Abstract
Aims:
Our study examined characteristics of adolescent and young adult study participants using gabapentinoids (gabapentin, pregabalin), with previous history of adolescent psychiatric inpatient hospitalization. Particular focus was on temporal association of age, at first prescription for gabapentinoids, to age at tobacco smoking initiation, regular alcohol use, diagnosis of substance dependence and prescriptions for benzodiazepines and opioids. Methods: The initial study population contained 508 adolescents (300 females, 208 males) admitted to psychiatric inpatient care in Oulu University hospital between the ages 13–17 years. Register-based follow-up information on prescriptions for gabapentinoids, benzodiazepines and opioids, as well as ICD-10 diagnosis for hospital-treated substance dependence, was obtained from the Finnish national health care registers. Results: The users of gabapentinoids accounted for 9.1% of the initial study population. Of adolescence-related characteristics, suicidal ideation, suicide attempts and non-suicidal self-injury was emphasized in females using gabapentinoids. The majority of participants using gabapentinoids had benzodiazepines (80.4%) and opioids (71.7%) as comorbid drugs. Initiation of tobacco smoking and alcohol use and first prescriptions for of benzodiazepines and opioids, and a diagnosis of substance dependence commonly predated first prescriptions for gabapentinoids. Conclusions: In clinical practice, the decision to prescribe gabapentinoids to adolescents or young adults must be made with caution, particularly for those with substance use problems and/or without a clinically approved indication.
1. Introduction
Gabapentin and pregabalin (collectively referred as gabapentinoids) are gamma-aminobutyric acid (GABA) analogues. They reduce calcium influx and neurotransmitter release in the central nervous system by binding to the alpha-2-delta subunit of voltage-gated calcium channels, which is believed to cause the antinociceptive, anticonvulsant and anxiolytic effects (Bockbrader et al., Citation2010).
Pregabalin is approved for use in the treatment of neuropathic pain and partial seizures. In the European Union, it is also approved for the treatment of generalized anxiety disorder (GAD) and, in the USA, for the treatment of fibromyalgia. Gabapentin is also used to treat neuropathic pain and epilepsy (Häkkinen et al., Citation2014). Initially, gabapentinoids were considered to have low misuse potential, but the risk for misuse was recognized in two early case reports (Markowitz et al., Citation1997; Reccoppa et al., Citation2004) and subsequent studies (Bastiaens et al., Citation2016; Evoy et al., Citation2017; Peckham et al., Citation2017). Swedish researchers found that pregabalin abuse and addiction were first reported in national registers in 2008 (Schwan et al., Citation2010). The same observation was made in Germany, where reports of pregabalin misuse were increasing from 2008 (Gahr et al., Citation2013). Chiappini and Schifano (Citation2016) reviewed all reports of misuse, abuse or dependence of gabapentin (2004–2015) and pregabalin (2006–2015) available from the European Medicine Agency (EudraVigilance) database. They summarized that 6.6% out of a total of 115,616 reports, were associated with gabapentin, and 4.8% out of 90,166 reports involved pregabalin, with >75% reported after the year 2012.
Gabapentinoid use has found to be associated with numerous adverse outcomes such as intentional drug overdoses (Daly et al., Citation2018). A recent large population cohort study from Sweden showed that, between the years 2006–2013, 5.2% of those who had been prescribed gabapentinoids had been treated for suicidal behavior or committed suicide (Molero et al., Citation2019). Additionally, gabapentinoid use was associated with unintentional overdoses, injuries and traffic incidents and offenses (Molero et al., Citation2019).
Despite the growing numbers of reports of their abuse, gabapentinoids are increasingly prescribed for patients. In the United Kingdom, prescriptions of pregabalin have increased by 350% over a five year period, the corresponding percentage for gabapentin being 150% (Spence, Citation2013). Gabapentioids are commonly misused in combination with other drugs, especially opioids (Evoy et al., Citation2017). Häkkinen et al. (Citation2014) found concurrent opioid use among 91.4% of people who misuse pregabalin and 87.5% of those who misuse gabapentin. Gabapentin overuse with opioids has been found to associate with increased health service utilization (Peckham et al., Citation2018). In their large population based study, Gomes et al. (Citation2017) found that gabapentin use was associated with a nearly 60% increase in the odds of opioid-related death, compared to no concomitant gabapentin use.
