Abstract
Background: Risky alcohol use is related to a variety of cognitive impairments, including memory and visuo-perceptual difficulties. Remarkably, no prior work has assessed whether usage of alcohol can predict difficulties perceiving facial identity. Objectives: Therefore, this study aimed to investigate whether riskier alcohol consumption predicted impairments in face perception and self-reported difficulties in face recognition. Results: Participants (N = 239, male = 77) were over 18 years old and had normal or corrected-to-normal vision. Alcohol use was assessed using the Alcohol Use Disorder Identification Test (AUDIT), while face recognition difficulties were determined by the 20-item Prosopagnosia Index questionnaire (PI20). A subsample of participants (N = 126, male = 51) completed the Cambridge Face Perception task (CFPT) to assess their face perception ability. Multiple linear regressions showed significant models of prediction on both face perception and face recognition when considering AUDIT score and age as predictors. Conclusion: This study suggested, for the first time, that risky alcohol use predicts both poorer visuo-perceptual processing for faces and self-reported difficulties in face recognition.
Introduction
Risky alcohol use is associated with changes in brain structures and related impairments of cognitive skills that are likely to increase the risk of dementia (Rehm et al., Citation2019). In this study, in line with the National Institute for Health and Care Excellence (NICE, 2010) definitions of hazardous and harmful drinking, risky drinking is considered that which may cause or increase the risk of harm. Different hypotheses have been proposed to describe which brain areas are more affected by alcohol consumption, such as the right hemisphere or the frontal lobes, which are thought to be the neural basis of the cognitive difficulties found in risky drinkers (Crowe et al., Citation2019; Oscar-Berman & Marinkovic, Citation2003). However, more diffuse brain atypicality may better explain the variety of neuropsychological impairments related to alcohol use and abuse, thus supporting a generalized, rather than specific, pattern of deficits in cognition (Crowe et al., Citation2019; Stavro et al., Citation2013;). In this perspective, people with disordered drinking habits show diminished functioning in global cognition, including: attention; executive functions such as verbal fluency, inhibitory control, problem solving, working memory (Nowakowska-Domagała et al., Citation2017; Nuyens et al., Citation2021; Woods et al., Citation2016), memory (Le Berre et al., Citation2017; Topiwala et al., Citation2017); and visuospatial and visuo-perceptual functions (Creupelandt et al., Citation2021).
To our knowledge, no studies have investigated whether alcohol consumption can predict impairments in facial identity processing. The Bruce and Young (Citation1986) model describes how successful face identification is a multistage process composed by an early low-level perceptual stage (apperceptive phase) followed by a recognition stage (associative phase; for a more comprehensive review of face identity processing models, see Bruce & Young, Citation1986; Haxby, Citation2002; Bernstein & Yovel, Citation2015). The perceptual processing of faces is the ability to perceive and discriminate subtle differences between faces, despite illumination/position changes (Tsao & Livingstone, Citation2008), requiring minimal demands of memory. For example, when judging whether two faces are of the same individual or not when presented at the same time. Differently, face recognition is the ability to correctly recognize people from their faces, allowing the viewer to make a judgment about whether a face has already been seen and hence whether it is stored in memory (Stantic et al., Citation2021).
Being able to properly process facial identity is a critical function in many daily contexts, from the ability to perceive and learn new faces, to recognizing familiar faces and creating social networks. Poor face identity processing may make it more complicated to create relationships both on a personal and professional level. Consequently, this may lead individuals to experience a sense of loneliness and affect their self-confidence. Additional consequences of difficulties in recognizing faces are feelings of embarrassment, guilt, and concern about offending others for not recognizing them. Moreover, poorer face identity recognition has been found to be associated with increased social anxiety which certainly impacts the quality of life (Davis et al., Citation2011; Yardley et al., Citation2008). Furthermore, the ability to accurately process facial identity has several implications for the legal system, from the management of high-level security works (e.g., police and border force officers) to the involvement in forensic settings (e.g., eyewitness testimony; Tindall et al., Citation2021; Jores et al., Citation2019). While risky drinking is associated with various cognitive deficits (Brennan et al., Citation2020; Le Berre et al., Citation2017), it is still unclear whether alcohol consumption can predict the processing of faces, and if problems do exist, whether they occur at the perceptual stage (i.e., detecting subtle differences between faces) and/or the subsequent recognition stage (i.e., memory component).
