ABSTRACT
Establish a quantitative analysis of multi-components by the single marker (QAMS) method for quality evaluation and validate its feasibilities by the simultaneous quantitative assay of four main components in Linderae Reflexae Radix. Four main components of pinostrobin, pinosylvin, pinocembrin, and 3,5-dihydroxy-2-(1-p-mentheneyl)-trans-stilbene were selected as analytes to evaluate the quality by RP-HPLC coupled with a UV-detector. The method was evaluated by a comparison of the quantitative results between the external standard method and QAMS with a different HPLC system. The results showed that no significant differences were found in the quantitative results of the four contents of Linderae Reflexae Radix determined by the external standard method and QAMS (RSD <3%). The contents of four analytes (pinosylvin, pinocembrin, pinostrobin, and Reflexanbene I) in Linderae Reflexae Radix were determined by the single marker of pinosylvin. This fingerprint was the spectra determined by Shimadzu LC-20AT and Waters e2695 HPLC that were equipped with three different columns.
GRAPHICAL ABSTRACT
Introduction
Chinese Herbal Medicine (CHM) contains multi-components, and the quality of CHM is highly related to its major active constituents; thus, in most cases, a quantitative analysis of these components is necessary. In fact, the short supply of reference substance and the high cost make the multiple detection unfeasible. So, it is urgently needed to develop a simple, economic way to control the multi-components by one marker. Wang proposed a new quality evaluation method for CHM, using one chemical reference substance to calculate multi-components simultaneously; the method was called quantitative analysis of multi-components with single marker (QAMS),[Citation1] and it needed only one reference, which was both economical and available. Commonly, QAMS was proceeded by high-performance liquid chromatography coupled with a UV-detector (HPLC-UV), which was the most frequently used method in the quality control of CHM.[Citation2,Citation3] The quantity assessment of Panax Ginseng and Panax notoginseng by ginsenosides (ginsenoside Rgl, Re, Rf, Rhl, Rbl, Rc, Rb2, Rb3, Rd) was performed successfully by this method.[Citation4] At the same time, four flavones in Radix Scutellariae were also simultaneously determined by QAMS.[Citation5] In Chinese pharmacopoeia (2015 edition), the content of tanshinone IIA and cryptotanshinone was determined with tanshinone I by this method in the crude drugs of Salvia miltiorrhizae radix et rhizome.[Citation6]
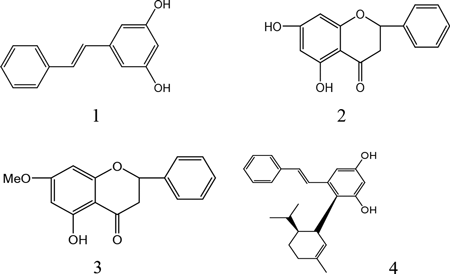
Linderae Reflexae Radix originates from the root of Lindera reflexa Hemsl; it is a newly discovered herbal drug, and there has been no record of its use in classical literature. Now, it is listed in the Dictionary of Chinese Medicine and is used for the treatment of gastritis and peptic ulcer.[Citation7] Linderae Reflexae Radix is the main material of Chinese patent medicine called “Weitongning Tablets,” which is the pill that is used for treating peptic ulcer and has been used in clinical practice for a few years. The quality of the drug was controlled by determining the content of pinostrobin, pinosylvin, or pinocembrin.[Citation8,Citation9] Recently, one new component was isolated from this drug by our lab; the new component was named as 3,5-dihydroxy-2-(1-p-mentheneyl)-trans-stilbene,[Citation10] and it has a high content in this crude drug. The four purified components found it difficult to get the standard reference from the market, so it is necessary to set up a simple and effective method to control the quality of multiple components by one marker.
In this article, a quantitative method for simultaneously determining the four analytes in Linderae Reflexae Radix was developed by the QAMS method. Pinostrobin was chosen as the internal standard; the relative correction factors (RCF) of the other three analytes were determined, and then, their content was calculated according to RCF. On the other hand, to validate the accuracy of the QAMS method, the contents of pinostrobin were authentically determined by the external standard method to compare the results determined by QAMS.