There is conflicting evidence as to whether misuse of gabapentinoids is more prevalent in males or females. Chiappini and Schifano (Citation2016) showed that the female/male ratio among those misusing gabapentinoids was 1.13 for pregabalin and 1.27 for gabapentin. Similarly, in Appalachian Kentucky, 77.8% of gabapentin users were women (Smith et al., Citation2015) and, in an addiction services population study from Ireland, 59% of participants with a positive test for pregabalin were female (McNamara et al., Citation2015). Conversely, in a German postmortem study, 64% of a total of 55 reports of pregabalin abuse/dependence involved males (Gahr et al., Citation2013). Studies from Sweden and Denmark have also reported a higher proportion of males using pregabalin at higher than the maximum allowed dose (Bodén et al., Citation2014; Schjerning et al., Citation2016).
A relatively young age seems to have a connection with the abuse of gabapentinoids. In a Finnish postmortem study (n = 359), the median age among gabapentinoid abusers was 30 (Häkkinen et al., Citation2014), and young age was also mentioned as a connective factor in pregabalin abuse by Bodén et al. (Citation2014). In a cohort study from the United Arab Emirates, pregabalin was found to be commonly abused in young individuals, with the mean age of first use being 20 years old (Alblooshi et al., Citation2016).
Although the misuse potential of gabapentinoids, as well as adverse outcomes such as suicidal behavior, are recognized in literature, less is known about specific psychosocial factors in childhood and adolescence associated with later gabapentinoid use. In this study, we examined characteristics of gabapentinoid (gabapentin, pregabalin) users with a preceding history of adolescent psychiatric hospitalization between the ages 13–17 years. A specific aim was to analyze the temporal association of age at first prescription for gabapentinoids to age at tobacco smoking initiation, regular alcohol use, diagnosis of substance dependence and prescriptions for benzodiazepines and opioids. The initial data, gathered during the adolescent psychiatric hospitalization period, was linked with the follow-up data, obtained from nationwide registers of prescriptions for reimbursable medicines and care registers for health care.
2. Materials and methods
2.1. Study participants
This study is part of a clinical follow-up project at Oulu University Hospital, Finland, which is designed to examine long-term outcomes of former adolescent psychiatric inpatients. The catchment area of Oulu University hospital covers the two sparsely populated northernmost provinces of Finland (the provinces of Oulu and Lapland), which covers 43% of the area of Finland. The initial study population consists of 508 adolescents (300 female and 208 male) admitted to psychiatric inpatient treatment between the ages 13–17 years, during a five-year study period from April 2001 to March 2006 (referred to from here on as index hospitalization). The study protocol was approved by the Ethics Committee of Oulu University Hospital, Finland.
In our study, the focus is to analyze participants using gabapentinoids exclusively, covering 9.1% (n = 46) of the initial study population. The initial data were gathered during the adolescent psychiatric hospitalization, between the ages 13–17 years, and was linked with nationwide follow-up data, obtained from the registers of purchases of reimbursable medicines and care register for health care. The data linkage was made by using unique personal identification code, assigned to each Finnish resident.
2.2. Register-based follow-up
2.2.1. Sources for follow-up data
The information on the use of gabapentinoids and other psychotropic medications, up to young adulthood, was obtained from the purchase register of reimbursable drugs, administered by the Social Insurance Institution (SII) of Finland (The Social Insurance Institution of Finland, 2020). The data available for this study included the exact purchase date and The Anatomical Therapeutic Chemical (ATC)-code (WHO Collaborating Center for Drug Statistics Methodology, Citation2020) of each psychotropic medication prescription, up to the end of 2012. The age of the study participants, at the end of follow-up time, varied between 20–30 years.