Such identity related processes are commonly related to the ability to process facial expressions (Biotti & Cook, Citation2016; Burns, Martin, et al., Citation2017), which has been reported to be dysfunctional in people with alcohol use disorder (Freeman et al., Citation2018; Hoffman et al., Citation2019; Leiker et al., Citation2019; Rupp et al., Citation2017). While it was historically believed that emotion perception dissociates from identity processing (Bruce & Young, Citation1986), modern neuropsychological (for reviews see Biotti & Cook, Citation2016; Burns, Martin, et al., Citation2017) and neuroimaging literature (Fox et al., Citation2009; Pitcher, Citation2014; Pitcher et al., Citation2008; Tsuchiya et al., Citation2008; Van den Stock et al., Citation2008) indicates emotion processing relies on neural networks that are partly involved in face identity processing. Thus, recent work has indicated that facial identity and emotion processing are not entirely independent. For example, while the fusiform face area (FFA) has been related to identity processing (Burns et al., Citation2019; Kanwisher et al., Citation1997), it may also be involved during expression processing (Bernstein & Yovel, Citation2015; Calder, Citation2011). Conversely, areas commonly related to expression recognition (e.g., Posterior superior temporal suclsus-pSTS-FA), show an activation during face identity processing (see Bernstein & Yovel, Citation2015; Calder, Citation2011; Calder & Young, Citation2005).
As a consequence of these findings, revised frameworks for face processing have been recently proposed by Duchaine and Yovel (Citation2015) in which both facial emotion and identity processes may be dependent upon shared underlying mechanisms. Therefore, if individuals with risky drinking behaviors exhibit problems in facial emotion perception (Capito et al., Citation2017; Freeman et al., Citation2018; Miller et al., Citation2015), and these deficits are due to neural abnormalities that overlap with brain regions associated with identity perception, then it seems plausible that riskier drinkers may also have difficulties when processing facial identity. We should therefore expect face perception and face recognition to be poorer in individuals with risky use of alcohol due to the damaging effects prolonged alcohol use may have on the brain.
Considering the generalized memory, visuo-perceptual, and emotion processing impairments in people with risky alcohol consumption, we expect increased alcohol use to predict difficulties in facial identity processing, consistent with the alcohol impairment hypothesis. We thus predict riskier drinkers should exhibit greater difficulties in face perception and face recognition than lighter drinkers. This study therefore aims to investigate whether riskier drinking habits predict poor face identity processing, using face perception and self-reported face recognition abilities as outcomes.
Material and method
Participants
Participants (N = 244, Male = 79, M Age = 27, SD = 11.28, Age range = 18–68) completed the experiment online via Testable (https://www.testable.org/; Rezlescu et al., Citation2020) and were reimbursed for their participation. Inclusion criteria required being over the age of 18 years-old and having normal or corrected-to-normal vision. Ethical approval for the study was provided both by Loughborough University and Edge Hill University. All participants provided informed consent before participating in the study.
Materials
The alcohol use disorders identification Test (AUDIT)
The AUDIT (Saunders et al., Citation1993) is a 10-item questionnaire created as a screening tool to measure an individual’s risk of alcohol harm by rating the frequency of alcohol-related behaviors (e.g., “How often do you have a drink containing alcohol?”; “How often during the last year have you had feeling of guilt or remorse after drinking?”). Responses to each item are rated from 0 (e.g., “never”) to 4 (e.g., “daily or almost daily”) and are summed to give a total score from 0 to 40. Total scores indicate low risk (0–7), increasing risk (8–15), higher risk (16–19), or possible dependence (≥20). The Cronbach’s alpha value in this sample was .84.
The 20-item prosopagnosia index (PI20)
The PI20 (Shah et al., Citation2015) is a standardized self-report questionnaire comprised of 20 items which aim to identify face recognition difficulties in daily life (e.g., “I often mistake people I have met before for strangers”; “I find it easy to picture individual faces in my mind”). In addition to detecting face processing difficulties in the neurotypical population, it can also identify people with developmental prosopagnosia, which is a characterized by an inability in recognizing faces (Bate et al., Citation2014; Burns, Bennetts, et al., 2017; Burns et al., Citation2014, Citation2022; Burns & Bukach, Citation2021, Citation2022; Childs et al., Citation2021; Corrow et al., Citation2016; Maw et al., Citation2023; Wilcockson et al., Citation2020). The PI20 has been shown to correlate with multiple measures of face memory, e.g., Cambridge Face Memory Task and Famous Faces Tests (Estudillo, Citation2021; Shah et al., Citation2015) supporting its validity on investigating face memory abilities, specifically on how people rate their own face recognition ability. It has recently been shown that the PI20 may be more effective than cognitive task-based approaches for detecting severe levels of face memory problems (Burns et al., Citation2022). Participants were asked to read each item and indicate how much they agree or disagree to each statement using a Likert scale from 1 (strongly disagree) to 5 (strongly agree). Scores range between 20 and 100, with high scores representing more difficulty with face recognition. The Cronbach’s alpha value in this sample was .86.
