?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A clinical trial is currently underway to examine the efficacy of using caffeine citrate to prevent intermittent hypoxemia in late preterm neonates. Determining caffeine concentration using saliva in this population would be preferable as it is less invasive than plasma sampling, but a suitable method of analysis is required. This paper presents the development and validation of a rapid, efficient and reproducible stability-indicating high-performance liquid chromatography (HPLC) method and extraction protocol for the quantification of caffeine present in saliva. The stability of extemporaneously prepared caffeine citrate solutions (at 20–25 °C) was determined, along with the stability of caffeine spiked saliva samples (at 20–25 and 2–8 °C), to ensure the suitability of the developed method in the analysis of clinical trial samples. Protein precipitation using acetonitrile ensured the complete removal of salivary proteins and resulted in extraction recovery of ≥95% for all samples. The HPLC assay following extraction was linear (R2>0.99) over the range 0.3–50 µg/mL (lower limit of quantification 0.3 µg/mL). The accuracy of the quality control samples was 94–100% and the relative standard deviation (RSD) was <7% for all samples. Caffeine-spiked saliva samples were stable for three freeze-and-thaw cycles pre-extraction and up to 72 hr post-extraction. The extraction and HPLC methods described were thus suitable for the analysis of clinical trial samples from the Latte Dosage Trial. All caffeine solutions were physically and chemically stable, with concentrations at the end of the three-month test period being within 4% of the baseline concentrations, indicating appropriateness for use as clinical trial medications.
Graphical Abstract
Introduction
Caffeine is a methylxanthine drug widely used in neonatal intensive care units for the prevention and treatment of apnea of prematurity and is one of the most cost-effective interventions in this population.[Citation1,Citation2] It has been used in neonatal units in both IV and oral formulations since the 1970s, and various commercial formulations are available in different countries. The Latte Dosage Trial, which is currently being conducted to examine the efficacy of using caffeine citrate to prevent intermittent hypoxemia in late preterm neonates, requires the use of caffeine citrate oral liquid at four different strengths to allow for trial blinding alongside a placebo.[Citation3] In New Zealand (NZ), caffeine citrate is available commercially in a single 20 mg/mL strength, so extemporaneous compounding by pharmacists at participating sites is required to obtain the appropriate strengths (5, 10, 15, and 20 mg/mL) for the trial to maintain blinding. The formulation of caffeine produced for the Latte Dosage Trial consists solely of caffeine citrate dissolved in water to avoid exposing preterm infants to unnecessary excipients. Before being used in a clinical setting, the storage stability of preservative-free caffeine solutions needs to be determined.
While plasma samples are most commonly used for the analysis of drug concentrations in pharmacokinetic studies, saliva has many advantages over plasma. Saliva is easily obtained by non-invasive techniques, increasing patient acceptability (especially where a large number of samples or children are involved) and decreasing the technical skills required for collection.[Citation4] These factors make it more suitable than plasma for use in neonatal clinical trial participants. Furthermore, the concentration in saliva reflects free or unbound drug concentrations in plasma, and as this represents the pharmacologically active drug concentration it may be more meaningful for some drugs than plasma concentrations, which often reflect both bound and unbound drugs.[Citation5] In both preterm neonates and adults, salivary caffeine concentrations are comparable to plasma concentrations.[Citation6–8]
Previously reported methods for the quantification of caffeine from biological samples have used phase separation techniques for the extraction of caffeine.[Citation9–11] This technique is difficult with saliva, as the clear and colorless nature of both phases makes it difficult to find the phase boundary during the extraction process. Saliva also contains a variety of endogenous substances, such as proteins, which need to be removed before chromatographic drug analysis.[Citation12] The incomplete removal of proteins from samples for high-performance liquid chromatography (HPLC) analysis impacts negatively on both column performance and the sensitivity of the analytical method.[Citation12]
This paper aimed to develop and validate a liquid chromatography assay for in vitro and in vivo quantification of caffeine and use this to determine the stability of caffeine citrate solution. The primary objectives were to (a) develop and validate an extraction protocol and liquid chromatography assay for the extraction and quantification of caffeine from human saliva, and (b) evaluate the chemical, physical and microbial stability of caffeine solutions.
