?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aqueous two-phase extraction (ATPE) has been extensively utilized for the extraction and separation of tiny-molecule substances as a new system (system with short-chain ethanol and inorganic salts). In this study, an innovative method of extracting anthocyanins from mulberry was developed, employing microwave-assisted extraction with ethanol/ammonium sulfate as a biphasic extractant. Response surface methodology (RSM) was utilized to optimize anthocyanin extraction conditions: 39% ethanol (w/w), 13% ammonium sulfate (w/w), and liquid-to-solid ratio of 45:1, microwave duration 3 min, microwave temperature 32 °C, and microwave power 480 Watt (W). High-performance liquid chromatography (HPLC) analysis demonstrated no significant differences in the structure of mulberry anthocyanins before and after MAATPE treatment, furthermore. The extraction behavior of MAATPE was due to hydrogen bonding, according to Fourier transform infrared spectroscopy (FT-IR). Scanning electron microscopy analysis found that MAATPE damaged the cell structure via a microwave enhancement effect, which was more favorable to anthocyanin dissolution than standard extraction methods. The DPPH free radical scavenging rate of mulberry extracts at 0.5 mg/mL was higher than that of vitamin C (96.4 ± 0.76%), and the ABTS free radical scavenging rate (82.52 ± 2.13%) was close to that of vitamin C, indicating that MAATPE-derived mulberry extracts have good antioxidant activity.
Introduction
Mulberry is an oval polymerized berry with a unique flavor and rich nutritional profile.[Citation1] It is abundant in vitamins,[Citation2] amino acids,[Citation3] base minerals, phenols,[Citation4] total flavonoids,[Citation5] and other active ingredients. Various studies demonstrate its traditional application besides indicating other potential therapeutic applications such as in liver protection,[Citation6] antibacterial,[Citation7] antithrombotic activities,[Citation8] antioxidative effects,[Citation9] and antiobesity actions.[Citation10] Mulberries possess numerous applications in the pharmaceutical and food preparation fields.[Citation11] Mulberry is one of the reservoirs for natural pigments and a rich source of anthocyanins, which provides a black-purple or purplish-red color to the fruit.[Citation12] Due to their high nutrient content and pharmacological properties, mulberry-derived anthocyanins are now extensively utilized in a variety of industries, including the food and pharmaceutical sectors.[Citation13] However, the main obstacles associated with using mulberry-extracted anthocyanins in the food business are higher production expenses and poorer yields from extraction.[Citation14] Anthocyanins are in greater demand globally, which has prompted additional research into ways to improve extraction, purification, and stabilization processes.[Citation15] Enzymatic extraction of mulberry anthocyanins and ultrasonic-assisted extraction are two common techniques. The enzyme-assisted extraction technique makes use of the enzyme’s specificity to break down the cell wall and promote the solvent to extract anthocyanins inside the cell. However, due to the lengthy extraction process and high solvent consumption, it is not regarded as an effective extraction technique. The cavitation action of ultrasound is used in the ultrasonic-assisted approach to break down cell walls to promote the release of anthocyanins from the cells. However, the ultrasonic-assisted top treatment is not consistent, resulting in insufficient sample extraction, which restricts the scope of its application.[Citation16,Citation17] Therefore, it is crucial to find an extraction technique for mulberry anthocyanins that is productive and recyclable.
Aqueous two-phase extraction (ATPE) is an extensively employed method for separating anthocyanins because of its simple and mild operating conditions, friendly to the environment, simplicity of expansion, and inexpensive costs.[Citation18] ATPE separates and purifies substances based on the difference in the selective distribution of components among the two phases. Anthocyanins are soluble in organic solvents. With the formation of two phases in ATPE, anthocyanins are enriched in the alcohol phase, and other substances such as polysaccharides and some impurities are enriched in the salt phase. With the gradual extension of methods of separation into the realms of extraction and purification, it has become an efficient single-step extraction method.[Citation19] At the same time, microwave-assisted extraction (MAE) has the potential to expedite the migration of specific molecules from substances to the environment.[Citation20] In recent years, a method known as MAATPE has grown in popularity, which incorporates MAE and ATPE. It attracted a lot of interest owing to the benefits of rapid heating minimized solvent damage, minimal use of energy, and regulated emissions.[Citation21] MAATPE can provide remaining thermal quick demixing effects by utilizing microwave-enhanced interactions dependent on the ability for biphasic extraction and microwave field intensifying.[Citation18] Up to now, the MAATPE method has yet to be applied for anthocyanin extraction from mulberry fruits.
The purposes of this work were to identify the optimal MAATPE conditions for anthocyanins from mulberry fruits, as well as to establish a green, quick, and efficient process for extracting anthocyanins from mulberry fruits. The parameters affecting MAATPE were investigated through a single-factor approach. Secondly, the concentration of ethanol and ammonium sulfate was optimized by response surface methodology (RSM), microwave temperature, and time to maximize the recovery rate of anthocyanins. By means of high-performance liquid chromatography (HPLC) and Fourier transform infrared spectroscopy (FT-IR), the obtained anthocyanins were characterized, and their main components and extraction behavior were clarified. Moreover, scanning electron microscopy (SEM) was utilized to investigate how various methods of extraction impacted the mulberry surface structure. DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS+ (2,2′-Azino-bis[3-ethylbenzothiazoline-6-sulfonic acid]) free radical scavenging evaluation were utilized as well to assess the antioxidant activity of mulberry anthocyanins.
Materials and methods
Sample preparation
Mulberry samples, sourced from Bozhou Xindi Ecological Agriculture Co., Ltd. All of the mulberry fruits in the batch were thoroughly cleaned, freeze-dried, crushed, and sieved through 100 mesh to obtain the fine mulberry powder, and, subsequently, until further usage, stored in a cool, dark space at 4 °C.