The Cambridge Face Perception Test (CFPT)
The Cambridge Face Perception Test (CFPT; Duchaine et al., Citation2007) is a computerized sorting task that assesses face perception abilities. During the task, participants were asked to order the likeness of six test faces to a target face image. On each of the 16 trials, a monochrome grayscale target face image was presented with a ¾ profile view above a line of six grayscale test faces in a random order. These pictures were made to look like the target to varying degrees of similarity, containing 88%, 76%, 64%, 52%, 40%, and 28% of the target face, respectively (see ). In eight trials all the targets and test images were presented upright, while in the other eight trials they were inverted (i.e., upside-down). Upright and inverted trials were intermixed and each trial lasted 60 s.
Figure 1. Images from an item in the Cambridge face perception Test. The numbers below each stimulus face is the percentage of the similarity with the target face above.
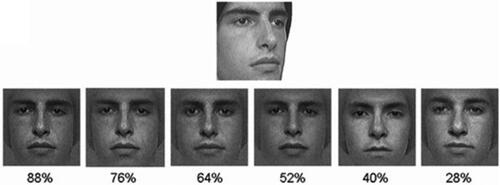
To complete the task, participants sorted the test faces by clicking on them and moving them to the desired location in order from the most resembling, to the least resembling, the target face. The score is calculated by summing the errors, that is the deviations from the correct order (e.g., if a face was two places away from its proper position, it added 2 to the score). Therefore, the higher the score, the poorer the performance. Upright and inverted scores are reported separately. Two practice trials (one upright and one inverted) were presented at the beginning to familiarize participants with the task. The Cronbach’s alpha values in this sample were .80 for CFPT for the upright faces and .67 for CFPT for the inverted faces.
Procedure
Participants were asked to read and agree to the informed consent form to participate in the study. Two hundred and forty-four participants were asked to estimate how many units of alcohol they consumed per week (one unit was equivalent to half pint, a small glass of wine, or a small spirit) and complete the AUDIT and the PI20. During data collection, the CFPT was later included so we had an objective measure of face processing abilities. Therefore, a subset of participants also completed the CFPT (N = 130) in order to obtain additional face perception information.
Results
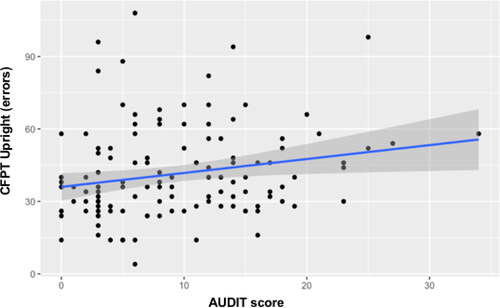
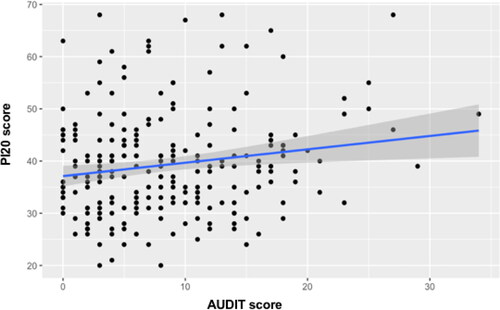
A priori power analysis using G*Power (Faul et al., Citation2007) for linear multiple regression, using a medium effect size (f2= .15), power set at 0.80, and alpha at 0.05, yielded a recommended total sample size of 68. Five out of 244 participants were removed from the analysis due to failures on the engagement trials which were provided to ensure individuals were properly paying attention when reading instructions throughout the tasks. Therefore, the final sample size consisted of 239 participants who completed the PI20 and a subsample of 126 participants who also completed the CFPT (see for descriptives of the sample). Three multiple linear regressions were performed to investigate whether a riskier alcohol consumption predicted difficulties in face recognition (i.e., PI20; see ), face perception of orthodox faces (CFPT for upright faces; see ), and face perception of inverted faces (CFPT for inverted faces; see ). RStudio (v. 1.4.1106) was used to run the analyses and create the graphs.
