief model predicts vaccination intentions against COVID-19: A survey experiment approach. Appl Psychol Health Well Being, 13(2), 469–484. doi:https://doi.org/10.1111/aphw.12262
- Zingg, A., & Siegrist, M. (2012). Measuring people’s knowledge about vaccination: Developing a one-dimensional scale. Vaccine, 30(25), 3771–3777. doi:https://doi.org/10.1016/j.vaccine.2012.03.014