Chemicals and reagents
The analytical reagents, which included ammonium sulfate, potassium chloride, concentrated hydrochloric acid, sodium acetate, and ethanol (absolute grade), were provided by Sinopharm Chemical Reagent Ltd. (Shanghai, China). DPPH, and ABTS+. MACKLIN Reagent Co., Ltd. (Shanghai, China) supplied the methanol and formic acid (HPLC grade).
Phase diagrams for the ATPE process
Utilizing the titration approach, the ethanol + ammonium sulfate + water systems’ phase diagrams were created.[Citation4] In the experiment, 20 mL of 0.25 g/m L ammonium sulfate solution was added to a 50 mL glass beaker, titrated with ethanol and continuously stirred until turbidity appeared. The phase separation was static, the entire mass of the beaker and the volume of ethanol consumed were recorded, the solution was then clarified by adding distilled water, and the quality of distilled water added was recorded. Repeat the preceding steps and collect adequate information to create the phase diagram. The mixture’s mass was determined on balance for analytical purposes with a precision of 0.0001 g. A thermostat water bath was employed to maintain the system temperature at 25 ± 0.1 °C.
MAATPE method
Adding 1 g mulberry powder and a certain amount of extractant to the three-well flask, the experiment was carried out in the MCR-3 atmospheric microwave chemical reactor equipped with a digital timer, a magnetic stirrer, a power and temperature controller (Gongyi Yu 'a Instrument Co., Ltd.). Experiments utilizing MAE were carried out at the MCR-3 atmospheric microwave chemical reactor. The sample was quickly chilled to room temperature (25 °C) in an ice water bath after extraction. Subsequently, it was moved to the liquid separation funnel for parvafacies. The amount of volume with the top and bottom phases was determined, and the anthocyanin concentration in the two phases was analyzed. Any residue accumulated at the two-phase interface is discarded. EquationEquation 1(1)
(1) was utilized to calculate the anthocyanin partition coefficient (K).
(1)
(1)
where CT and CB represent the equilibrium amount of anthocyanin in the top and bottom phases, correspondingly.
Recovery (Y) was calculated by multiplying the anthocyanin concentration partitioned in the top and bottom phases with the total amount of anthocyanin. The amount of volume in the top and bottom phases is represented by VT and VB, respectively. EquationEquation 2(2)
(2) was utilized to calculate it.
(2)
(2)
Estimation of mulberry anthocyanins
The pH-differential methods were employed to estimate the number of anthocyanins.[Citation22] To the 1 mL water solution of extract, 4 mL of 0.025 M potassium chloride (pH = 1.0) and 0.4 M sodium acetate (pH = 4.5) buffer were added. Using a UV–Vis spectrophotometer, the mixture’s absorbance was subsequently measured at 520 nm and 700 nm. EquationEquation 3(3)
(3) was employed to calculate the anthocyanin content generally based on the cyanidin-3-O-glucoside equivalent.
(3)
(3)
where A represents absorbance, f for dilution multiple, MW for cyanidin-3-O-glucoside’s molecular weight (449.2 g/mol), and
for cyanidin-3-O-glucoside’s molar extinction coefficient of 26,900.
Single-factor experiments
The impact of significant variables on Y and K was examined in determining the optimum extraction conditions for mulberry anthocyanins including varied ethanol and (NH4)2SO4 concentrations at 32–40% (w/w) and 12–16% (w/w), respectively. Besides, other MAATPE-associated parameters were also studied, such as microwave temperature (25–45 °C), time (2–6 min), power (240–560 W), and liquid-to-solid ratio (40–60 g/g). Three replicas of each experiment were run throughout.
Experimental design for optimization of extraction parameters
RSM was employed to evaluate the single-factor experiment’s results to identify the optimal conditions for mulberry anthocyanin extraction. The following four independent factors were taken into account in a Box–Behnken design: A, the concentration of ethanol (%, w/w): 36, 38, and 40; B, the concentration of ammonium sulfate (%, w/w): 12, 13, and 14; C, the microwave’s temperature (°C), 25, 30, and 45; and D, the microwave’s time (min), 2, 3, and 4; at three levels of variation (). The design involved 29 experiments, containing 24 factorial experiments and 5 repetitions at the center point. To calculate the likelihood of pure mistakes, experiments were carried out at the design’s core. Each experiment was carried out at random.
Table 1. The design approach and experimental result of response surface methodology.
HPLC analysis
For the evaluation of the anthocyanins from mulberry, an ultraviolet detector-equipped Agilent Infinity 1200 chromatograph (Agilent Technologies Co., Ltd., USA) and Venusil ASB C18 (4.6 × 250 mm, 5 μm) chromatographic column were utilized. The injection volume was 20 L, the instrument’s detection wavelength was 520 nm, and the solution speed was 1.5 mL/min. The column’s temperature was selected at 35 °C. Formic acid (solvent A) and formic acid methanol (solvent B) constituted the eluent solvents, both at 1% (v/v) concentration. The gradient elution conditions were the following: (a) from 8% to 12% B in 0–2 min, (b) from 12% to 18% B in 2–5 min, (c) from 18% to 20% B in 5–10 min, (d) from 20% to 25% B in 10–12 min, (e) from 25% to 30% B in 12–15 min, (f) from 30% to 45% B in 15–18 min, (g) from 45% to 80% B in 18–20 min, from 80% to 8% B in 20–22 min, (h) 8% B in 22–30 min.
FT-IR analysis
The following methodology was employed to bring the extracted mulberry anthocyanins to FT-IR analysis for structural characterization. Before being crushed and flattened into a 1-mm wafer and determined in a VERTEX 80 V FT-IR spectrometer (Bruker, Germany), KBr powder was incorporated into a small amount of the dry material.