Table 1. Descriptive statistics for alcohol consumption variables (units per week; alcohol use disorders identification test (AUDIT)), 20-item prosopagnosia test (PI20), and Cambridge face perception Test (CFPT).
Table 2. Regression coefficients of alcohol consumption (AUDIT) and age on face recognition (PI20).
Table 3. Regression coefficients of alcohol consumption (AUDIT) and age on face perception (CFPT upright, CFPT inverted).
Given that face processing abilities have been reported to decline as people age (Konar et al., Citation2013), two predictors of Age and AUDIT scores were included in the analyses. The first regression considered the PI20 scores as dependent variable. The model explained 7.4% of the variation in PI20 scores with both predictors making a significant positive contribution. However, alcohol consumption seemed to be a slightly stronger predictor (see ). A regression was then conducted replacing the PI20 with the CFPT upright (errors) as dependent variable. This model was also significant, accounting for 4.8% of the variation in CFPT errors. The standardized Betas of Age and AUDIT scores seemed roughly similar in accounting for the model, albeit with neither predictor appearing significant (see ). Finally, a regression was performed considering the CFPT inverted (errors) as the dependent variable while age and AUDIT score stayed as predictors. Unlike the previous regressions, this model was not significant with only 0.6% of the variance explained (see ).
In summary, these results showed how riskier alcohol consumption (i.e., AUDIT) and age predicted poorer face recognition (i.e., PI20, see ) and face perception of orthodox faces (CFPT for upright faces, see ), but not face perception of inverted faces.
Discussion
The current study investigated whether riskier drinking habits can predict face perception deficits (CFPT) and self-reported face recognition difficulties (PI20). First, supporting our hypothesis, riskier drinking behavior caused an increasing difficulty in perceiving upright facial identity. Second, general difficulties with face recognition (i.e., the PI20) were also predicted by alcohol consumption, with risky drinkers reporting more face-related complaints. While causality cannot be established, these results are consistent with risky drinking causing changes in the initial phases of facial identity perception (i.e., apperceptive difficulties) and the associative phase (e.g., recollecting a person’s identity; Bruce & Young, Citation1986). As we found more face perception difficulties and daily troubles with faces in risky drinkers, these two stages may be similarly affected. Although age has been reported to influence the decline of face identity processing (Konar et al., Citation2013, Obermeyer et al., Citation2012), our results showed that alcohol consumption (AUDIT score) represents the strongest contribution in our model to predict face recognition. However, both age and AUDIT scores seem to have similar contributions to predict face perception when both are taken into account resulting in a significant model.
Moreover, the effect that alcohol has in subordinate processing (e.g., psychomotor speed, attention, and executive functions) might have influenced the relationship between alcohol consumption and face perception (Burns & Wilcockson, Citation2019). However, if that was the case, we should have seen a significant prediction of risky drinking habits on the inverted CFPT scores given that these processes are similarly involved in the perception of upright and inverted images. Thus, it seems intuitive to assume that riskier drinking impacts face perception abilities, which in turn causes self-reported problems with daily life. However, even if subordinate processes such as attention are causing some of these effects, particularly the link between consumption and the CFPT, it does not change the fact the outcomes are the same, i.e., risky drinkers exhibit problems with face perception and self-reported problems in daily life. Future studies may wish to explore the potential role that alterations in attention are having in driving these effects.