Experimental
Reagents and chemicals
The Linderae Reflexae Radix was collected from Xinxian, Henan province, in August 2010; the roots of Lindera reflexa Hemsl were taken and chopped into pieces. All the samples were identified by professor Chen, and the voucher specimens were deposited at Pharmacy College of Henan University of Traditional Chinese Medicine.
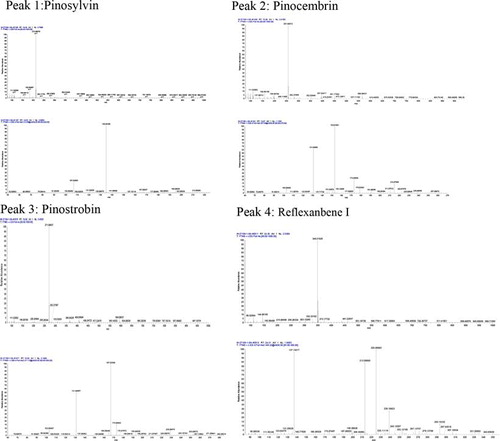
Standard references of pinostrobin, pinosylvin, pinocembrin, and 3,5-dihydroxy-2-(1-p-mentheneyl)-trans-stilbene (names as Reflexanbene I) were isolated from Linderae Reflexae Radix in our lab. All were identified by 1H-NMR, 13C-NMR, HSQC, and HMBC, and their purity was more than 98%[Citation10]; the chemical structure of the four analytes is shown in . The mass spectrum of each peak fraction from the sample was identified by LC–ESI–MSn experiments that were performed on a Thermo Fisher LTQ-Orbitrap XL Hybrid mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) that was equipped with an electrospray ionization (ESI) source connected to the UHPLC instrument. Methanol was HPLC grade (TEDIA, USA), and others reagents were analytical grade (Shield, Tianjin). Deionized water was purified with a Milli-Q Academic ultra-pure water system (Billerica, MA, USA).
Apparatus
A Shimadzu LC-20AT HPLC system is equipped with an SPD-20A UV-detector and a CBM-102 workstation. A Waters e2695 HPLC system consists of a DAD detector, a quota pump, and the workstation was supplied by the manufacturer.
Relative correction factors calculation
Within the linear range, the concentration of one component is in direct proportion to the response of the detector; the equation is W = fA (W is concentration, A is the peak area). In case of the quality assessment of multi-components of TCM, the characteristics component that has an economical reference standard is used as the internal standard; then, the RCF of test components to the characteristic component is established; finally, the contents of test components is calculated by their RCF. The equation is as follows:
From the earlier formula
Standard solution preparation
The stock standards of pinostrobin, pinosylvin, pinocembrin, and Reflexanbene I were accurately weighed, then dissolved in methanol, and mixed; the standard solution contained 0.502, 0.418, 0.103, and 0.505 mg/mL, respectively.
Sample solution preparation
Approximately 3.0 g of sample powders of Linderae Reflexae Radix were accurately weighed and extracted with 12 times the amount of 70% ethanol; ultrasonic extraction was conducted for 1 h; the extraction was repeated thrice; and the combined filtrate was evaporated to dryness. The accurately weighed 0.100 g was extracted, dissolved in methanol, filtered, and diluted to 50 mL with methanol. The solution was strained through a filter with 0.45-um pores and was used as the sample solution.
HPLC condition
The Shimadzu HPLC system: column: phenomenex luna C18 (5µ, 250 × 4.60 mm), wavelength: 297 nm, oven temperature: 30°C, flow: 1.0 ml/min, injection: 10 µl; the mobile phase was performed as indicated in . The chromatograms of the standard solution and the sample solution are shown in . The mass spectrum of each peak of the sample is shown in . The ESI source parameters were set as follows: ion spray voltage, 4.5 kV; capillary temperature, 350°C; capillary voltage, 15 V; tube lens voltage, 80 V. sheath (N2); and auxiliary gases (He) flow rate, 25 and 3 arbitrary units, respectively. The Orbitrap mass analyzer was operated in the positive ion mode, with a mass range of 80–2000. Accurate mass analyses were calibrated according to the manufacturer's guidelines using a standard mixture solution of caffeine, MRFA, and Ultramark 1621.