Scanning electron microscope (SEM) analysis
The microstructures on the surface of mulberry were examined utilizing a Zeiss EVO 18 electron microscope with scanning technology (Zeiss, Germany). The powder was spray-gold treated with the SBC-12 Ion Sputtering Coater from Beijing, China. A 20.0 kV voltage source was employed for examining the sprayed material in the Zeiss EVO 18's empty room.
DPPH assay
The previously published method was utilized to calculate the free radical-scavenging DPPH capacity.[Citation23] Briefly, 10 mL of ethanol and 0.8 mL of 0.15 mM DPPH were utilized to homogenize the sample water solution. A small amount of this combination was stored in the dark and incubated at room temperature for 30 min.
UV–Vis spectrophotometer was employed to measure the mixture’s fluorescence at 517 nm. The reference substance utilized was water-soluble vitamin C. EquationEquation 4(4)
(4) was applied to calculate the activity of radical scavenging in the format indicated below:
(4)
(4)
Where A0 represents the mixture’s absorbance in the absence of samples, A1 the mixture’s absorbance with the experiment’s sample and the DPPH solution were both present, while A2 indicated the fluorescence of the mixture that did not include the DPPH solution.
ABTS assay
The assay was based on a previous procedure for ABTS cation radical (ABTS+) decolorization after reduction to ABTS.[Citation24] For the production of ABTS+, 2.45 mM potassium persulphate aqueous solution was combined with 7 mM ABTS solution and incubated for 12–16 hr at room temperature. Furthermore, phosphate buffer has been mixed with the ABTS+ sample to produce an absorbance of 0.70 ± 0.02 at 734 nm. The absorbance (A1) was then determined by mixing 2.5 mL of diluted ABTS+ solution with 0.2 mL of the sample solution for 6 min at room temperature. For the control, 0.2 mL of ethanol was utilized in place of the sample, and the absorbance A2 was derived at 734 nm. As in the DPPH experiment, water-soluble vitamin C was employed as a control material. EquationEquation 5(5)
(5) was applied to calculate the radical scavenging activity:
(5)
(5)
where A1 was the absorption coefficient of the combination containing both the test sample and the ABTS+ solution and A2 represented the absorbing capacity of the mixture containing only the test material and no ABTS+ solution.
Results and discussion
Preparation for phase diagram
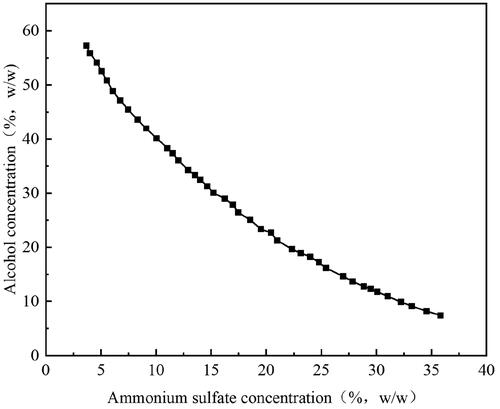
The ATPE system composed of ethanol/ammonium sulfate was investigated using a phase diagram created using cloud point titration. The ethanol/ammonium sulfate systems displayed two different zones delimited by a curve, as shown in . The aqueous ethanol phase was at the top of this zone, while ammonium sulfate phase was at the bottom. The top restriction of ammonium sulfate concentration is roughly 40% (w/w) for both ATPE and ethanol concentration is roughly 60% (w/w) for ATPE (ethanol/ammonium sulfate) (). These results matched with previous studies.[Citation25]
Single-factor experiment
Ethanol concentration
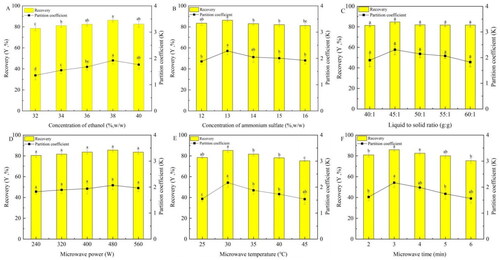
The effects of ethanol concentration on K and Y factors were associated with mulberry anthocyanins (). Aqueous two-phase separation was not observed in the system at an ethanol concentration of less than 18%. Moreover, ammonium sulfate precipitation occurred in the aqueous two-phase system, with ethanol concentration exceeding 40%. The Y and K showed significant enhancement at an increasing ethanol concentration of 32–38% (p < 0.05), depicting maximum values of 86.42 ± 1.65% and 1.922 ± 0.13, respectively, at 38% (w/w) concentration. The solvent utilized for the extraction process with microwaves is determined by the intended component’s solubility as well as the solvent’s ability to permeate the sample while interacting with it.[Citation25] The factors Y and K decreased considerably when the ethanol concentration was beyond 38% (p < 0.05). Due to the rise in ethanol content, the top phase incorporates decreased microwave energy. As a consequence, for additional optimization examinations, the ethanol concentration of 38% (w/w) was retained.
Ammonium sulfate concentration
The MAATPE process has been studied in terms of ammonium sulfate concentration (). With increasing ammonium sulfate concentration, Y and K showed a tendency to increase initially and subsequently drop (p < 0.05). The volume of the top phase shrank when the sulfate of ammonium concentration increased, which reduced the amount of anthocyanin in the top phase.[Citation26] Hence, for further optimization, the ammonium sulfate concentration was maintained at 13% (w/w).