Prior work has found many different predictors for face processing abilities. For example, individuals who are extraverted appear to exhibit superior face recognition skills (Lander & Poyarekar, Citation2015; Li et al., Citation2010). Similarly, people with lower levels of autistic traits (i.e., who take a greater interest in social information) also seem better at recognizing faces (Halliday et al., Citation2014; although see Rhodes et al., Citation2013). Moreover, those who grew up in larger cities also seem more skilled when judging facial identity (Balas & Saville, Citation2017; Sunday et al., Citation2019). Here, we add to this literature of face processing predictors by showing greater levels of risky drinking predict poorer face perception skills and more prevalent symptoms of prosopagnosia. This may have a negative impact on the general quality of life increasing feelings of loneliness and social anxiety which, in turn, may lead to an unproductive increment of drinking as a coping strategy (Villarosa-Hurlocker et al., Citation2019) resulting in an unhealthy vicious circle. In this study, an objectively validated self-report index for face recognition (i.e., PI20) and an experimental task for face perception (i.e., CFPT) were used to reduce fatigue and distractions in participants who completed the study online. However, future studies could use additional experimental tasks to investigate further aspects of face identity processing in more detail (e.g., CFMT-Cambridge Face Memory Task, Duchaine & Nakayama, Citation2006; GFMT-Glasgow Face Matching Task, Burton et al., Citation2010; UNSW Face Test, Dunn et al., Citation2020). Similarly, it would be beneficial to know if facial identity and emotion related problems in heavier drinkers extend into difficulties perceiving other face traits, such as attractiveness (Burns et al., Citation2021; Ying et al., Citation2019; Citation2020) or trustworthiness (Krumhuber et al., Citation2007; Todorov et al., Citation2008; Wilson & Rule, Citation2015).
In conclusion, our findings suggested that risky use of alcohol predicts both poorer visuo-perceptual processing for faces and difficulties in recognizing faces in daily life. Our results provide partial support for the alcohol impairment hypothesis which states risky alcohol use is related to deficits in a broad range of cognitive processes. These outcomes might also have important implications in many security and forensic situations such as the reliability of eyewitness testimony or the ability to identify faces by law enforcement policymakers. In the view of the legal system’s assumption, previous studies mostly investigated the effect of acute intoxication reporting reduced completeness and impaired quality of testimony in intoxicated eyewitness or victims (Altman et al., Citation2018; Attwood et al., Citation2015; Jores et al., Citation2019; La Rooy et al., Citation2013). However, this study suggests that riskier drinkers’ performance might be negatively affected in identifying faces regardless of their present status of intoxication. To our knowledge, this is the first time that face identity processing has been explored in relation to alcohol consumption in a non-addicted sample of drinkers. Additional studies are needed to more fully understand the nature of these impairments and their consequences.
Ethical approval
This study has been conducted in accordance with the Declaration of Helsinki. Ethical approval for the study was provided both by Loughborough University and Edge Hill University.
Informed consent
All participants provided informed consent before participating in the study.
CRediT authorship contribution statement
Denise Dal Lago: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Writing-Original draft preparation, Writing-Review and Editing, Visualization, Project administration. Edwin Burns: Conceptualization, Writing-Review and Editing, Supervision, Funding acquisition. Elizabeth Gaunt: Investigation. Emma Peers: Investigation. Robin C. Jackson: Writing-Review and Editing, Supervision. Thomas D. W. Wilcockson: Conceptualization, Writing-Review and Editing, Supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Altman, C. M., Schreiber Compo, N., McQuiston, D., Hagsand, A. V., & Cervera, J. (2018). Witnesses’ memory for events and faces under elevated levels of intoxication. Memory, 26(7), 946–959. https://doi.org/10.1080/09658211.2018.1445758