The follow-up data of inpatient care and specialized level outpatient visits was obtained from the nationwide Care Register for Health Care (Care Register for Health Care, Citation2020), administered by the Finnish National Institute for Health and Welfare (THL). The CRHC data includes all hospital discharges, both in general and specialized health care, from year 1969 onwards, and all outpatient visits in specialized health care from 1998 onwards. The diagnoses in the CRHC are coded according to ICD-10 diagnostic classification (ICD-8: 1969–1986, ICD-9: 1987–1995, ICD-10: 1996 onwards). The CRHC data for the current study was available up to the end of 2016.
2.2.2. Assessment for use of gabapentinoids
Gabapentinoid use included purchases of physician-prescribed pregabalin (ATC-code: N03AX16) and gabapentin (ATC-code: N03AX12). A study subject was defined as being a gabapentinoid user if at least one prescription for gabapentinoids was identified from that purchase register of reimbursable medicines, by the end of follow-up period. The age (in years) at first prescription, and the total number of prescriptions of pregabalin and/or gabapentin, was calculated for each participant using gabapentinoids.
Comorbid physician-prescribed medicines focused on the use of benzodiazepines (ATC-codes: N05BA, N03AE01) and opioids (ATC-codes: N02AA, N02AB, N02AC, N02AE, N02AJ, N02AX,). Age at first prescription, and number of prescriptions of benzodiazepines and opioids, was determined for each user of gabapentinoids.
The indication for physician-prescribed gabapentinoids was evaluated, based on the data from the CRHC. The main researcher (JP) interrogated the data to identify the conditions clinically indicated to be treated using gabapentinoids (epilepsy, neuropathic pain or generalized anxiety disorder). Diagnoses of substance dependence disorder (ICD-10: F1x.2) and onset age of substance dependence disorder were also extracted from the CRHC.
2.3. Adolescence-related data
2.3.1. Research instruments
All adolescents were interviewed during their adolescent inpatient hospitalization, between the ages 13–17 years, using the semi-structured Schedule for Affective Disorder and Schizophrenia for School-Age Children Present and Lifetime (K-SADS-PL). K-SADS-PL is a diagnostic interview designed to assess psychopathology of children and adolescents, according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (Ambrosini, Citation2000; American Psychiatric Association, Citation2000; Kaufman et al., Citation1997). The adolescents’ interviews were conducted by the treating physician, or by trained medical students. Parents or guardians were also interviewed, in order to complete missing or otherwise incomplete information based on adolescent’s interview.
The European Addiction Severity Index (EuropASI) interview was used to collect information on parents’ employment status and psychiatric- and substance use related problems (Kokkevi & Hartgers, Citation1995). The EuropASI contains questions on various areas of life, such as parental psychiatric and substance problems, and it has been demonstrated to be a reliable instrument for research purposes (Kokkevi & Hartgers, Citation1995).
The level of nicotine dependence (ND) was measured using the modified Fagerström Tolerance Questionnaire (mFTQ) for adolescents (Prokhorov et al. Citation2017). The total score of the mFTQ can range from 0 to 9. The cutoffs (Prokhorov et al., Citation2000), indicating level of dependence, for the total score are as follows: 0–2 = no ND, 3–5 = moderate ND, and 6–9 = high ND. Nonsmokers are included in the category of “no ND”.
2.3.2. Adolescence-related characteristics
2.3.2.1. Family-related factors
The information on family type, at admission to adolescent inpatient care, was based on the K-SADS-PL. Family type was categorized into: two parent family (family with two biological parents or blended family with one biological parent and cohabiting spouse), single parent family (one biological parent), and out-of-home placement (foster family, child welfare unit, other home environment). Information on parents’ employment status (at least one parent in full- or part time work; yes/no), as well as parental psychiatric and substance use related problems, were obtained from the EuropASI. Parental psychopathology was based on the adolescents’ self-reporting as to whether they perceived their parent(s) to have required treatment (yes/no) for psychiatric or substance use related (alcohol, drugs) problems.