Liquid-to-solid ratio
Liquid-to-solid ratio in the range of 40–60 g/g was used to research the effects on K and Y of mulberry anthocyanin in both phases (). According to the figure, the highest K value was 2.308 ± 0.2, and the highest Y value was 84.68 ± 1.73%. In addition, a higher liquid-to-solid ratio contributed to a larger concentration gradient between the solvent and solid, which in turn decreased the extraction medium’s viscosity, increased diffusion, and improved the recovery of mulberry anthocyanins.[Citation17] However, the overall difference in K and Y values was insignificant at the selected range of liquid-solid ratio (p > 0.05). Thus, the ratio was kept constant at 45 g/g for subsequent optimization studies.
Microwave power
Analyzing the consequences of various microwave intensities on the Y and K of the MAATPE process according to , the variation in K and Y values was insignificant (p > 0.05) when microwave power increased from 240 to 560 W. This outcome matched what Zhang et al. and Ma et al. had previously reported.[Citation27,Citation28] The MAE system with a temperature sensor regulates the microwave power; if the temperature rises over a certain threshold, an adequate temperature remains maintained by automatically decreasing the irradiation power. Therefore, a microwave power operating at 480 W was selected for subsequent studies.
Microwave temperature
displayed the differential partitioning of mulberry anthocyanins employing MAATPE at varying microwave temperatures. shows a maximum of 84.539 ± 1.78% and 2.169 ± 0.18, respectively, at 30 °C. This finding could be attributed to the decreased solvent viscosity and surface tension at higher temperatures, resulting in enhanced solubility and diffusion coefficient.[Citation15] Additionally, the recovery rate of mulberry anthocyanin declined as the temperature exceeded 30 °C. The reduced recovery rate may be ascribed to the heat resistance of anthocyanin and the degradation of some heat-sensitive components within anthocyanin caused by increasing temperature.[Citation29]
Microwave time
demonstrated the influence of microwave irradiation time on Y and K factors of mulberry anthocyanins in the MAATPE process, revealing that both factors increased within the first 3 min, exhibiting the highest values of 85.939 ± 1.38% and 2.169 ± 0.18 for Y and K, respectively, at 3 min. A further increase in time could result in internal overheating caused by cell rupture and enhanced solubility of active components, thus releasing anthocyanin into the extraction medium.[Citation30] Consequently, anthocyanin degradation may occur beyond 3 min of extraction time due to prolonged excessive heating.[Citation31]
Verification of optimum conditions
Multiple factors influence the process of extraction of active ingredients from plant fruits. In the MAATPE system, several parameters impacting the extraction process, which include ethanol concentration, ammonium sulfate concentration, microwave temperature, and microwave time, are often regarded as critical. In the following work, the effect of all these parameters on anthocyanin recovery and partition coefficient during extraction was noteworthy except liquid-to-solid ratio (45 g/g) and microwave power (480 W) (p > 0.05). As a consequence, the RSM design was chosen to employ the four extraction parameters of ethanol and ammonium sulfate concentration, microwave temperature, and microwave time.
Statistical analysis and model fitting
Employing the Design Expert 8.0 application, a variety of regression analyses was carried out on the experiment results, and an equation of the second order of equations was fitted to create a connection between the various factors and the responses. EquationEquation 6(6)
(6) represents the final recovery (Y) rate model as follows:
(6)
(6)
where Y represents the recovery rate (%), A represents the ethanol concentration (%,w/w), B represents the ammonium sulfate concentration (%,w/w), C represents the microwave temperature (°C), and D represents the microwave time (min).
ANOVA analysis related to anthocyanin recovery rate
presents the ANOVA results for determining the coefficients in the regression model. Understanding the patterns of interaction between independent variables was evaluated statistically utilizing p-values. The model’s appropriateness was demonstrated by the observation of a p-value of less than 0.0001. The correlation coefficient R2 (99.01%) and the adjusted determination coefficient R2 (98.01%) demonstrated the model’s great dependability. Additionally, the model’s accuracy and dependability were acceptable, as demonstrated by an insignificant lack of fit with a p-value of 0.1877. Ammonium sulfate concentration value was discovered to be the most significant component that impacts the anthocyanin recovery, followed by microwave temperature, ethanol concentration, and microwave time, according to the significance of the coefficients of regression of the polynomial quadratic model. In addition, the interactions between the parameters AC, AD, BC, BD, and CD were extremely significant (p < 0.01) as well as The quadratic term (A2, B2, C2, D2, was very significant (p < 0.01). As a result, the model was statistically accurate and reliable enough.
Table 2. ANOVA of the mulberry anthocyanin recovery test.
RSM analysis
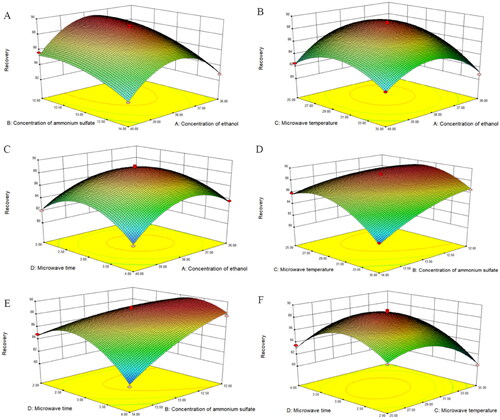
The effect of interaction between factors on anthocyanin extraction was evaluated based on response surface analysis diagrams, where the surface steepness indicated a more significant interaction between the two factors.[Citation32] The interaction diagrams presented in six pairs of variables impacted the recovery of mulberry anthocyanins, as shown in . The response surface contours were all convex and had maximum points in the experimental domain, proving that the factor range was appropriate. Nevertheless, the response surface curves of the predictors for the two phases differed slightly (). Finally, predicted values for the four variables were obtained. According to the Box–Behnken test and regression equation results, the following were the optimal conditions for extracting mulberry anthocyanins utilizing MAATPE: ethanol concentration (38.89%, w/w), ammonium sulfate concentration (13.35%, w/w), temperature (32.43 °C), and time (3.44 min), with an overall recovery rate of 85.22%. Considering the instrument limitation for parameters, experiment operability, and cost, the following are the optimum process parameters: ethanol at 39% (w/w), (NH4)2SO4 at 13% (w/w), microwave temperature of 32 °C, and microwave exposure for 3 min. Under modified extraction conditions, the experimental verification value of anthocyanin recovery was 86.35 ± 0.32%, which corresponded to the anticipated value. The findings indicated that the prediction model was both dependable and successful.