Experimental methods
Materials
Caffeine citrate used in the formulation of the oral liquid preparations and standards for analysis was purchased from ACROS Organics, Fisher Scientific (Belgium). Water for irrigation was used in the extemporaneous formulation of the oral liquids, in keeping with usual compounding practice at the local hospital, and was purchased from Baxter Healthcare (Australia). Acetonitrile (Merck, Germany) was analytical grade and Milli-Q water for HPLC was obtained from Millipak (Millipore, 0.22 µm). Blank saliva samples for method validation and stability evaluation were obtained by direct expectoration from six healthy adult volunteers who had abstained from caffeine-containing food and beverages for a period of at least 24 hr before morning collection. Each volunteer provided a single sample of at least 4 mL for use in this research, and all samples were combined to give a standardized saliva mix for validation experiments [Health and Disability Ethics Committee (Northern A), New Zealand; reference number 18/NTA129].
Chromatographic conditions
An Agilent 1260 HPLC machine (Agilent Corporation, Germany) was used for sample analysis. A Kinetex C18 column (5 µm, 4.6 × 150 mm) was used with a mobile phase comprising acetonitrile-water (1:9 v/v) and the flow rate set at 0.9 mL/min. An injection volume of 20 µL was used, and the UV absorbance was measured at 273 nm using a photodiode array (PDA) detector. This chromatographic method was validated for both caffeine solutions (see Supplementary Data) and human saliva samples and was applied to determine the stability of caffeine in solution for use as a trial medication, as well as to analyze saliva samples from trial participants.
Preparation of calibration and quality control (QC) standards in saliva
Ten-fold concentrated caffeine citrate solutions ranging from 3 to 500 µg/mL were prepared from a 1 mg/mL stock solution of caffeine citrate in water. Blank saliva was spiked with the appropriate volume of respective spiking solution to prepare triplicates of seven calibration standards at 0.3, 0.6, 2, 5, 10, 25, and 50 µg/mL. Similarly, three different QC standards: 1, 15, and 30 µg/mL, were spiked in triplicate as standards to determine the accuracy and precision of the method.
Extraction of caffeine from saliva
Samples were prepared as follows: 50 µL of saliva was mixed with 50 µL of Milli-Q water and 900 µL acetonitrile. The mixture was vortex mixed at 2000 rpm for 3 min at room temperature (22 °C) then centrifuged at 19,100 rcf at 4 °C for 10 min. The supernatant (975 µL) was transferred to a new Eppendorf tube and evaporated under a gentle stream of nitrogen at 42 °C. The residue was then reconstituted using 50 µL water followed by vortex mixing at 2000 rpm for 3 min at room temperature (22 °C), then centrifuged at 19,100 rcf at 4 °C for 10 min. A 20 µL aliquot of the supernatant was injected for HPLC analysis.
The extraction recoveries of spiked quality control (QC) saliva samples prepared at three different concentration levels (1, 15, and 30 µg/mL) were evaluated by injecting nine samples of each concentration. The extraction recoveries were calculated using Equation (1) below.[Citation13]
(1)
(1)
Method validation
Following extraction, the method for saliva sample analysis was validated according to the Food and Drug Administration (FDA) guidelines for the validation of bioanalytical methods.[Citation13] The following tests were repeated daily for three consecutive days.
Sensitivity
The sensitivity of the method was determined by calculating the limit of quantitation (LOQ) based on the standard deviation (SD) of the lowest non-zero calibrator and the slope of the linearity curve as described in Equation (2). The signal-to-noise ratio of the lowest concentration (0.3 µg/mL) was also determined.[Citation13]
(2)
(2)
Linearity
Linearity demonstrates that the sample solutions are within a concentration range where analyte response is directly proportional to concentration. This was assessed by analyzing freshly prepared spiked saliva samples (n = 7) ranging from 0.3 to 50 µg/mL daily for three consecutive days. A linearity standard curve was obtained by plotting the peak area against concentration.
Selectivity and specificity
Blank saliva samples (n = 3) were compared and evaluated for the interference of endogenous compounds during analysis to establish the selectivity of the method. Specificity was evaluated based on the selectivity of the method and the ability to detect caffeine citrate (±20% LLOQ) from the sample matrix. The chromatograms of blank samples and spiked samples (0.3 µg/mL; n = 3) were analyzed and compared to determine the specificity of the method.