2.3.2.2. Adolescent psychiatric disorders
The DSM-IV-based psychiatric disorders in adolescence were based on the diagnostic K-SADS-PL interview. These disorders were categorized into five major categories: anxiety disorders, affective disorders, psychotic disorders, conduct disorders and substance use related disorders. An adolescent may have had diagnoses belonging to multiple categories of psychiatric disorders.
2.3.2.3. Suicidality
As part of K-SADS-PL interview, the adolescents were asked about their suicidal ideation, non-suicidal self-injury (NSSI) and suicide attempts. Suicidal ideation was defined as being present if the thoughts of suicide had been recurrent and the adolescent has planned the method of suicide. NSSI covered non-suicidal physical self-damaging acts, without intent to die, and was defined as being present if there were at least four self-damaging acts in the previous year. Suicide attempt was defined as being present if the adolescent had performed a life-threatening attempt, with definite suicidal intent, or with potentially medically lethal consequences.
2.3.2.4. Smoking and nicotine dependence
The information on regular smoking (at least one cigarette per day) and onset age of regular smoking was based on the K-SADS-PL interview. The level of nicotine dependence (ND) was measured with the sum-score of the mFTQ: no ND (nonsmokers, sum-score of the mFTQ, 0–2), moderate ND (sum-score 3–5), and high ND (sum-score 6–9).
2.3.2.5. Alcohol abuse in adolescence
The diagnoses of alcohol abuse (DSM-IV: 305.00) and onset age of alcohol abuse during adolescence were obtained from the K-SADS-PL.
2.4. Statistical methods
Group differences in categorical variables were assessed using Pearson Chi-square test or Fisher’s exact test and in continuous variables with Student’s t-test or Mann-Whitney U-test. The statistical software used in analyses was IBM SPSS Statistics 25. All test were two-tailed and a limit for statistical significance was set at p < 0.05.
3. Results
3.1. Sample characteristics
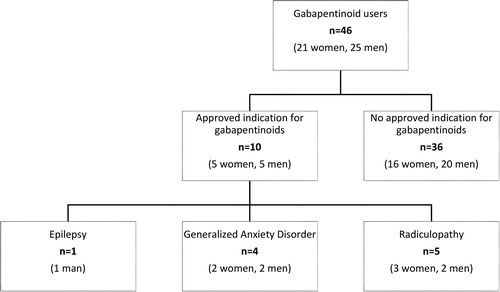
Of the initial study population (n = 508), 46 (9.1%) subjects received prescriptions for gabapentinoids during the follow-up time, up to young adulthood. As seen in , one fifth (n = 10, 21.7%) of participants using gabapentinoids were evaluated as having an approved clinical indication for their gabapentinoid prescription. One male participant was prescribed pregabalin for his epilepsy. Four participants using gabapentinoids had been diagnosed with generalized anxiety disorder (GAD). Among them, one patient had received a GAD diagnosis before first prescription for gabapentinoids, while in the remaining three patients, the time from first gabapentinoid prescription to subsequent GAD diagnosis varied from 15 months to 5.5 years. Further, five participants using gabapentinoids had a diagnosis of radiculopathy, which may cause neurological pain and, consequently, is an approved indication for gabapentinoid use. In all of these cases, however, the radiculopathy diagnosis was made after the first prescription for gabapentinoids and, generally, the time from first gabapentinoid prescription to diagnosis of radiculopathy varied from 2 months to 3 years.
3.2. Adolescence-related characteristics and gabapentinoid use
presents adolescence-related socio-demographic and clinical characteristics of the participants using gabapentinoids. Female participants showed a notable higher prevalence of suicidal ideation (66.7% vs. 24.0%, p = 0.004), suicide attempts (57.1% vs. 12.0%, p = 0.001) and NSSI (66.7% vs. 12.0%, p < 0.001) than male participants.
Table 1. Adolescence-related socio-demographic and clinical characteristics of participants using gabapentinoids.
3.3. Comorbid drug use and substance dependence
As seen in , the majority of participants using gabapentinoids had used pregabalin (82.6%), comorbid drug use was common (opioids 71.7%, benzodiazepines 80.4%), and they had been diagnosed with ICD-10 substance dependence (87.0%) by young adulthood. A statistically significant male preponderance was found in the amount of gabapentin users (56.0% in men vs. 19.0% in women, p = 0.011).