- Attwood, A. S., Catling, J. C., Kwong, A. S. F., & Munafò, M. R. (2015). Effect pf 7.5.% carbon dioxide (CO2) inhalationand ethnicity on face memory. Physiology & Behavior, 147, 97–101. https://doi.org/10.1016/j.physbeh.2015.04.027
- Balas, B., & Saville, A. (2017). Hometownsize affects the processing of naturalistic face variability. Vision Research, 141, 228–236. https://doi.org/10.1016/j.visres.2016.12.005
- Bate, S., Cook, S. J., Duchaine, B., Tree, J. J., Burns, E. J., & Hodgson, T. L. (2014). Intranasal inhalation of oxytocin improves face processing in developmental prosopagnosia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 50, 55–63. https://doi.org/10.1016/j.cortex.2013.08.006
- Bernstein, M., & Yovel, G. (2015). Two neural pathways of face processing: A critical evaluation of current models. Neuroscience and Biobehavioral Reviews, 55, 536–546. https://doi.org/10.1016/j.neurbiorev.2015.06.010
- Biotti, F., & Cook, R. (2016). Impaired perception of facial emotion in developmental prosopagnosia. Cortex, 81, 126–136. https://doi.org/10.1016/j.cortex.2016.04.008
- Brennan, S., McDonald, S., Page, M., Reid, J., Ward, S., Forbes, A., & McKenzie, J. (2020). Long-term effects of alcohol consumption on cognitive function: A systematic review and dose-response analysis of evidence published between 2007 and 2018. Systematic Reviews, 9(1), 33. https://doi.org/10.1186/s13643-019-1220-4
- Bruce, V., & Young, A. (1986). Understanding face recognition. British Journal of Psychology, 77(Pt 3), 305–327. https://doi.org/10.1111/j.2044-8295.1986.tb02199.x
- Burns, E. J., & Bukach, C. M. (2021). Face processing predicts reading ability: Evidence from prosopagnosia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 145, 67–78. https://doi.org/10.1016/j.cortex.2021.03.039
- Burns, E. J., & Bukach, C. M. (2022). Face processing still predicts reading ability: Evidence from developmental prosopagnosia. A reply to Gerlach and Starrfelt (2022). Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 154, 340–347. https://doi.org/10.1016/j.cortex.2022.06.008
- Burns, E. J., & Wilcockson, T. D. (2019). Alcohol usage predicts holistic perception: A novel method for exploring addiction. Addictive Behaviors, 99, 106000. https://doi.org/10.1016/j.addbeh.2019.05.024
- Burns, E. J., Arnold, T., & Bukach, C. M. (2019). P-curving the fusiform face area: Meta-analyses support the expertise hypothesis. Neuroscience and Biobehavioral Reviews, 104, 209–221. https://doi.org/10.1016/j.neubiorev.2019.07.003
- Burns, E. J., Bennetts, R. J., Bate, S., Wright, V. C., Weidemann, C. T., & Tree, J. J. (2017b). Intact word processing in developmental prosopagnosia. Scientific Reports, 7(1), 1683. https://doi.org/10.1038/s41598-017-01917-8
- Burns, E. J., Gaunt, E., Kidane, B., Hunter, L., & Pulford, J. (2022). A new approach to diagnosing and researching developmental prosopagnosia: Excluded cases are impaired too. Behavior Research Methods, 1–24. https://doi.org/10.3758/s13428-022-02017-w
- Burns, E. J., Martin, J., Chan, A. H., & Xu, H. (2017). Impaired processing of facial happiness, with or without awareness, in developmental prosopagnosia. Neuropsychologia, 102, 217–228. https://doi.org/10.1016/jneuropsychologia.2017.06.020
- Burns, E. J., Tree, J. J., & Weidemann, C. T. (2014). Recognition memory in developmental prosopagnosia: Electrophysiological evidence for abnormal routes to face recognition. Frontiers in Human Neuroscience, 8, 622. https://doi.org/10.3389/fnhum.2014.00622
- Burns, E. J., Yang, W., & Ying, H. (2021). Friend effects framework: Contrastive and hierarchical processing in cheerleader effects. Cognition, 212, 104715. https://doi.org/10.1016/j.cognition.2021.104715
- Burton, A. M., White, D., & McNeil, A. (2010). The Glasgow Face Matching Test. Behavior Research Methods, 42(1), 286–291. https://doi.org/10.3758/brm.42.1.286
- Calder, A. J., others. (2011). Does facial identity and facial expression recognition involve separate visual routes? In Andrew J. Calder (Ed.), Oxford handbook of face perception. Oxford Library of Psychology.