Accuracy and precision
The accuracy (% A) of the method is the degree of closeness of the measured concentration of the analyte to the true concentration of the analyte in QC samples.[Citation13] Accuracy was determined by analyzing nine QC samples at three different concentration levels (1, 15, and 30 µg/mL), then calculated using Equation (3) below.[Citation13]
(3)
(3)
Precision is the degree of closeness between the results. Intra-day and inter-day precision were determined by analysis of three separate QC samples at each of the three different concentrations (1, 15, and 30 µg/mL), both over a single day and for three consecutive days, respectively, and calculating the relative standard deviations (RSD). According to the FDA guidelines, the accuracy and precision of a method are considered acceptable provided the RSD values remain below 15%.[Citation13]
Sample storage and post-extraction stability
The stability of caffeine stored in an auto sampler (without temperature control) was determined by reinjecting the QC caffeine samples (n = 3 for each concentration) over a period of 72 h. Freeze-and-thaw stability of spiked saliva samples was also determined at three QC concentrations in triplicate to determine the stability of caffeine over three freeze-and-thaw cycles (−20 °C to ambient temperature). Stability was considered adequate provided the accuracy at each concentration level remained within ± 15%.[Citation13]
Longer-term storage of spiked saliva samples was assessed at two concentrations (0.5 µg/mL and 3 µg/mL; n = 3 each) using blank saliva samples as negative controls. Samples were stored at room temperature (20–25 °C) and under refrigeration (2–8 °C). A portion of each sample was withdrawn immediately following preparation (time 0), on days 1, 2, and 7 and analyzed with the developed method to determine the quantity of caffeine remaining following storage at each of the conditions.
Stability analysis of extemporaneously prepared caffeine citrate formulation
Sample preparation
Samples were prepared using standard hospital extemporaneous compounding protocols. First, a stock solution of caffeine citrate 20 mg/mL was prepared using water for irrigation. The stock solution was further diluted with water for irrigation as required to give 5, 10, 15, and 20 mg/mL solutions. Six separate samples were prepared at each strength and stored individually in amber polypropylene medicine bottles. From these, three samples at each strength were labeled and used for HPLC stability testing, and the remaining three samples were labeled and used for pH and organoleptic analysis. An additional five samples of the 20 mg/mL formulation were prepared for microbial analysis. All samples were stored at room temperature (20–25 °C) under fluorescent lighting in the local hospital pharmacy to mimic in-use conditions.
HPLC assessment
Evaluation of chemical stability was performed at pre-determined intervals (weekly for one month, then fortnightly for one month, then 3 months from the date of preparation). At each timepoint, samples were withdrawn from the three storage bottles and analyzed directly using the HPLC parameters and methods described in the Chromatographic conditions section above. Details of the validation of this method are provided as a supplement to this paper.
Organoleptic and pH assessment
At each time point, 2–3 mL of sample was decanted into a test tube for analysis. Samples were visually examined and photographed against both light and dark backgrounds for clarity, color, precipitation, or other visual change, and assessed for any olfactory change. In addition, the pH of each sample was measured using a pH meter (SevenEasy pH meter, Mettler-Toledo Inc., Columbus, OH, USA). To ensure the accuracy of the measurement, the pH meter was calibrated using standard buffer solutions at pH 4, 7, and 10 on the day of stability assessment.