Table 2. Use of gabapentinoids (pregabalin, gabapentin), opioids and benzodiazepines, and diagnosis of substance dependence up to young adulthood.
3.4. Temporal profile of substance misuse among participants using gabapentinoids
outlines the gender-specific temporal profiles of substance use among participants using gabapentinoids. Regardless of gender, substance use began with early initiation of smoking and regular use of alcohol, followed by a diagnosis of substance dependence and, further, prescriptions for benzodiazepines and opioids, and then subsequent prescriptions for gabapentinoids. shows that smoking initiation, regular use of alcohol, diagnosis of substance dependence, and first prescriptions for benzodiazepines and opioids, occurred statistically significantly at a younger age compared to first prescription for gabapentinoids, except in age at first prescription for opioids in women.
Figure 2. Age at initiation of smoking, alcohol abuse diagnosis, diagnosis for substance dependence, and first prescription for benzodiazepines, opioids and gabapentinoids, by gender among participants using gabapentinoids.
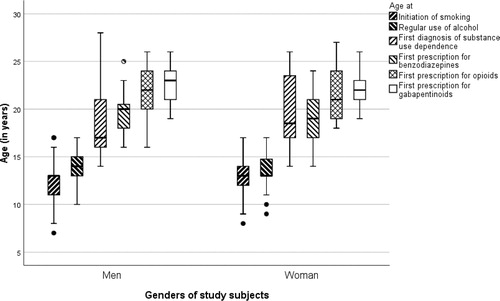
Table 3. Difference (in years), from initiation of smoking, alcohol abuse diagnosis, substance dependence diagnosis and first prescription for benzodiazepines or opioids to first prescription for gabapentinoids.
4. Discussion
The results of our explorative follow-up study of former adolescent psychiatric inpatients, demonstrated that one fifth of participants using gabapentinoids had an approved indication for their gabapentinoid prescriptions. Regardless of gender, the temporal profile of substance use was clear, beginning with early initiation of smoking and regular use of alcohol, followed by a diagnosis of substance dependence, prescriptions for benzodiazepines and opioids and eventual prescriptions for gabapentinoids.
In our study, an approved clinical indication for gabapentinoids (e.g. epilepsy, GAD, radiculopathy) was evaluated as being present in only 22% of participants using gabapentinoids, and the vast majority of them had both benzodiazepines and opioids as comorbid drugs and physician-diagnosed substance dependence. The results of a systematic review of studies on gabapentinoid abuse summarized the risk factors for gabapentinoid misuse to include a history of substance abuse, particularly opioids, and psychiatric co-morbidities (Evoy et al., Citation2017). It has been suggested that gabapentionds are misused, not only for their euphoric or sedative effects, but also to relieve the symptoms of opioid withdrawal (Bastiaens et al., Citation2016; Hägg et al., Citation2020), one vulnerable population being former prisoners (Bastiaens et al., Citation2016). Interestingly, the ICD-10 diagnosis of substance dependence of our study subjects was set approximately three years before their first gabapentinoid prescriptions. Thus, our study findings, from a clinical sample of former adolescent psychiatric inpatients, highlights the potential for misuse of gabapentinoids and, above all, emphasizes the importance of carefully considering prescribing gabapentinoids to individuals at high-risk of drug abuse (Chiappini & Schifano, Citation2016; Bonnet & Scherbaum, Citation2017), including patients with chronic pain (Anantharamu & Govind, Citation2018).
Of the adolescence-related characteristics of participants using gabapentinoids examined in our study, suicidal behavior and affective and anxiety disorders were characterized by female gender. In addition, smoking, nicotine dependence, alcohol abuse, and diagnoses of conduct and/or substance use disorders in adolescence were apparent, but independent of the gender of participants using gabapentinoids. Doctors may consider gabapentinoids to be less addictive compared to benzodiazepines and, therefore, prefer them when treating anxiety symptoms among patients with substance misuse. This may partly explain why the age difference between substance dependence diagnosis and initiation of gabapentinoid use was nearly twice as high in males (4 years) than in females (2.2 years). This difference may be explained by their comorbid depression, anxiety and suicidality which may serve as plausible reasons to seek prescriptions for gabapentinoids. There is only limited evidence supporting off-label use of gabapentinoids (Goodman & Brett, Citation2019). However, it is possible that, in some cases, prescriptions may have been based on justifiable off-label indications and clinical response and that potential adverse events have been properly assessed.