Figure 3. Response surface analysis for the extraction variables on the recovery. (A) Recovery versus ethanol concentration and ammonium sulfate concentration, (B) recovery versus ethanol concentration and microwave temperature, (C) recovery versus ethanol concentration and microwave time, (D) recovery versus ammonium sulfate concentration and microwave temperature, (E) recovery versus ammonium sulfate concentration and microwave time, and (F) recovery versus microwave temperature and microwave time.
HPLC and FT-IR spectroscopy analysis
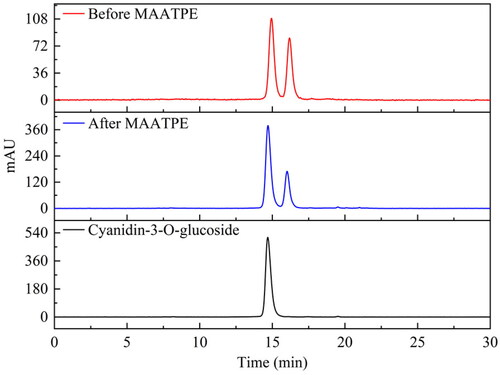
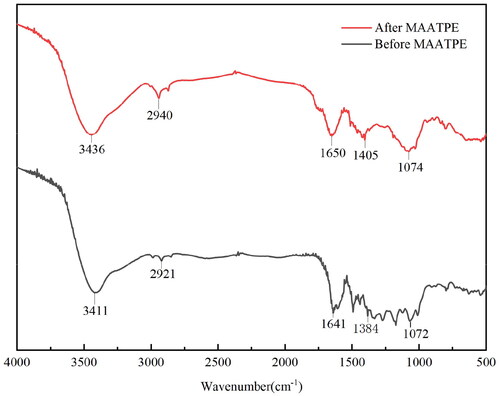
shows the HPLC results before and after MAATPE. Each chromatogram exhibited two peaks with nearly identical retention times, and it proved that the anthocyanins obtained by MAATPE had no detectable alterations in structure.[Citation33] The primary components of mulberry anthocyanin are cyanidin-3-O-glucoside, compared to the cyanidin-3-O-glucoside chromatogram. MAATPE enriched the cyanidin-3-O-glucoside compared with the before MAATPE extraction. Furthermore, through investigating the structure and functional groups of the extract, FT-IR spectra were utilized to further understand the MAATPE mechanism.[Citation34] The adsorption band around at 3436 cm−1 and 3411 cm−1 demonstrated that the O–H stretching vibration was detected ().[Citation35] Moreover, the absorption peak appeared at 1390 cm−1 representing the O–H bending vibration.[Citation36] The symmetric and asymmetrical CH stretching vibrations were attributed to the peak near 2921 cm−1, while CH symmetric stretching might be the cause for the peak at 2940 cm−1.[Citation37] Besides, the characteristic wavelength adjacent to 1070 cm−1 represented C–O stretching vibration. These findings indicate some differences between the chemical structures of extracts before and after MAATPE and suggest that the main mechanism behind the extraction characteristic of the MAATPE process is hydrogen bonding.
SEM analysis
SEM was employed to analyze the impacts of various methods of extraction on the surface structure of mulberry. includes the control group, MAE group, ATPE group, and MAATPE group. From the SEM images of , it can be found that compared with MAE and ATPE, the surface of the sample after MAATPE extraction showed a more porous and looser microstructure, which is attributed to the fact that in a specific microwave field, the strength of the connection between molecules can be considerably improved, which causes cell bursting and solute release.[Citation20] MAATPE incorporates the positive aspects of MAE and ATPE, not only improves the extraction rate through the microwave enhancement effect but also separates anthocyanins from mulberry powder through biphasic extraction performance. Therefore, MAATPE is an effective alternative method for extracting anthocyanins from mulberry.
Antioxidant activity of mulberry extract
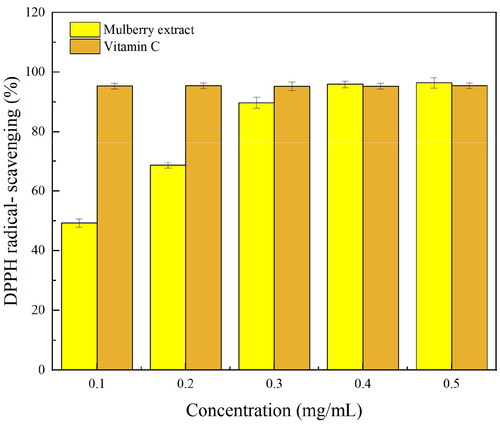
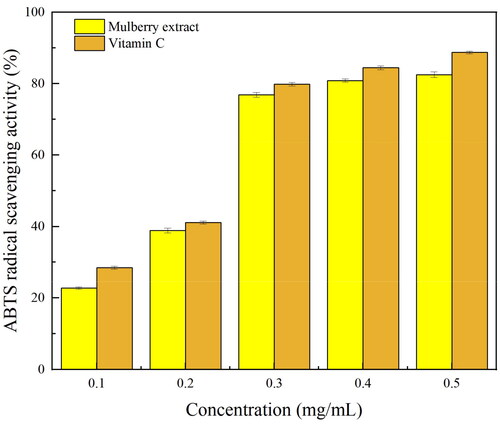
The mulberry extract obtained through the MAATPE method was assessed for its scavenging activity on DPPH and ABTS free radicals. By transferring a hydrogen atom or an electron to a sample, the ABTS and DPPH experiments are utilized frequently to measure the antioxidant capacity of water-soluble or lipid-soluble compounds.[Citation38] The absorption of hydrogen by DPPH free radicals, which results in the transformation of DPPH into a nonradical state, is the process by which DPPH free radicals are scavenged. indicated that a dose range of 0.10–0.50 mg/mL considerably increased the ability of anthocyanin to scavenge DPPH free radicals, reaching 96.4 ± 0.76% at 0.50 mg/mL anthocyanin concentration. This was higher than the antioxidant capability of vitamin C at 0.40 mg/mL. ABTS radicals react with antioxidants possessing the hydrogen-donating ability to generate colorless ABTS. The sample’s antioxidant capability was assessed by maintaining track of the change in absorption values. The scavenging effect of anthocyanin on ABTS free radicals enhanced with its increasing concentration (), achieving activity of up to 82.52 ± 2.13% at 0.50 mg/mL concentration. The results demonstrated the anthocyanin obtained from the MAATPE's high antioxidant activity, and mulberry anthocyanins are consequently a viable source of pigments and natural antioxidants for future natural antioxidant research and development.