Microbial analysis
To allow for comparison with the commercially available formulation, a sample of the 20 mg/mL caffeine citrate formulation was submitted to an accredited contracting laboratory at each of 0, 7, 14, 21, and 28 days after compounding. These samples were tested for the presence of Cronobacter spp., Listeria spp., Salmonella spp., Bacillus cereus, Coagulase-positive Staphylococci, Coliforms, Escherichia coli, and yeasts and molds. This includes pathogens specified by the European Pharmacopeia in the section on the microbiological quality of aqueous preparations for oral use, plus others to known to be pathogenic in neonates, and/or to have been identified as contaminants in medications or neonatal food products.[Citation14–16]
Results and discussion
Development and selection of extraction protocol
Biological matrices, such as saliva, contain many proteins which, unless removed in a pretreatment step, may precipitate on contact with the mobile phase within an HPLC system, degrading the performance of the HPLC column.[Citation17] The extraction method for caffeine in saliva was adapted from that used by Perera et al.[Citation9] Ethyl acetate has been used widely for the extraction of polar analytes from biological samples by liquid-liquid extraction technique as it is effective in extracting compounds with a broad range of polarity.[Citation18] However, preliminary trials for extraction of caffeine from saliva using ethyl acetate as described by Perera et al.[Citation9] were complicated by difficulties establishing solvent boundaries between the saliva solution and ethyl acetate, and correspondingly challenges in removing only the organic phase for further processing, due to the colorless nature of both phases. Therefore, an alternative method was developed for the extraction of caffeine by protein precipitation using acetonitrile. Acetonitrile has the highest efficiency in protein removal[Citation19] and is, hence, the most commonly used organic solvent for protein precipitation in bioanalysis.[Citation17] Acetonitrile is water-miscible, allowing it to mix freely with saliva, where it displaces water from the solvation layer of the proteins causing aggregation and precipitation. At a ratio of 2:1 acetonitrile: aqueous phase, the resulting supernatant is essentially protein-free.[Citation17] Precipitation of salivary proteins using acetonitrile was found to be simple, uniform, and repeatable. A small amount of water (50 µL) was added to increase the solubility of caffeine during the extraction process, with the organic to inorganic solvent ratio being maintained at 1:9 to ensure maximum removal of proteins.[Citation17]
Extraction recovery
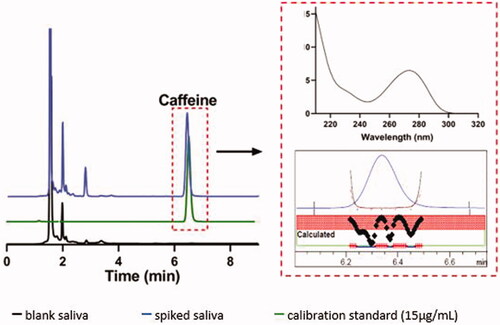
The extraction recovery of QC samples was found to be 95–111% at 1, 15, and 30 µg/mL ().
Table 1. Accuracy, precision, and extraction recovery data for caffeine citrate at concentrations of 1, 15, and 30 µg/mL.
The final extraction method described above was simple, rapid, and accurate while not requiring particularly specialized equipment, thus making it suitable for the analysis of a large number of samples from a clinical trial in a standard laboratory.
Method validation
The developed method was found to be simple, rapid, sensitive, and reproducible with caffeine eluting at 6.5 ± 0.2 min. The results of various validation parameters are discussed below.
Sensitivity
Using Equation (2) described above, the developed method was found to be sensitive in quantifying caffeine concentration to 0.3 µg/mL This was further confirmed by calculating the signal-to-noise ratio at this concentration, which was found to be above 10, indicating adequate sensitivity at this concentration. The LOQ of the developed method was thus considered to be 0.3 µg/mL.
Linearity
The plasma concentration vs. peak area plot was found to be linear for caffeine (n = 3) within the concentration range of 0.3–50 µg/mL in saliva with an R2 value of 0.9975. The regression equation is shown below (EquationEquation 4(4)
(4) ).
(4)
(4)
This concentration range is expected to cover the range of observed concentrations of caffeine in the saliva of mothers and babies participating in a clinical trial of caffeine citrate in late preterm babies, based on previous reports of salivary caffeine levels equivalent to 2.48–18.44 µg/mL in the saliva of lactating women, following ingestion of a variety of caffeine-containing beverages,[Citation20] and neonatal salivary concentrations of 0.4–36.8. µg/mL in a previously reported clinical trial.[Citation21]
Selectivity and specificity
The developed method was found selective for caffeine in the presence of other endogenous compounds. Analysis of multiple blank samples showed that there was no interference from the endogenous compounds in saliva ().
Accuracy and precision
The method was accurate and precise at three different concentrations (). The accuracy was between 94 and 100%. The precision (percentage bias) was below 7, less than the ±15% provided for in the FDA guidelines[Citation13] for both inter- and intra-day samples, indicating that the method is repeatable and reproducible.
Sample storage and post-extraction stability
The stability of caffeine-spiked saliva samples was determined both before and after extraction to mimic conditions during collection and transportation periods as well as sample analysis. The concentration of caffeine in saliva samples was found to remain stable with storage at both room temperature and under refrigeration for a period of 7 days ().
Table 2. Stability of caffeine in saliva under refrigeration (2–8 °C), at room temperature (20–25 °C), and frozen (−20 °C).