There are limitations of our study that must be acknowledged. The nationwide CRHC data comprehensively covers all treatments occurring in inpatient settings and at specialized level outpatient clinics. The data for outpatient visits in primary care and private clinics was not available for this study and, thus, may have caused an underestimation of the number of cases with a clinical indication for gabapentinoid prescriptions. Secondly, since our study utilized retrospective register based information on gabapentinoid prescriptions, we did not have any laboratory tests available to verify blood levels of gabapentinoids. Third, gabapentinoids are also purchased illegally on the black market, and the people making these purchases are even more likely to be misusing gabapentinoids. Therefore, our results may underestimate the true levels of gabapentinoid use of our study participants. Further, the data on prescribed medication did not include information on exact dosages and the number of pills in a package and, thus, it was not possible to analyze the true extent of gabapentinoid use. Moreover, pregabalin was first licensed for marketing in the EU in 2004 (European Medicines Agency, Citation2020) and, therefore, our results of temporal profile of initiating psychotrophic drugs may be inflated. Our study sample of adolescents and young adults using gabapentinoids was extracted from a clinical sample of former adolescent psychiatric inpatients and, therefore, the results are not generalizable to the general population of the same age.
The strengths of this study, are its use of the follow-up data from the nationwide Finnish registers, which have been shown to be a valid source for information in scientific research (Miettunen et al., Citation2011). In Finland, physician-prescribed gabapentinoids are sold only in pharmacies and, thus, our study includes comprehensive data of prescriptions for gabapentinoids over the study period. A further strength is that adolescent-related characteristics and psychiatric disorder were assessed using widely used and reliable research instruments, including the K-SADS-PL (Kaufman et al., Citation1997) and the EuropASI (Kokkevi & Hartgers, Citation1995).
4. Conclusions
Our study findings suggest that gabapentinoids are highly associated with the use of other psychotropic drugs. Therefore, the potential benefits and risks should be carefully weighed when considering gabapentinoid prescriptions for adolescents and young adults with substance use problems. Our study is an important addition to the previous literature, since gabapentinoid misuse among adolescents or young adults is a neglected topic of research. The clinical importance and relevance of our study is emphasized by the increasing trend in gabapentinoid use Finland (Finnish Medicines Agency, Citation2016).
Acknowledgements
This work received support from The Alma and K.A. Snellman Foundation, Oulu, Finland.
Declaration of interest
The authors report no conflict of interest
References
- Alblooshi, H., Hulse, G. K., El Kashef, A., Al Hashmi, H., Shawky, M., Al Ghaferi, H., Al Safar, H., & Tay, G. K. (2016). The pattern of substance use disorder in the United Arab Emirates in 2015: Results of a National Rehabilitation Centre cohort study. Substance Abuse Treatment, Prevention, and Policy, 11, 19. https://doi.org/10.1186/s13011-016-0062-5
- Ambrosini, P. J. (2000). Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS). Journal of the American Academy of Child and Adolescent Psychiatry, 39(1), 49–58. https://doi.org/10.1097/00004583-200001000-00016
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders. Text revision (DSM-IV-TR). 4th ed. American Psychiatric Association.