Conclusion
In this study, the MAATPE mulberry anthocyanin processing was optimized with the RSM model. The structure of anthocyanins obtained by MAATPE had no obvious change significantly before and after extraction and enriched the cyanidin-3-O-glucoside. The mechanism study shows that the hydrogen bond effect promotes the extraction behavior of MAATPE. Compared with MAE and ATPE extraction, MAATPE combines the advantages of microwave extraction and ATPE, not only improving the extraction rate through the microwave enhancement effect but also separating anthocyanins from mulberry powder through biphasic extraction performance. In addition, the mulberry anthocyanins obtained by MAATPE have high antioxidant activity. MAATPE consequently provides a green, rapid, and efficient mulberry anthocyanin extraction method.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Hao, J. Y.; Gao, Y. F.; Xue, J. B.; Yang, Y. N.; Yin, J. N.; Wu, T.; Zhang, M. Phytochemicals, Pharmacological Effects and Molecular Mechanisms of Mulberry. Foods, 2022, 11, 1170. DOI: 10.3390/foods11081170.
- Sireesha, G.; Sri, N. K. V. Nutrient and Qualitative Phytochemical Analysis-Evaluation of Antimicrobial Activity and Development of Products with Mulberry Leaves (Mulberry indica L.). CNF, 2021, 17, 708–715. DOI: 10.2174/1573401316999201112093131.
- Li, D.; Ma, B.; Xu, X. F.; Chen, G.; Li, T.; He, N. J. MMHub, a Database for the Mulberry Metabolome. Database, 2020, 2020, baaa011. DOI: 10.1093/database/baaa011.
- Li, Z. G.; Jiang, B.; Zhang, D. J.; Xiu, Z. L. Aqueous Two-Phase Extraction of 1,3-Propanediol from Glycerol-Based Fermentation Broths. Sep. Purif. Technol., 2009, 66, 472–478. DOI: 10.1016/j.seppur.2009.02.009.
- Panyatip, P.; Padumanonda, T.; Yongram, C.; Kasikorn, T.; Sungthong, B.; Puthongking, P. Impact of Tea Processing on Tryptophan, Melatonin, Phenolic and Flavonoid Contents in Mulberry (Morus alba L.) Leaves: Quantitative Analysis by LC-MS/MS. Molecules, 2022, 27, 4979. DOI: 10.3390/molecules27154979.
- Yu, Y. F.; Chen, Y. H.; Shi, X. P.; Ye, C.; Wang, J. W.; Huang, J. Z.; Zhang, B.; Deng, Z. Y. Hepatoprotective Effects of Different Mulberry Leaf Extracts against Acute Liver Injury in Rats by Alleviating Oxidative Stress and Inflammatory Response. Food Funct., 2022, 13, 8593–8604. DOI: 10.1039/d2fo00282e.
- Ma, Z. L.; Liu, Y.; Feng, X.; Ibrahim, S. A.; Huang, W. Effects of Different Carriers on Physicochemical and Antioxidant Properties of Freeze-Dried Mulberry Powder. Food Process Preserv., 2022, 46, 16322. DOI: 10.1111/jfpp.16322.
- Khalifa, I.; Zhu, W.; Li, K. K.; Li, C. M. Polyphenols of Mulberry Fruits as Multifaceted Compounds: Compositions, Metabolism, Health Benefits, and Stability: A Structural Review. J. Funct. Foods, 2018, 40, 28–43. DOI: 10.1016/j.jff.2017.10.041.
- Gao, H. Q.; Guo, M. M.; Wang, L. Q.; Sun, C.; Huang, L. X. Identification and Antioxidant Capacity of Free and Bound Phenolics in Six Varieties of Mulberry Seeds Using UPLC-ESI-QTOF-MS/MS. Antioxidants (Basel), 2022, 11, 1764. DOI: 10.3390/antiox11091764.
- Manzoor, M. F.; Hussain, A.; Tazeddinova, D.; Abylgazinova, A.; Xu, B. Assessing the Nutritional-Value-Based Therapeutic Potentials and Non-Destructive Approaches for Mulberry Fruit Assessment: An Overview. Comput. Intell. Neurosci., 2022, 2022, 6531483–6531416. DOI: 10.1155/2022/6531483.
- Jan, B. S.; Parveen, R.; Zahiruddin, S.; Khan, M. U.; Mohapatra, S.; Ahmad, S. Nutritional Constituents of Mulberry and Their Potential Applications in Food and Pharmaceuticals: A Review. Saudi J. Biol. Sci., 2021, 28, 3909–3921. DOI: 10.1016/j.sjbs.2021.03.056.
