Abstract
Alismatis Rhizoma is a perennial herb originating from the rhizomes of Alisma orientalis (Sam) Juzep and the same species which have been used to treat seborrheic dermatitis, eczema, polydipsia, and pedal edema. We aimed to determine the concentrations of the compounds alisol B and alisol B acetate present in a sample of the herb using high-performance liquid chromatography coupled with a photodiode array detector. We selected methanol as the optimal solvent considering the structures of alisol B and alisol B acetate. We estimated the proportion of alisol B and alisol B acetate in a standard extract to be 0.0434% and 0.2365% in methanol, respectively. To optimize extraction, we employed response surface methodology to determine the yields of alisol B and alisol B acetate, which mapped out a central composite design consisting of 15 experimental points. The extraction parameters were time, concentration, and sample weight. The predicted concentration of alisol B derivatives was estimated to be 0.2388% under the following conditions: 81 min of extraction time, 76% of methanol concentration, and 1.52 g of sample weight.
INTRODUCTION
Traditional herbal medicines are among the most comprehensive, well-documented folk medicines in human history. Since ancient times, many Oriental countries have used traditional herbal medicine to treat a vest number of diseases, as well as to maintain health.[ Citation1-3 ] Although many traditional herbal medicines have shown efficacy in clinical trials, they all contain a complex array of compounds that are rarely standardized. Liquid chromatography is a powerful analytical tool for complex mixtures of compounds such as those found in traditional herbal medicines.[ Citation 4 ] In recent years, traditional herbal medicines have been standardized using many innovative techniques based on liquid chromatography including capillary electrophoresis, LC-MS/MS, micellar electrokinetic capillary chromatography, high-speed counter-current chromatography, and so on.[ Citation 5 , Citation 6 ]
Alismatis Rhizoma is a perennial herb that originates from the rhizomes of Alisma orientale Juzepczuk (Alismataceae) and the same species.[ Citation 7 , Citation 8 ] This herb is included in several Oriental preparations, such as Taeg-Sa-San and O-Ryeing-San, that have been used to treat condition such as seborrheic dermatitis, eczema, polydipsia, pedal edema, and so on.[ Citation9-11 ] In addition, the dried rhizome of this plant is a well-known East-Asian medicine that has long been used for infections, inflammation, allergies, nephrolithiasis, diuresis, atherosclerosis, hyperlipidemia, and diabetes in China, Japan, Korea, and North America.[ Citation 9 , Citation12-14 ]
Alismatis Rhizoma contains triterpenoids including alisol A, B, C, alisol A 24-acetate, alisol B 23-acetate, alismol, alismoxide, epigalisol A, and the saccharides such as D-glucose, D-fructose, sucrose, and so on.[ Citation 8 , Citation 9 , Citation 12 , Citation14-16 ] In particular, alisol B acetate is an important compound for which several biological activities have been identified, including inhibition of lipopolysaccharide-induced mRNA expression of inducible nitric oxide synthase and nitric oxide production. It has also been found to inhibit complement fixation and antibody-mediated allergic reaction.[ Citation 3 ]
Several earlier reports have defined the respective contents of alisol A 24-acetate, alisol B 23-acetate, alisol C 23-acetate, and alismol in Alismatis Rhizoma using the HPLC-UV and HPLC-ESI-MS technique.[ Citation 9 , Citation 13 , Citation 14 , Citation 17 , Citation 18 ] However, there have been no previous studies on the optimal conditions of alisol derivatives. In this study, we investigated the optimal extraction conditions of Alismatis Rhizoma using response surface modeling based on the concentrations of alisol B and alisol B acetate.
EXPERIMENTAL
Plant Material
Specimens of Alismatis Rhizoma collected in Suncheon (Jeonman, Korea) were identified and dried by professor Young Bae Seo of Daejeon University. A voucher specimen of this material (ALR 09) was deposited at the herbarium of the Herbal Resources Research Center at the Korea Institute of Oriental Medicine (KIOM), Korea.
Chemicals
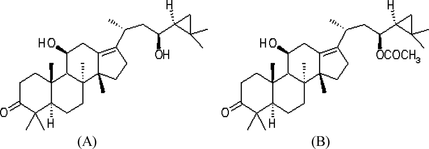
The standard compounds alisol B and alisol B acetate (Figure ) were purchased from Wako Pure Chemical Industries (Osaka, Japan). HPLC grade methanol, ethanol, 2-propanol, n-hexane, dichloromethane, acetonitrile, and distilled water were obtained from J. T. Baker Inc. (Phillipsburg, NJ, USA).