Figure 2. Chromatograms of four mixed reference standards (a) and samples of Linderae Reflexae Radix (b) 1. Pinosylvin 2. Pinocembrin 3. Pinostrobin 4. Reflexanbene I.
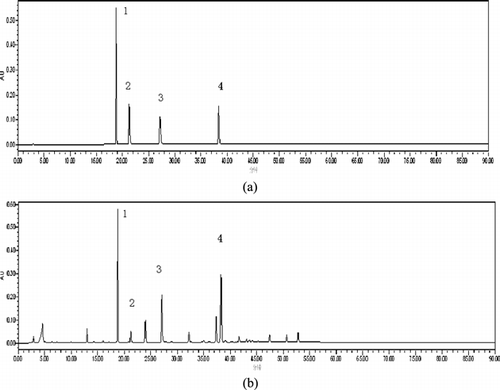
Table 1. Gradient elution of mobile phase.
Results and discussion
Calibration and validation
Calibration
The prepared standard solutions of 4, 8, 10, 15, and 20 µl were injected; the standard curves for each component were plotted by using linear regression of the peak area versus concentration. The coefficient of correlation (R2) was used to judge the linearity. The calibrations are shown in .
Table 2. Calibration data of four standards.
RCF calculation
Using pinostrobin as an internal standard to calculate the RCF of pinosylvin, pinocembrin, and Reflexanbene I to pinostrobin, the results are shown in .
Table 3. Relative correction factors.
Intro-day precision and inter-day precision
One sample solution was injected continuously for six times in one day to test the intro-day precision. The peak areas of pinostrobin, pinosylvin, pinocembrin, and Reflexanbene I were calculated; the RSDs were 1.49, 1.74, 0.97, and 1.04%, respectively. In addition, one sample solution was injected three times for five consecutive days to test the inter-day precision; the peak areas of pinostrobin, pinosylvin, pinocembrin, and Reflexanbene I were calculated, and their RSDs were 2.03, 3.50, 3.74, and 2.58%, respectively.
Stabiltity
One sample solution was injected into HPLC at 0, 2, 4, 8, 16, 24, and 48 h to investigate the stability of the sample. The peak areas of pinostrobin, pinosylvin, pinocembrin, and Reflexanbene I were calculated; their RSDs were 2.24, 2.57, 1.31, and 0.95%, respectively.
Repeatability
The repeatability was evaluated by performing six reduplicate experiments of the extraction method, and the six test solutions were injected into the HPLC system. The peak areas of pinostrobin, pinosylvin, pinocembrin, and Reflexanbene I were calculated; their RSDs were 2.35, 1.50, 1.34, and 2.17%, respectively.
Recovery
Six extraction samples were accurately weighed and dissolved in methanol; then, the known content of the three standard solutions was spiked into six sample solutions. The content of pinostrobin, pinosylvin, pinocembrin, and Reflexanbene I in the six test solutions was calculated; their recoveries were 99.48, 100.72, 99.51, and 99.42%, respectively, and their RSDs were1.72, 1.82, 2.10, and 1.65%, respectively.
Contrast of QAMS method and external standard method
The external standard method was also performed to compare the result with the QAMS method. The external standard method was used to verify the accuracy of the QAMS method; the results are shown in . No significant differences were found in the quantitative results of the three contents, and the RSDs were within 3%.
Table 4. Results comparison between external standard method and QAMS.
RCF stability study
RCF calculation with different column
Three columns of Phenomenex luna C18(5µ, 250 × 4.60 mm), Elite C18(5µ, 250 × 4.60 mm), and Agilent TC-C18 (5µ, 250 × 4.60 mm) were chosen to compare the differences of RCF; the standard solutions of 4, 8, 10, 15, and 20 μl were injected into the HPLC system; and the results are shown in .
Table 5. RCF calculated with different columns.
RCF calculation with different instruments
Six sample solutions were chosen to determine the content of pinostrobin, pinosylvin, and pinocembrin by the HPLC instrument of Waters e2695 and to compare it with the content determined by Shimadzu LC-20AT; meanwhile, the two instruments were equipped with three different columns, respectively. The QAMS method was used to calculate the content; the results are shown in .
Table 6. The pinostrobin content determined by different instruments (%).