- Zhou, X. J.; Zhu, C. T.; Zhang, L. Y.; You, S.; Wu, F. A.; Wang, J. Enrichment and Purification of Red Pigments from Defective Mulberry Fruits Using Biotransformation in a Liquid-Liquid-Solid Three-Phase System. Environ. Sci. Pollut. Res. Int., 2021, 28, 24432–24440. DOI: 10.1007/s11356-020-08731-2.
- Guo, Y. Y.; Zhang, H.; Shao, S.; Sun, S.; Yang, D. Y.; Lv, S. W. Anthocyanin: A Review of Plant Sources, Extraction, Stability, Content Determination and Modifications. Int. J. Food Sci. Tech., 2022, 57, 7573–7591. DOI: 10.1111/ijfs.16132.
- Zhang, B.; Jiang, X. Z.; Huang, G. Q.; Xin, X. D.; Attaribo, T.; Zhang, Y. Y.; Zhang, N.; Gui, Z. Z. Enhancement of Stability and Antioxidant Activity of Mulberry Anthocyanins through Succinic Acid Acylation. Food Technol. Biotechnol., 2022, 60, 321–329. DOI: 10.17113/ftb.60.03.22.7203.
- Odabaş, H. İ.; Koca, I. Simultaneous Separation and Preliminary Purification of Anthocyanins from Rosa Pimpinellifolia L. fruits by Microwave Assisted Aqueous Two-Phase Extraction. Food Bioprod. Process., 2021, 125, 170–180. DOI: 10.1016/j.fbp.2020.11.007.
- Bi, Y. H.; Chi, X. W.; Zhang, R.; Lu, Y. H.; Wang, Z. Y.; Dong, Q.; Ding, C. X.; Yang, R. L.; Jiang, L. Highly Efficient Extraction of Mulberry Anthocyanins in Deep Eutectic Solvents: Insights of Degradation Kinetics and Stability Evaluation. Innovative Food Sci. Emerg. Technol., 2020, 66, 102512. DOI: 10.1016/j.ifset.2020.102512.
- Li, Y.; Han, L.; Ma, R. J.; Xu, X. Y.; Zhao, C. P.; Wang, Z. F.; Chen, F.; Hu, X. S. Effect of Energy Density and Citric Acid Concentration on Anthocyanins Yield and Solution Temperature of Grape Peel in Microwave-Assisted Extraction Process. J. Food Eng., 2012, 109, 274–280. DOI: 10.1016/j.jfoodeng.2011.09.021.
- Zhou, S. Y.; Wu, X. H.; Huang, Y. X.; Xie, X. J.; Lin, Y. Y.; Fan, H. J.; Luo, L. C.; Zhang, W.; Tang, J. Z. Microwave-Assisted Aqueous Two-Phase Extraction of Alkaloids from Radix Sophorae Tonkinensis with an Ethanol/Na2HPO4 System: Process Optimization, Composition Identification and Quantification Analysis. Ind. Crops Prod., 2018, 122, 316–328. DOI: 10.1016/j.indcrop.2018.06.004.
- Ran, L.; Yang, C.; Xu, M. L.; Yi, Z. B.; Ren, D. B.; Yi, L. Z. Enhanced Aqueous Two-Phase Extraction of Proanthocyanidins from Grape Seeds by Using Ionic Liquids as Adjuvants. Sep. Purif. Technol., 2019, 226, 154–161. DOI: 10.1016/j.seppur.2019.05.089.
- Cheng, Z. Y.; Song, H. Y.; Cao, X. L.; Shen, Q. H.; Han, D. D.; Zhong, F. L.; Hu, H. B.; Yang, Y. J. Simultaneous Extraction and Purification of Polysaccharides from Gentiana Scabra Bunge by Microwave-Assisted Ethanol-Salt Aqueous Two-Phase System. Ind. Crops Prod., 2017, 102, 75–87. DOI: 10.1016/j.indcrop.2017.03.029.
- Xie, X. J.; Zhu, D.; Zhang, W.; Huai, W. B.; Wang, K.; Huang, X. W.; Zhou, L.; Fan, H. J. Microwave-Assisted Aqueous Two-Phase Extraction Coupled with High Performance Liquid Chromatography for Simultaneous Extraction and Determination of Four Flavonoids in Crotalaria sessiliflora L. Ind. Crops Prod., 2017, 95, 632–642. DOI: 10.1016/j.indcrop.2016.11.032.
- Mercedes, B. F. A.; Santos, G. S. J.; Nydia, C. B. O.; Isabel, S. M. D.; Jaime, L. C.; Karina, B. R. A. Validation of a Micro-Assay Based on the pH Differential Method to Quantify Total Monomeric Anthocyanins in Red Cabbage (Brassica oleracea Var. capitata f Rubra). Food Measure, 2022, 16, 3967–3976. DOI: 10.1007/s11694-022-01505-z.
- Wang, Y. S.; Song, K. Y.; Kim, Y. Effects of Thermally Treated Mulberry Leaves on the Quality, Properties, and Antioxidant Activities of Yogurt. Food Process. Preserv., 2022, 46, 16139. DOI: 10.1111/jfpp.16139.
- Paunovic, S. M.; Maskovic, P.; Milinkovic, M. Antioxidant and Biological Activities of Black Mulberry (Morus nigra L.) Fruit Depending on Altitude. Erwerbs-Obstbau, 2022, 64, 663–671. DOI: 10.1007/s10341-022-00763-x.