- Calder, A. J., & Young, A. W. (2005). Understanding the recognition of facial identity and facial expression. Nature Reviews. Neuroscience, 6(8), 641–651. https://doi.org/10.1038/nrn1724
- Capito, E. S., Lautenbacher, S., & Horn-Hofmann, C. (2017). Acute alcohol effects on facial expressions of emotions in social drinkers: A systematic review. Psychology Research and Behavior Management, 10, 369–385. https://doi.org/10.2147/PRBM.S146918
- Childs, M. J., Jones, A., Thwaites, P., Zdravković, S., Thorley, C., Suzuki, A., Shen, R., Ding, Q., Burns, E., Xu, H., & Tree, J. J. (2021). Do individual differences in face recognition ability moderate the other ethnicity effect? Journal of Experimental Psychology. Human Perception and Performance, 47(7), 893–907. https://doi.org/10.1037/xhp0000762
- Corrow, S. L., Dalrymple, K. A., & Barton, J. J. S. (2016). Prosopagnosia: Current perspective. Eye and Brain, 8, 165–175. https://doi.org/10.2147/EB.S92838
- Creupelandt, C., Maurage, P., & DˈHondt, F. (2021). Visuoperceptive impairments in severe alcohol use disorders: A critical review of behavioural studies. Neuropsychology Review, 31(3), 361–384. https://doi.org/10.1007/s11065-020-09469
- Crowe, S., Cammisuli, D., & Stranks, E. (2019). Widespread cognitive deficits in alcoholism persistent following prolonged abstinence: An updated meta-analysis of studies that used standardised neuropsychological assessment tools. Archives of Clinical Neuropsychology, 35(1), 31–45. https://doi.org/10.1093/arclin/acy106
- Davis, J. M., McKone, E., Dennett, H., O’Connor, K. B., O’Kearney, R., & Palermo, R. (2011). Individual differences in the ability to recognise facial identity are associated with social anxiety. PloS One, 6(12), e28800. https://doi.org/10.1371/journal.pone.0028800
- Duchaine, B., & Nakayama, K. (2006). The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia, 44(4), 576–585. https://doi.org/10.1016/j.neuropsychologia.2005.07.001
- Duchaine, B., & Yovel, G. (2015). A revised neural framework for face processing. Annual Review of Vision Science, 1, 393–416. https://doi.org/10.1146/annurev-vision-082114-035518
- Duchaine, B., Germine, L., & Nakayama, K. (2007). Family resemblance: Ten family members with propopagnosia and within-class object agnosia. Cognitive Neuropsychology, 24(4), 419–430. https://doi.org/10.1080/02643290701380491
- Dunn, J. D., Summersby, S., Towler, A., Davis, J. P., & White, D. (2020). UNSW Face Test: A screening tool for super-recognizers. PloS One, 15(11), e0241747. https://doi.org/10.1371/journal.pone.0241747
- Estudillo, A. J. (2021). Self-reported face recognition abilities for own and other-race faces. Journal of Criminal Psychology, 11(2), 105–115. https://doi.org/10.1108/JCP-06-2020-0025
- Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioural, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146
- Fox, C. J., Moon, S. Y., Iaria, G., & Barton, J. J. (2009). The correlates of subiective perception of identity and expression in the face network: An fMRI adaptation study. NeuroImage, 44(2), 569–580. https://doi.org/10.1016/j.neuroimage.2008.09.011
- Freeman, C. R., Wiers, C. E., Sloan, M. E., Zehra, A., Ramirez, V., Wang, G. J., & Volkow, N. D. (2018). Emotion recognition biases in alcohol use disorder. Alcoholism: Clinical and Experimental Research, 42(8), 1541–1547. https://doi.org/10.1111/acer.13802
- Halliday, D. W. R., MacDonald, S. W. S., Scherf, K. S., & Tanaka, J. W. (2014). A reciprocal model of face recognition and autistic traits: Evidence from an individual differences perspective. PloS One, 9(5), e94013. https://doi.org/10.1371/journal.pone.0094013
- Haxby, J. V., Hoffman, E. A., & Gobbini, M. I. (2002). Human neural systems for face recognition and social communication. Society of Biological Psychiatry, 51(1), 59–67. https://doi.org/10.1016/S0006-3223(01)01330-0
- Hoffman, L., Lewis, B., & Nixon, S. (2019). Neurophysiological and interpersonal correlates of emotional face processing in alcohol use disorder. Alcoholism, Clinical and Experimental Research, 43(9), 1928–1936. https://doi.org/10.1111/acer.14152
- Jores, T., Collof, M. F., Kloft, L., Smailes, H., & Flowe, H. D. (2019). A meta-analysis of the effects of acute alcohol intoxication on witness recall. Applied Cognitive Psychology, 33(3), 334–343. https://doi.org/10.1002/acp.3533
- Kanwisher, N., McDermott, J., & Chun, M. M. (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience, 17(11), 4302–4311. https://doi.org/10.1523/JNEUROSCI.17-11-04302.1997