Caffeine in saliva was stable for three freeze-and-thaw cycles (−20 °C to ambient temperature), indicating that freezing and thawing of clinical trial samples for analysis will not affect the results. In addition, caffeine was stable at all three concentrations (1, 15, and 30 µg/mL) for at least two days post-extraction when kept in the auto sampler (). This indicates post-extraction stability of caffeine in all the samples until analysis of the final sample in the sequence, despite the temperature not being controlled in the auto sampler.
Table 3. Stability evaluation of extracted caffeine samples from saliva kept in auto sampler for 72 hr and for three freeze-and-thaw cycles (from −20 °C to room temperature) for n = 3 samples each.
Summary of method validation for determination of caffeine in saliva
The validation testing described above provides data to support the developed method for the quantification of caffeine in human saliva. This method will be used to quantify salivary caffeine levels in infants participating in a randomized controlled trial of caffeine for the prevention of intermittent hypoxemia, and their mothers.[Citation3] While it would have been preferable to perform this validation using neonatal saliva as well as adult saliva, ethical and practical issues preclude this, and any differences between the two matrices are likely to be minor.
The stability testing of saliva samples spiked with caffeine citrate confirms that the caffeine concentration in saliva is not significantly affected by storage at room temperature or under refrigeration, so delays in the transport of clinical trial samples to the laboratory for analysis will not significantly alter the results obtained from the analysis. Stability through freeze-thaw cycles indicates that freezing of samples until the end of the trial before analyzing them (as is required to avoid breaking the trial blinding) will not alter the results obtained.
Stability analysis of extemporaneously prepared caffeine citrate formulation
Organoleptic assessment
Throughout the three months of storage, all solutions remained clear and colorless with no visible precipitation. There was no noticeable odor from any of the tested solutions at any point during the study. This is in keeping with reported results from stability studies on preserved caffeine citrate solution.[Citation22]
pH assessment
During the three-month period of stability testing, no significant changes in pH were observed in any of the four concentrations of caffeine citrate solution (). Our results are similar to those reported by Barnes et al. in their stability testing of a 10 mg/mL preserved caffeine citrate solution.[Citation22]
Table 4. pH of caffeine citrate samples [mean (SD); n = 3] stored at room temperature for up to 3 months.
HPLC analysis
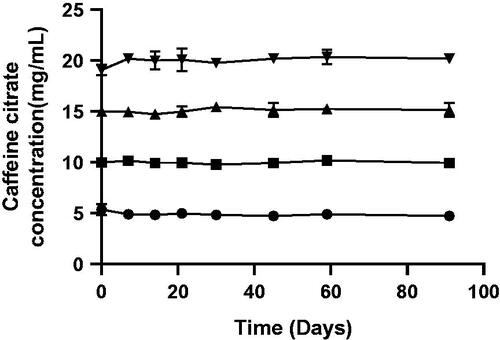
Calibration curve construction demonstrated that detection of caffeine citrate in solution was linear across the range tested, from 1 to 25 µg/mL. Throughout the trial period, all strengths of the caffeine citrate solution retained adequate potency (), defined as deviating by not more than 10% of the initial concentration[Citation23] with no new peaks observed on the chromatogram. After three months, the 5, 10, 15, and 20 mg/mL solutions were found to have mean (SD) caffeine concentrations of 5.0 (0.1), 9.9 (0.2), 15.0 (0.1), and 20.3 (0.5) mg/mL remaining, respectively, which was within 4% of corresponding baseline concentrations.
Microbial analysis
None of the 20 mg/mL samples, prepared under standard extemporaneous compounding conditions at the local hospital pharmacy, showed any growth for Cronobacter spp., Listeria spp., Salmonella spp., Bacillus cereus, Coagulase-positive Staphylococci, Coliforms, Escherichia coli or yeasts and molds when stored in amber polypropylene medicine bottles under florescent lighting at room temperature for time periods of up to 28-days post-manufacture. As solutions were extemporaneously compounded and did not contain any preservative, use beyond 28 days post-manufacture would not be considered in clinical practice.
Summary of stability analysis of extemporaneously prepared caffeine citrate formulation
As blinding requirements for the Latte Dosage Trial require the use of caffeine citrate oral liquid at four different strengths alongside a water placebo, and such products are not available commercially, we required a stable formulation of caffeine citrate that could be easily extemporaneously compounded by pharmacists at participating sites. As caffeine is both highly water soluble and known to be stable,[Citation22,Citation24] we formulated caffeine citrate as a simple aqueous solution to minimize the exposure of neonates to unnecessary pharmaceutical excipients. At 20 mg/mL this gave a formulation with comparable appearance and pH as that available commercially in NZ.