Table 7. The pinosylvin content determined by different instruments (%).
Table 8. The pinocembrin content determined by different instruments (%).
Table 9. The Reflexanbene I content determined by different instruments (%).
Retention time difference of target peaks
The main problem of the QAMS method is how to accurately locate the remaining three target peaks of pinosylvin, pinocembrin, and Reflexanbene I by only one single reference substance of pinostrobin. To solve this problem, the retention time difference and the retention time ratio were introduced; the two parameters could be used as the position markers of the other three target peaks. At the same time, three different columns from two different HPLC instruments were investigated to verify the two parameters; the result is shown in and , which indicate that the retention time difference is more accurate. Thus, the retention time difference was chosen to be another position marker of the target peaks when the reference was unavailable.
Table 10. Retention time difference of the three components.
Table 11. Retention time ratio of the three components.
Discussion
Pinostrobin was chosen as the internal standard, because it was completely separated from pinosylvin and pinocembrin, and its resolution was more than 1.5. At the same time, the content of pinostrobin was more than 4%; a large amount of high-purity pinostrobin was isolated from Linderae Reflexae Radix to ensure its use for determination.
In this study, the feasibility and applicability of the QAMS method was explored; the accuracy, stability, and repeatability of this method were also verified. It is clear that the QAMS method can be a quantitative method for simultaneously determining the three analytes in Linderae Reflexae Radix for quality control; the contents of pinosylvin and pinocembrin were determined through the RCF to pinostrobin. It is feasible to control the quality of Linderae Reflexae Radix by determining the multi-components by one marker of pinostrobin when short of the reference substances of pinosylvin and pinocembrin. In addition, the QAMS method is a helpful one for improving the quality control not only for Linderae Reflexae Radix but also for other complex herbal drugs.
References
- Wang, Z. M.; Gao H. M.; Fu, X. T. Multi-components quantitation by one marker new method for quality evaluation of Chinese herbal medicine. Chin. J. Chin. Mater. Med. 2006, 31 (23), 1925–1928.
- Li, L.; Zhang, J. L.; Sheng, X. Y.; Guo, D. A.; Wang, Q.; Guo, H. Z. Simultaneous quantification of six major active saponins of Panax notoginseng by high-performance liquid chromatography-UV method. J. Pharmaceut. Biomed. 2005, 38 (1), 45–51.
- Song, X. Y., Li, Y. D., Shi, Y. P.; Jin, L.; Chen J. Quality control of Chinese traditional medicines: A review. Chin. J. Nat. Med. 2013, 11 (6), 596–607.
- Zhu, J. J.; Wang, Z. M.; Kuang, Y. H. A quantitative method using one marker for simultaneous assay of ginsenosides in Panax ginseng and P. notoginseng Acta pharm sin. Acta Pharm. Sin. 2008, 43 (12), 1211–1216.
- Zhu, J. J.; Wang, Z. M.; Zhang, Q. W. A quantitative method for simultaneous assay of four flavones with one marker in Radix Scutellariae. China Chin. J. Chin. Mater. Med. 2009, 34 (24), 3229–3234.
- The State Pharmacopoeia Commission of PR China, Pharmacopoeia of People's Republic of China, vol. I, China Medical Science Press, Peking, 2015, p. 76.
- Jiangsu New Medical College, Dictionary of Chinese Medicine. Shanghai Scientific & Technical Publishers, Shanghai, 1975, p. 204.
- Pan, Y. F.; Lu, Y. B.; Zhang, J. M. Determine the pinostrobin content in Lindera reflexa Hemsl by HPLC method. Chin. Tradit. Herb. Drug 2004, 35 (9), 1059–1060.
- Lei, J. W.; Guo, Y. L.; Bai, Y. Establish the Quantitative Model of 3,5-didroxystilbene in Lindera reflexa Hemsl by Near-infrared Spectroscopy. Chin. J. Exp. Tradit. Med. Form 2011, 17 (17), 75–77.
- Chen, S. Q.; Wang, L. L.; Zhang, W. Q.; Wei, L. Y. Secondary Metabolites from the Root of Lindera reflexa Hemsl. Fitoterapia 2015, 105 (9), 222–227.