Sample Preparation for Optimal Solvent
Alismatis Rhizoma was blended using a blender (Shinil Industrial Co., Ltd., Gyeonggi-do, Korea) and ground into fine powder through the sieve. To determine the optimum solvent, aliquot of 1.0 g powdered sample was extracted in two one-hour ultrasonic waves in 25 mL of solvent including methanol, ethanol, 2-propanol, n-hexane, and dichloromethane and filled precisely in a 50 mL volumetric flask. Prior to injection, 2.0 mL were filtered through a 0.2-µm membrane filter (PALL Corporation, Ann Arbor, MI, USA). Extraction samples were analyzed in three independent variables. After selecting the optimal solvent, samples were experimented by response surface methodology.
Experimental Design and Response Surface Methodology
The three measured variables in this experiment were extraction time (X1), methanol concentration (X2), and sample weight (X3). We assigned five levels (−2, −1, 0, 1, 2) to both dependent variables: the concentrations of alisol B and alisol B acetate (Table ). The experimental 15 factors were planed central composite design (CCD) factors, in accordance with n = 2k + 2k + n0 where k is the number of factors.[ Citation19-23 ] Next, regression coefficients for intercept, linear, quadratic, and interaction terms where β0, βi (i = 1, 2, 3), and βij (i = 1, 2, 3; j = 1, 2, 3, i ≤ j) were performed following this function (1):
TABLE 1 Experimental Range and Value of Independent Variable in the Central Composite Design for Extraction Conditions
HPLC-PAD Analysis
The HPLC system consisted of a Waters Alliance 2695 system coupled with a 2996 photodiode array detector. Data processing was carried out with Empower software (Waters Co., Milford, MA, USA). Separation was performed with Atlantis dC18 (4.6 × 250 mm, 5 µm particle size, no. 186001346, Waters Co., Milford, MA, USA), XTerra RP18 (4.6 × 250 mm, 5 µm particle size, no. 186000498, Waters Co., Milford, MA, USA), and Luna 5 µ C18(2) 100A (4.6 × 250 mm, 5 µm particle size, no. 00G-4252-E0, Phenomenex Co., Torrance, CA, USA) columns. The solvents ratio for the 40 min mobile phase was A:B:C = 61:7:32 (A = acetonitrile, B = methanol, C˭H2O). The UV wavelength was scanned over the range of 190–400 nm and recorded at 203 nm. The flow rate was 0.6 mL/min, and the injection volume was 20 µL. Sample peaks were assigned according to retention time and the UV spectra of the two standard compounds in the chromatogram.
Method Validation
Two stock solutions, 1.2 mg of alisol B and 1.0 mg of alisol B acetate, were dissolved in 10 mL methanol, respectively. We carried out serial dilutions in construct standard calibration curves in which concentrations of standard solution alisol B and alisol B acetate were 120, 60, 12, 1.2, and 0.4 µg/mL and 100, 50, 10, 1.0, and 0.5 µg/mL, respectively. Five different concentrations of the two standard compounds were injected in triplicate. The precisions of the standard compounds were determined for intra-day and inter-day variability in triplicate. Accuracy was calculated using observed concentrations and nominal concentrations. The limited of detection (LOD) and limited of quantitation (LOQ) were calculated using 3.3 * σ/s and 10 * σ/s, where σ is the standard deviation and s is slope of regression equation.[ Citation 24 ]
RESULTS AND DISCUSSION
Method Validation
Three differential columns were used to test the samples: XTerra RP18 (4.6 × 250 mm, 5 µm), Luna 5 µ C18(2) 100A (4.6 × 250 mm, 5 µm), and Atlantis dC18 (4.6 × 250 mm, 5 µm). The optimal column was selected based on a comparative chromatogram of the retention times, separations, resolutions, and shapes of the alisol B and alisol B acetate peaks (Figure ). On the XTerra RP18, Luna 5 µ C18(2) 100A, and Atlantis dC18 columns, alisol B was detected at approximately 15, 27, and 25 min, respectively. Under the same column conditions, alisol B acetate was showed at about 21, 35, and 32 min, respectively. The two standard compounds showed tailing peaks in the Atlantis dC18 column condition. The peak shapes were good in the other two columns, however we selected the XTerra RP18 column because of its reduced analytical time.[ Citation 25 , Citation 26 ] Our sample of Alismatis Rhizoma contained alisol B and alisol B acetate at approximately 15 and 21 min in the XTerra RP18 column (Figure ).