- Anantharamu, T., & Govind, M. A. (2018). Managing chronic pain: Are gabapentinoids being misused? Pain Management, 8(5), 309–311. https://doi.org/10.2217/pmt-2018-0018
- Bastiaens, L., Galus, J., & Mazur, C. (2016). Abuse of gabapentin is associated with opioid addiction. Psychiatric Quarterly, 87(4), 763–767. https://doi.org/10.1007/s11126-016-9421-7
- Bockbrader, H. N., Wesche, D., Miller, R., Chapel, S., Janiczek, N., & Burger, P. (2010). A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clinical Pharmacokinetics, 49(10), 661–669. https://doi.org/10.2165/11536200-000000000-00000
- Bodén, R., Wettermark, B., Brandt, L., & Kieler, H. (2014). Factors associated with pregabalin dispensing at higher than the approved maximum dose. European Journal of Clinical Pharmacology, 70(2), 197–204. https://doi.org/10.1007/s00228-013-1594-5
- Bonnet, U., & Scherbaum, N. (2017). How addictive are gabapentin and pregabalin? A systematic review. European Neuropsychopharmacology, 27(12), 1185–1215. https://doi.org/10.1016/j.euroneuro.2017.08.430
- Care Register for Health Care. (2020). Retrieved November 8, 2020, from https://thl.fi/en/web/thlfi-en/statistics/information-on-statistics/register-descriptions/care-register-for-health-care
- Chiappini, S., & Schifano, F. (2016). A decade of gabapentinoid misuse: An analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs, 30(7), 647–654. https://doi.org/10.1007/s40263-016-0359-y
- Daly, C., Griffin, E., Ashcroft, D. M., Webb, R. T., Perry, I. J., & Arensman, E. (2018). Intentional drug overdose involving pregabalin and gabapentin: Findings from the National Self-Harm Registry Ireland, 2007–2015. Clinical Drug Investigation, 38(4), 373–380. https://doi.org/10.1007/s40261-017-0616-y
- European Medicines Agency. (2020). Retrieved November 8, 2020, from https://www.ema.europa.eu/en/medicines/human/EPAR/lyrica
- Evoy, K. E., Morrison, M. D., & Saklad, S. R. (2017). Abuse and misuse of pregabalin and gabapentin. Drugs, 77(4), 403–426. https://doi.org/10.1007/s40265-017-0700-x
- Finnish Medicines Agency (Fimea). (2016). Drug consumption in 2016. Retrieved November 8, 2020, from https://www.fimea.fi/web/en/databases_and_registeries/consumption
- Gahr, M., Freudenmann, R. W., Hiemke, C., Kölle, M. A., & Schönfeldt-Lecuona, C. (2013). Pregabalin abuse and dependence in Germany: Results from a database query. European Journal of Clinical Pharmacology, 69(6), 1335–1342. https://doi.org/10.1007/s00228-012-1464-6
- Gomes, T., Juurlink, D. N., Antoniou, T., Mamdani, M. M., Paterson, J. M., & van den Brink, W. (2017). Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study. PLOS Medicine, 14(10), e1002396. https://doi.org/10.1371/journal.pmed.1002396
- Goodman, C. W., & Brett, A. S. (2019). A clinical overview of off-label use of gabapentinoid drugs. JAMA Internal Medicine, 179(5), 695–701. https://doi.org/10.1001/jamainternmed.2019.0086
- Hägg, S., Jönsson, A. K., & Ahlner, J. (2020). Current evidence on abuse and misuse of gabapentinoids. Drug Safety, 43(12), 1235–1254. https://doi.org/10.1007/s40264-020-00985-6
- Häkkinen, M., Vuori, E., Kalso, E., Gergov, M., & Ojanperä, I. (2014). Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic Science International, 241, 1–6. https://doi.org/10.1016/j.forsciint.2014.04.028
- Kaufman, J., Birmaher, B., Brent, D., Rao, U., Flynn, C., Moreci, P., Williamson, D., & Ryan, N. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. https://doi.org/10.1097/00004583-199707000-00021
- Kokkevi, A., & Hartgers, C. (1995). EuropASI: European adaption of a multidimensional assessment instrument for drug and alcohol dependents. European Addiction Research, 1(4), 208–210. https://doi.org/10.1159/000259089
- Markowitz, J. S., Finkenbine, R., Myrick, H., King, L., & Carson, W. H. (1997). Gabapentin abuse in a cocaine user: Implications for treatment?Journal of Clinical Psychopharmacology, 17(5), 423–424. https://doi.org/10.1097/00004714-199710000-00012
- McNamara, S., Stokes, S., Kilduff, R., & Shine, A. (2015). Pregabalin abuse amongst opioid substitution treatment patients. Irish Medical Journal, 108(10), 309–310.