In keeping with the commercially available formulation, the prepared solutions were stored at room temperature. Refrigerated testing was considered unnecessary, as the commercial product was known to be stable at room temperature, and storage is easier both within the hospital and for patients at home if refrigeration is not required. The findings of the stability testing reported above provide reassurance that caffeine citrate, when compounded extemporaneously as an aqueous solution, is stable at the concentrations tested and is suitable for use in the Latte Dosage Trial.
Conclusion
The method presented above for the extraction and quantification of caffeine in human saliva was found to be simple, rapid, accurate, and reproducible, and hence suitable for the analysis of clinical trial samples. Salivary caffeine is stable for at least three freeze-and-thaw cycles and at least seven days at both room temperature and under refrigeration, meaning delays in the transport of samples from the point of collection to the laboratory, and freezing before analysis at the end of the trial will not affect salivary caffeine concentrations. Extemporaneously compounded caffeine citrate aqueous solutions are chemically and physically stable at room temperature for at least three months, with no growth of organisms of interest on microbial testing for at least one month after manufacture.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Eichenwald, E. C.; Watterberg, K. L.; Aucott, S.; Benitz, W. E.; Cummings, J. J.; Goldsmith, J.; Poindexter, B. B.; Puopolo, K.; Stewart, D. L.; Wang, K. S. Apnea of Prematurity. Pediatrics 2016, 137, 1–7. DOI: 10.1542/peds.2015-3757.
- Dukhovny, D.; Lorch, S. A.; Schmidt, B.; Doyle, L. W.; Kok, J. H.; Roberts, R. S.; Kamholz, K. L.; Wang, N.; Mao, W.; Zupancic, J. A. F. Economic Evaluation of Caffeine for Apnea of Prematurity. Pediatrics 2011, 127, e146–e155. DOI: 10.1542/peds.2010-1014.
- Oliphant, E. A.; McKinlay, C. J. D.; McNamara, D. G.; Alsweiler, J. M. Caffeine Prophylaxis to Improve Intermittent Hypoxaemia in Infants Born Late Preterm: A Randomised Controlled Dosage Trial (Latte Dosage Trial). BMJ Open 2020, 10, e038271. DOI: 10.1136/bmjopen-2020-038271.
- Gorodischer, R.; Burtin, P.; Hwang, P.; Levine, M.; Koren, G. Saliva versus Blood Sampling for Therapeutic Drug Monitoring in Children: Patient and Parental Preferences and an Economic Analysis. Ther. Drug Monit. 1994, 16, 437–536. DOI: 10.1097/00007691-199410000-00001.
- Horning, M. G.; Brown, L.; Nowlin, J.; Lertratanangkoon, K.; Kellaway, P.; Zion, T. E. Use of Saliva in Therapeutic Drug Monitoring. Clin. Chem. 1977, 23, 157–164. DOI: 10.1093/clinchem/23.2.157.
- Dobson, N. R.; Liu, X.; Rhein, L. M.; Darnall, R. A.; Corwin, M. J.; McEntire, B. L.; Ward, R. M.; James, L. P.; Sherwin, C. M. T.; Heeren, T. C.; et al. Salivary Caffeine Concentrations Are Comparable to Plasma Concentrations in Preterm Infants Receiving Extended Caffeine Therapy. Br. J. Clin. Pharmacol. 2016, 82, 754–761. DOI: 10.1111/bcp.13001.
- Alkaysi, H. N.; Salem, M. S.; El-Sayed, Y. M. High Performance Liquid Chromatographic Analysis of Caffeine Concentrations in Plasma and Saliva. J. Clin. Pharm. Ther. 1988, 13, 109–110. DOI: 10.1111/j.1365-2710.1988.tb00165.x.
- Carrillo, J. A.; Christensen, M.; Ramos, S. I.; Alm, C.; Dahl, M. L.; Benítez, J.; Bertilsson, L. Evaluation of Caffeine as an In Vivo Probe for CYP1A2 Using Measurements in Plasma, Saliva, and Urine. Ther. Drug Monit. 2000, 22, 409–417. DOI: 10.1097/00007691-200008000-00008.