FIGURE 2 Chromatogram of the suitable analytical column for alisol A (the front peak) and alisol B acetate (the back peak): XTerra RP 18 (A), Atlantis dC18 (C), and Luna 5 µ C18(2) 100A. (Color figure available online.)
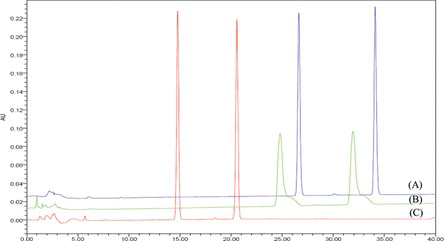
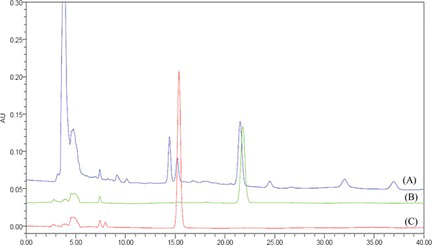
FIGURE 3 Chromatogram of sample for Alismatis Rhizoma (A), alisol B acetate (B), and alsiol B (C) in XTerra RP18 column condition. (Color figure available online.)
The concentrations of alisol B and alisol B acetate ranged from 0.4–120 µg/mL and 0.5–100 µg/mL, and the regressive equations (coefficient of correlation) were calculated at y = 76030.232x + 16057.012 (R2 = 1.000) and y = 59806.416x + 9131.577 (R2 = 1.000), respectively (Table ).
TABLE 2 Linearity and Precision of Alisol B and Alisol B Acetate
Limits of detection (LOD) for alisol B and alisol B acetate were 0.082 µg/mL and 0.114 µg/mL, respectively. Limits of quantitation (LOQ) for alisol B and alisol B acetate were 0.249 µg/mL and 0.344 µg/mL, respectively. The RSDs of the intra-day and inter-day variabilities for two standard compounds were less than 1.0% and 5.0%, respectively (Table ). Accuracy was determined using the spike test, in which we added 12 µg/mL of standard alisol B and 50 µg/mL of standard alisol B acetate. The means of recovery of alisol B and alisol B acetate were measured as 103.6% and 106.4%, respectively. This method demonstrated good linearity, precision, and accuracy.
TABLE 3 Intra-Day and Inter-Day Variability for Concentrations of Alisol B and Alisol B Acetate
Optimization of Extraction Solvent
We determined the contents of alisol B and alisol B acetate using the following six solvents: methanol, ethanol, 2-propanol, n-hexane, dichloromethane, and acetonitrile. To select the optimal extraction solvent, we calculated the total extracted concentrations of the two compounds. In dichloromethane, alisol B was not detected, and only a trace of alisol B acetate was detected. In the alcohol solvents and acetonitrile, the compounds were both present in high concentrations. Methanol yielded the highest concentration of alisol B derivatives, approximately 0.2799% (Table ). The extraction temperature, time, solvent concentration, and sample weight have an effect on the yield of a natural product. In particular, mixed solvents of a lower alcohol and water have been generally used for the extraction of natural products.[ Citation 23 ] Among the various alcohols, methanol was selected by preliminary test. A methanol extraction was detected to the highest contents of alisol B and alisol B acetate.
TABLE 4 Contents of Alisol A and Alisol B Acetate for Alismatis Rhizoma in Various Solvents
Fitting the Models
The quantities of alisol B and alisol B acetate in Alismatis Rhizoma obtained from all of the experiments are listed in Table . These experimental data were used to calculate the coefficient of the second-order polynomial equation as shown in Table . In this model, the significances of the effect on the respective response variables increased with a greater regression coefficient and a lower p-value.[ Citation 27 ] The R2 value, which is always between 0 and 1, suggests a stronger and more predictive model the closer is the value to 1.00.[ Citation 28 ] The regression coefficient, R2 was calculated to be 0.9922 using the sum of the two components in our experimental model. The F-test calculated a high F-value (F = 56.40) and a low p-value (p < 0.001) for our model, indicating a significant effect.[ Citation 29 ] These values indicate that the second-order polynomial model adequately represented the experimental data.