- Zhang, D. Y.; Zu, Y. G.; Fu, Y. J.; Wang, W.; Zhang, L.; Luo, M.; Mu, F. S.; Yao, X. H.; Duan, M. H. Aqueous Two-Phase Extraction and Enrichment of Two Main Flavonoids from Pigeon Pea Roots and the Antioxidant Activity. Sep. Purif. Technol., 2013, 102, 26–33. DOI: 10.1016/j.seppur.2012.09.019.
- Xu, Y. Y.; Qiu, Y.; Ren, H.; Ju, D. H.; Jia, H. L. Optimization of Ultrasound-Assisted Aqueous Two-Phase System Extraction of Polyphenolic Compounds from Aronia Melanocarpa Pomace by Response Surface Methodology. Prep. Biochem. Biotechnol., 2017, 47, 312–321. DOI: 10.1080/10826068.2016.1244684.
- Zhang, W.; Zhu, D.; Fan, H. J.; Liu, X. Q.; Wan, Q.; Wu, X. H.; Liu, P.; Tang, J. Z. Simultaneous Extraction and Purification of Alkaloids from Sophora Flavescens Ait. by Microwave-Assisted Aqueous Two-Phase Extraction with Ethanol/Ammonia Sulfate System. Sep. Purif. Technol., 2015, 141, 113–123. DOI: 10.1016/j.seppur.2014.11.014.
- Ma, F.-Y.; Gu, C.-B.; Li, C.-Y.; Luo, M.; Wang, W.; Zu, Y.-G.; Li, J.; Fu, Y.-J. Microwave-Assisted Aqueous Two-Phase Extraction of Isoflavonoids from Dalbergia odorifera T. Chen Leaves. Sep. Purif. Technol., 2013, 115, 136–144. DOI: 10.1016/j.seppur.2013.05.003.
- Chen, X.; Guan, Y. M.; Zeng, M. M.; Wang, Z. J.; Qin, F.; Chen, J.; He, Z. Y. Effect of Whey Protein Isolate and Phenolic Copigments in the Thermal Stability of Mulberry Anthocyanin Extract at an Acidic pH. Food Chem., 2022, 377, 132005. DOI: 10.1016/j.foodchem.2021.132005.
- Shukla, S.; Lohani, U. C.; Shahi, N. C.; Dubey, A. Extraction of Natural Pigments from Red Sorghum (Sorghum bicolor) Husk by Ultrasound and Microwave Assisted Extraction: A Comparative Study through Response Surface Analysis. J. Food Process. Eng., 2022, 45, 14130. DOI: 10.1111/jfpe.14130.
- Lin, L.; Yang, W.; Wei, X.; Wang, Y.; Zhang, L.; Zhang, Y. S.; Zhang, Z. M.; Zhao, Y.; Zhao, M. J. Enhancement of Solasodine Extracted from Fruits of Solanum nigrum L. by Microwave-Assisted Aqueous Two-Phase Extraction and Analysis by High-Performance Liquid Chromatography. Molecules, 2019, 24, 2294. DOI: 10.3390/molecules24122294.
- Yang, S.-X.; Liu, B.; Tang, M.; Yang, J.; Kuang, Y.; Zhang, M.-Z.; Zhang, C.-Y.; Wang, C.-Y.; Qin, J.-C.; Guo, L.-P.; Zhao, L.-C. Extraction of Flavonoids from Cyclocarya Paliurus (Juglandaceae) Leaves Using Ethanol/Salt Aqueous Two-Phase System Coupled with Ultrasonic. J. Food Process. Preserv., 2020, 44, e14469. DOI: 10.1111/jfpp.14469.
- Wu, Y.; Wang, Y.; Zhang, W.; Han, J.; Liu, Y.; Hu, Y.; Ni, L. Extraction and Preliminary Purification of Anthocyanins from Grape Juice in Aqueous Two-Phase System. Sep. Purif. Technol., 2014, 124, 170–178. DOI: 10.1016/j.seppur.2014.01.025.
- Zhao, Y. M.; Wang, J.; Wu, Z. G.; Yang, J. M.; Li, W.; Shen, L. X. Extraction, Purification and anti-Proliferative Activities of Polysaccharides from Lentinus Edodes. Int. J. Biol. Macromol., 2016, 93, 136–144. DOI: 10.1016/j.ijbiomac.2016.05.100.
- Dil, E. A.; Asfaram, A.; Sadeghfar, F. Magnetic Dispersive Micro-Solid Phase Extraction with the CuO/ZnO@Fe3O4-CNTs Nanocomposite Sorbent for the Rapid Pre-Concentration of Chlorogenic Acid in the Medical Extract of Plants, Food, and Water Samples. Analyst, 2019, 144, 2684–2695. DOI: 10.1039/c8an02484g.
- Dankar, I.; Haddarah, A.; Omar, F. E. L.; Pujola, M.; Sepulcre, F. Characterization of Food Additive-Potato Starch Complexes by FTIR and X-Ray Diffraction. Food Chem., 2018, 260, 7–12. DOI: 10.1016/j.foodchem.2018.03.138.
- Cai, L. L.; Zou, S. S.; Liang, D. P.; Luan, L. B. Structural Characterization, Antioxidant and Hepatoprotective Activities of Polysaccharides from Sophorae Tonkinensis Radix. Carbohydr. Polym., 2018, 184, 354–365. DOI: 10.1016/j.carbpol.2017.12.083.
- Braga, M. B.; Veggi, P. C.; Codolo, M. C.; Giaconia, M. A.; Rodrigues, C. L.; Braga, A. R. C. Evaluation of Freeze-Dried Milk-Blackberry Pulp Mixture: Influence of Adjuvants over the Physical Properties of the Powder, Anthocyanin Content and Antioxidant Activity. Food Res. Int., 2019, 125, 108557. DOI: 10.1016/j.foodres.2019.108557.