- Perera, V.; Gross, A. S.; McLachlan, A. J. Caffeine and Paraxanthine HPLC Assay for CYP1A2 Phenotype Assessment Using Saliva and Plasma. Biomed. Chromatogr. 2010, 24, 1136–1144. DOI: 10.1002/bmc.1419.
- Newton, R.; Broughton, L. J.; Lind, M. J.; Morrison, P. J.; Rogers, H. J.; Bradbrook, I. D. Plasma and Salivary Pharmacokinetics of Caffeine in Man. Eur. J. Clin. Pharmacol. 1981, 21, 45–52. DOI: 10.1007/BF00609587.
- Jordan, N. Y.; Mimpen, J. Y.; van den Bogaard, W. J. M.; Flesch, F. M.; van de Meent, M. H. M.; Torano, J. S. Analysis of Caffeine and Paraxanthine in Human Saliva with Ultra-High-Performance Liquid Chromatography for CYP1A2 Phenotyping. J. Chromatogr. B 2015, 995–996, 70–73. DOI: 10.1016/j.jchromb.2015.05.020.
- McDowall, R. D.; Doyle, E.; Murkitt, G. S.; Picot, V. S. Sample Preparation for the HPLC Analysis of Drugs in Biological Fluids. J. Pharm. Biomed. Anal. 1989, 7, 1087–1096. DOI: 10.1016/0731-7085(89)80047-0.
- US Food and Drug Administration; U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Veterinary Medicine (CVM). Bioanalytical Method Validation: Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, 2018.
- Dong, Y.; Speer, C. P. Late-Onset Neonatal Sepsis: Recent Developments. Arch. Dis. Child Fetal Neonatal Ed. 2015, 100, F257–F263. DOI: 10.1136/archdischild-2014-306213.
- Council of Europe. European Pharmacopoeia (Ph. Eur.), 10th ed.; Council of Europe: Strasbourg, 2019.
- New Zealand Ministry of Health. Communicable Disease Control Manual | Ministry of Health NZ; New Zealand Ministry of Health: Wellington, 2012.
- Li, W.; Jian, W.; Fu, Y. Basic Sample Preparation Techniques in LC‐MS Bioanalysis. In Sample Preparation in LC‐MS Bioanalysis; Wiley: Hoboken, NJ, 2019; pp 1–30. DOI: 10.1002/9781119274315.ch1.
- Bogialli, S.; Corcia, A.; Di; Nazzari, M. Extraction Procedures. In Food Toxicants Analysis; Elsevier: Amstadam, The Netherlands, 2007; pp 269–297. DOI: 10.1016/B978-044452843-8/50010-9.
- Polson, C.; Sarkar, P.; Incledon, B.; Raguvaran, V.; Grant, R. Optimization of Protein Precipitation Based upon Effectiveness of Protein Removal and Ionization Effect in Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B 2003, 785, 263–275. DOI: 10.1016/S1570-0232(02)00914-5.
- Berlin, C. M.; Denson, M. H.; Daniel, C. H.; Ward, R. M. Disposition of Dietary Caffeine in Milk, Saliva, and Plasma of Lactating Women. Pediatrics 1984, 73, 59–63. DOI: 10.1542/peds.73.1.59.
- Chaabane, A.; Chioukh, F. Z.; Chadli, Z.; Ben Fredj, N.; Ben Ameur, K.; Ben Hmida, H.; Boughattas, N. A.; Monastiri, K.; Aouam, K. Therapeutic Drug Monitoring of Caffeine in Preterm Infants: Could Saliva Be an Alternative to Serum? Therapie 2017, 72, 685–689. DOI: 10.1016/j.therap.2017.06.004.
- Barnes, A. R.; Hebron, B. S.; Smith, J. Stability of Caffeine Oral Formulations for Neonatal Use. J. Clin. Pharm. Ther. 1994, 19, 391–396. DOI: 10.1111/j.1365-2710.1994.tb00699.x.
- Mehta, A. C. Practice Research: Strategies for Stability Studies on Hospital Pharmaceutical Preparations. Int. J. Pharm. Pract. 2011, 2, 49–52. DOI: 10.1111/j.2042-7174.1993.tb00720.x.
- Sigma-Aldrich. Caffeine Solution (C6035)–Datasheet. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/195/539/c6035-slbj7833vdat.pdf (accessed Jun 17, 2022).