TABLE 5 Central Composite Design Matrix of Three Test Variables and Experimental Results from the Response Variables
TABLE 6 Analysis of Variables for Regression Model of Dependent Y Variable in Extraction Conditions
Response Surface Methodology
Equation (Equation2) shows the relationship between the contents of the two compounds and their extraction factors.
The significance of each coefficient was determined using Student's t-test and the P-value. Significance of the corresponding coefficient increased with a greater t-test value and a lower p-value.[ Citation 23 ] With regard to extraction concentration, both the linear and quadratic terms were significant. Methanol concentration had a significant negative quadratic effect on the yields of alisol B and alisol B acetate, although this effect did not achieve significance. Extraction time showed no significant effects (Table ).
TABLE 7 Regression Result from the Data of CCD Experiments
Figure shows the response surface plot of the yields of alisol B and alisol B acetate according to extraction time (X1), extraction concentration (X2), and sample weight (X3). Figure , B shows that the content of alisol B derivatives tends to increase at higher concentrations of methanol. Figure shows that content did not strongly correlate with extraction time or sample weight.
FIGURE 4 Response surface plots of the yields of alisol B and alisol B acetate (Y) as a parameters of X1 (extraction time), X2 (extraction concentration, and X3 (sample weight). (Color figure available online)
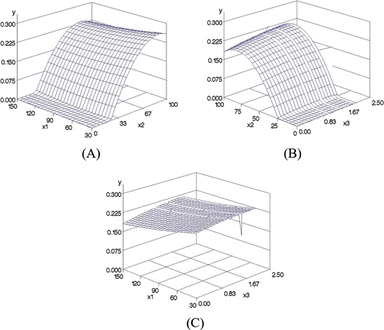
The maximum content of alisol B derivatives predicted using response surface methodology was 0.2388% with 81 min of extraction time in 76% methanol for 1.52 g of sample weight. The actual maximum content was measured to be 0.2405% with 90 min of extraction time in 100% of methanol for 1.5 g of sample weight. Thus, while the predicted extraction concentration differed slightly from the experimental value, the predicted extraction time and sample weigh were similar to the experimental values.
CONCLUSION
We carried out response surface methodology analysis to optimize the extraction conditions for alisol B derivatives. An experimental model yielded a regression coefficient of 0.9922 and a significance level less than 0.05%. The extraction concentration was significantly correlated to the linear and quadratic terms, but no other parameters were significant. The maximum content of alisol B derivatives predicted by response surface methodology was 0.2388% with 81 min of extraction time in 76% methanol using 1.52 g of sample weight. Thus, while the predicted methanol concentration differed slightly from the experimental values, the predicted extraction time and sample weights were similar to the experimental values. It is clear from our study that methanol concentration directly affects the amounts of alisol B and alisol B acetate extracted from a sample of Alismatis Rhizoma.
ACKNOWLEDGMENT
This study was supported by project, “Construction of the Basis for Practical Application of Herbal Resources (K10020)” as well as the project “A study for selection and quality control of premium herbal medicines” at the Korea Institute of Oriental Medicine, funded by the Ministry of Education, Science and Technology and by the Ministry of Health & Welfare of Korea.
Notes
a RSD (%) = 100 × SD/mean.
b Accuracy (%) = {1-(nominal – mean of measured concentration)/nominal concentration}×100.
c AB = abbreviation of alisol B.
d ABA = abbreviation of alisol B acetate.
a N.D. = abbreviation of not detected.
a N.D. = abbreviation of not detected.
**P-value < 0.01. ***P-value < 0.001.
**P-value <0.01.
REFERENCES
- Hu , Y. M. ; Wu , G. H. ; Sze , S. C.-W. ; Ye , W. ; Tong , Y. Quality Assessment of Cortex Phellodendri by high-Performance Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry . Biomed. Chromatogr. 2010 , 24 , 438 – 453 .
- Monden , T. ; Hosoya , T. ; Nakajima , Y. ; Kishi , M. ; Satoh , T. ; Hashimoto , K. ; Kasai , K. ; Yamada , M. ; Mori , M. Herbal Medicine, Hachimi-jio-gan and Its Component Cinnamomi Cortex Activate the Peroxisome Proliferator-Activated Receptor Alpha in Renal Cells . Endocr. J. 2008 , 55 ( 3 ), 529 – 533 .