- Miettunen, J., Suvisaari, J., Haukka, J., & Isohanni, M. (2011). Use of register data for psychiatric epidemiology in the Nordic countries: Textbook of psychiatric epidemiology. In M. Tsuang, M. Tohen, and P. Jones (Eds.), Textbook of psychiatric epidemiology. Wiley-Balckwell.
- Molero, Y., Larsson, H., D’Onofrio, B. M., Sharp, D. J., & Fazel, S. (2019). Associations between gabapentinoids and suicidal behaviour, unintentional overdoses, injuries, road traffic incidents, and violent crime: Population based cohort study in Sweden. BMJ (Clinical Research ed.), 365, l2147. https://doi.org/10.1136/bmj.l2147
- Peckham, A. M., Fairman, K. A., & Sclar, D. A. (2017). Prevalence of gabapentin abuse: Comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Investig, 37(8), 763–773. https://doi.org/10.1007/s40261-017-0530-3
- Peckham, A. M., Fairma, K. A., & Sclar, D. A. (2018). All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: A retrospective cohort analysis of a commercially insured US population. Drug Safety, 41(2), 213–228. https://doi.org/10.1007/s40264-017-0595-1
- Prokhorov, A. V., De Moor, C., Pallonen, U. E., Hudmon, K. S., Koehly, L., & Hu, S. (2000). Validation of the modified Fagerström tolerance questionnaire with salivary cotinine among adolescents. Addictive Behaviors, 25(3), 429–433. https://doi.org/10.1016/S0306-4603(98)00132-4
- Prokhorov, A. V., Khalil, G. E., Foster, D. W., Marani, S. K., Guindani, M., Espada, J. P., Gonzálvez, M. T., Idrisov, B., Galimov, A., Arora, M., Tewari, A., Isralowitz, R., Lapvongwatana, P., Chansatitporn, N., Chen, X., Zheng, H., & Sussman, S. (2017). Testing the nicotine dependence measure mFTQ for adolescent smokers: A multinational investigation. The American Journal on Addictions, 26(7), 689–696. https://doi.org/10.1111/ajad.12583
- Reccoppa, L., Malcolm, R., & Ware, M. (2004). Gabapentin abuse in inmates with prior history of cocaine dependence. The American Journal on Addictions, 13(3), 321–323. https://doi.org/10.1080/10550490490460300
- Schjerning, O., Pottegård, A., Damkier, P., Rosenzweig, M., & Nielsen, J. (2016). Use of pregabalin - A nationwide pharmacoepidemiological drug utilization study with focus on abuse potential. Pharmacopsychiatry, 49(4), 155–161. https://doi.org/10.1055/s-0042-101868
- Schwan, S., Sundström, A., Stjernberg, E., Hallberg, E., & Hallberg, P. (2010). A signal for an abuse liability for pregabalin-results from the Swedish spontaneous adverse drug reaction reporting system. European Journal of Clinical Pharmacology, 66(9), 947–953. https://doi.org/10.1007/s00228-010-0853-y
- Smith, R. V., Lofwall, M. R., & Havens, J. R. (2015). Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. The American Journal of Psychiatry, 172(5), 487–488. https://doi.org/10.1176/appi.ajp.2014.14101272
- Spence, D. (2013). Bad medicine: Gabapentin and pregabalin. BMJ (Clinical Research ed.), 347, f6747. https://doi.org/10.1136/bmj.f6747
- The Social Insurance Institution of Finland (KELA). (2020). Statistics on reimbursements for prescription medicines. Last modified: October 2019; Updated: October 2012. Retrieved November 8, 2020, from http://www.kela.fi/web/en/492
- WHO Collaborating Center for Drug Statistics Methodology. (2020). Retrieved November 8, 2020, from https://www.whocc.no/