- Huang , Y.-T. ; Huang , D.-M. ; Chueh , S.-C. ; Teng , C.-M. ; Cuh , J.-H. Alisol B Acetate, a Triterpene from Alismatis rhizome, Induces Bax Nuclear Translocation and Apoptosis in Human Hormone-Resistant Prostate Cancer PC-3 Cells . Cancer Lett. 2006 , 231 , 270 – 278 .
- Chen , L. ; Qi , J. ; Chang , Y.-X. ; Zhu , D. ; Yu , B. Identification and Determination of the Major Constituents in Traditional Chinese Medicinal Formula Danggui-Shaoyao-San by HPLC-DAD-ESI-MS/MS . J. Pharm. Biomed. Anal. 2009 , 50 , 127 – 137 .
- Yu , K. ; Gong , Y. ; Lin , Z. ; Cheng , Y. Quantitative Analysis and Chromatographic Fingerprinting for the Quality Evaluation of Scutellaria baicalenesis Georgi Using Capillary Electrophoresis . J. Pharm. Biomed. Anal. 2007 , 43 , 540 – 548 .
- Zgórka , G. Retention Behavior of Silica-Bonded and Novel Polymeric Reversed-Phase Sorbents in Studies on Flavones as Chemotaxonomic Marker of Scutellaria L. Genus . J. Chromatogr. A. 2006 , 1120 , 230 – 236 .
- Lee , J. C. ; Lee , E. ; Lee , Y.-C. Effects of Rhizoma Alismatis on Lipid Composition and TBARS Concentration in Rat Fed High Fat Diet . Kor. J. Herbology 2008 , 23 ( 3 ), 113 – 117 .
- Kim , S. E. ; Rhyu , D. Y. ; Yokozawa , T. ; Park , J. C. Antioxidant Effect of Alisma plantago-aquatica var . orientale and Its Main Component. Kor. J. Pharmacogn. 2007 , 38 ( 4 ), 372 – 375 .
- Ahn , M.-J. ; Lee , C. H. ; Shin , Y.-W. ; Chun , M.-S. ; Kim , C. Y. ; Kim , J.. Determination of Alisol B 23-Acetate and Alisol C 23-Acetate in Alismatis Rhizoma by HPLC-ESI-MS . Nat. Prod. Sci. 2008 , 14 ( 3 ), 152 – 155 .
- Lee , S.-I. Studies on the Diuretic Action of Oryeongsan and Kami-Oryengsan . Kor. J. Pharmacog. 1981 , 12 ( 1 ), 31 – 43 .
- Jo , Y. D. Nephroprotective Effects of Taeksa-San Aqueous extracts on cisplatin-induced rat acute renal failure . Degree of Master, Deagu Haany Unversity , 2009 , pp 1 – 2 .
- Lee , J.-H. ; Lee , Y.-J. ; Kang , S.-W. ; Kim , Y. ; Shin , M. ; Hong , M. ; Seo , E.-K. ; Kim , S.-H. ; Nah . S.-Y. ; Bae , H. Effects of Protostane-Type Triterpenoids on the 5-HT3A Receptor-Mediated Ion Current in Xenopus oocytes . Brain Res. 2010 , 1331, 20–27 .
- Fong , W.-F. ; Wang , C. ; Zhu , G.-Y. ; Leung , C.-H. ; Yang , M.-S. ; Cheung , H.-Y. Reversal of Multidrug Resistance in Cancer Cells by Rhizoma Alismatis Extract . Phytomedicine 2007 , 14 , 160 – 165 .
- Kim , G. S. ; Kim , J. K. ; Seong , J. D. ; Park , C. K. ; Suh , H. S. ; Kwack , Y. H. Effects of Cultural Condition on the Yield and Contents of Effective Components in Alisma orientale (Samuels.) Juzepcz . Kor. J. Med. Crop Sci. 1996 , 4 ( 2 ), 139 – 144 .
- Choi , S.-S. The Effects of Alisma Canaliculatum Butanol Fraction with Selenium on Glycogen Level, Lipid Metabolism and Lipid Perosidation in Streptozotocin-Induced Diabetic Rats . Kor. J. Nutr. 2004 , 37 ( 1 ), 15 – 22 .
- Toh , C.-A. Pharmacognostical Studies on Alisma Plants . Kor. J. Pharmacogn. 1995 , 26 ( 4 ), 411 – 418 .
- Makabel , B. ; Zhao , Y. ; Wang , B. ; Bai , Y. ; Zhang , Q. ; Wu , L. ; Lv , Y. Stability and Structure Studies on Alisol A 24-Acetate . Chem. Pharm. Bull. 2008 , 56 ( 1 ), 41 – 45 .
- Wang , C. ; Zhang , J.-X. ; Shen , X.-L. ; Wan , C.-K. ; Tse , A. K.-W. ; Fong , W.-F. Reversal of P-glycoprotein-Mediated Multidrug Resistance by Alisol B 23-Acetate . Biochem. Pharmacol. 2004 , 68 , 843 – 855 .
- Kim , K.-L. ; Jeong , J.-S. Sensory Characteristics of Doenjang Supplemented with Sage Powder as Assessed by Response Surface Methodology . Kor. J. Food Preserv. 2010 , 17 ( 20 ), 243 – 249 .
- Shin , H.-H. ; Kang , M.-J. ; Cho , H.-Y. ; Kim , B.-C. ; Cho , E.-K. Optimization of Extraction Conditions for Houttuynia cordata Thunb and Saururus chinensis Bail Mixture by Response Surface Methodology. Food Eng. Prog. 2008, 12 (4), 247–255.
- Park , J.-W. ; Kim , H.-S. ; Park , I.-B. ; Shin , G.-W. ; Lee , Y.-J. ; Jo , Y.-C. Optimization of Ethanol Extraction Conditions from Glasswort (Salicirnia herbacea) Using Response Surface Methodology . Kor. J. Food Preserv. 2009 , 16 ( 3 ), 376 – 384 .
- Park , K.-J. ; Lim , J.-H. ; Kim , B.-K. ; Jeong , J.-W. ; Kim , J.-C. ; Lee , M.-H. ; Cho , Y.-S. ; Jung , H. Optimization of Extraction Conditions to Obtain Functional Components from Buckwheat (Fagopyrum esculentum M.) Sprouts, Using Response Surface Methodology . Kor. J. Food Preserv. 2009 , 16 ( 5 ), 734 – 741 .
- Kim , S.-H. ; Kim , H.-K. ; Yang , E. S. ; Lee , K. Y. ; Kim , S. D. ; Kim , Y. C. ; Sung , S. H. Optimization of Pressurized Liquid Extraction for spicatoside A in Liriope platyphylla . Sep. Purif. Technol. 2010 , 71 , 168 – 172 .
- Lee , S. M. ; Lee , H. B. ; Lee , C. G. A Convenience UPLC/PDA Method for the Quantitative Analysis of Panaxfuraynes A and B from Panax ginseng . Food Chem. 2010 , 123 , 955 – 958 .
- Yun , H.-J. ; Yun , S.-M. ; Lee , M.-H. ; Son , S.-W. Determination of Eugenol in Eugenia caryophyllata by High-Performance Liquid Chromatography with Photodiode Array Detection and Method Validation . Kor. J. Vet. Res. 2008 , 48 ( 1 ), 9 – 15 .
- Yun , H.-J. ; Yun , S.-M. ; Lee , M.-H. ; Son , S.-W. Determination of Amitraz by High-Performance Liquid Chromatography with Photodiode Array Detection and Method Validation . Kor. J. Vet. Res. 2008 , 48 ( 1 ), 33 – 38 .
- Yang , B. ; Liu , X. ; Gao , Y. Extraction Optimization of Bioactive Compounds (Crocin, Geniposide and Total Phenolic Compounds) from Gardenia (Gardenia jasminoides Ellis) Fruits with Response Surface Methodology . Innov. Food Sci. Emerg. Technol. 2009 , 10 , 610 – 615 .
- Vohra , A. ; Satyanarayana , T. Statistical Optimization of the Medium Components by Response Surface Methodology to Enhance Phytase Production by Pichia anomala . Proc. Biochem. 2002 , 37 , 999 – 1004 .
- Zhong , K. ; Wang , Q. Optimization of Ultrasonic Extraction of Polysaccharides from Dried Longan Pulp Using Response Surface Methodology . Carbohyd. Polym. 2010 , 80 , 19 – 25 .