- Konar, Y., Bennett, P. J., & Sekuler, A. B. (2013). Effects of aging on face identification and holistic face processing. Vision Research, 88, 38–46. https://doi.org/10.1016/j.visres.2013.06.003
- Krumhuber, E., Manstead, A. S., Cosker, D., Marshall, D., Rosin, P. L., & Kappas, A. (2007). Facial dynamics as indicators of trustworthiness and cooperative behavior. Emotion, 7(4), 730–735. https://doi.org/10.1037/1528-3542.7.4.730
- La Rooy, D., Nicol, A., & Terry, P. (2013). Intoxicated eyewitnesses: The effect of alcohol on eyewitness recall across repeated interviews. Open Journal of Medical Psychology, 02(03), 107–114. https://doi.org/10.4236/ojmp.2013.23017
- Lander, K., & Poyarekar, S. (2015). Famous face recognition, face matching, and extraversion. Quarterly Journal of Experimental Psychology, 68(9), 1769–1776. https://doi.org/10.1080/17470218.2014.988737
- Le Berre, A., Fama, R., & Sullivan, E. (2017). Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: A critical review to inform future research. Alcoholism, Clinical and Experimental Research, 41(8), 1432–1443. https://doi.org/10.1111/acer.13431
- Leiker, E., Meffert, H., Thornton, L., Taylor, B. K., Aloi, J., Abdel-Rahim, H., Shah, N., Tyler, P. M., White, S. F., Blair, K. S., Filbey, F., Pope, K., Dobbertin, M., & Blair, R. J. R. (2019). Alcohol use disorder and cannabis use disorder symptomatology in adolescents are differentially related to dysfunction in brain regions supporting face processing. Psychiatry Research. Neuroimaging, 292, 62–71. https://doi.org/10.1016/j.pscychresns.2019.09.004
- Li, J., Tian, M., Fang, H., Xu, M., Li, H., & Liu, L. (2010). Extraversion predicts individual differences in face recognition. Communicative & Integrative Biology, 3(4), 295–298. https://doi.org/10.4161/cib.3.4.12093
- Maw, K., Burns, E., & Beattie, G. (2023). Prosopagnosia is highly comorbid in developmental coordination disorder (DCD). PsyArXiv
- Miller, M. A., Bershad, A. K., & de Wit, H. (2015). Drug effects on responses to emotional facial expressions: Recent findings. Behavioural Pharmacology, 26(6), 571–579. https://doi.org/10.1097/FBP.0000000000000164
- National Institute for Health and Care Excellence. (2010). Alcohol-use disorders: Diagnosis and management of physical complications [NICE Guideline No.CG100]. https://www.nice.org.uk/guidance/cg100
- Nowakowska-Domagała, K., Jabłkowska-Górecka, K., Mokros, Ł., Koprowicz, J., & Pietras, T. (2017). Differences in the verbal fluency, working memory and executive functions in alcoholics: Short-term vs. long-term abstainers. Psychiatry Research, 249, 1–8. https://doi.org/10.1016/j.psychres.2016.12.034
- Nuyens, F., Billieux, J., & Maurage, P. (2021). Time perception and alcohol use: A systematic review. Neuroscience and Biobehavioral Reviews, 127, 377–403. https://doi.org/10.1016/j.neubiorev.2021.04.027
- Obermeyer, S., Kolling, T., Schaich, A., & Knopf, M. (2012). Differences between old and young adults’ ability to recognize human faces underlie processing of horizontal information. Frontiers in Aging Neuroscience, 4, 3. https://doi.org/10.3389/fnagi.2012.00003
- Oscar-Berman, M., & Marinkovic, K. (2003). Alcoholism and the brain: An overview. Alcohol Research and Health, 27, 125–133.
- Pitcher, D. (2014). Facial expression recognition takes longer in the posterior superior temporal sulcus than in the occipital face area. The Journal of Neuroscience, 34(27), 9173–9177. https://doi.org/10.1523/JNEUROSCI.5038-13.2014
- Pitcher, D., Garrido, L., Walsh, V., & Duchaine, B. C. (2008). Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. The Journal of Neuroscience, 28(36), 8929–8933. https://doi.org/10.1523/JNEUROSCI.1450-08.2008
- Rehm, J., Hasan, O., Black, S., Shield, K., & Schwarzinger, M. (2019). Alcohol use and dementia: A systematic scoping review. Alzheimer’s Research & Therapy, 11(1), 1. https://doi.org/10.1186/s13195-018-0453-0
- Rezlescu, C., Danaila, I., Miron, A., & Amariei, C. (2020). More time for science: Using Testable to create and share behavioural experiments faster, recruit better participants, and engage students in hands-on research. Progress in Brain Research, 253, 243–262. https://doi.org/10.1016/bs.pbr.2020.06.005
- Rhodes, G., Jeffery, L., Taylor, L., & Ewing, L. (2013). Autistic traits are likely to reduced adaptive coding of face identity and selectively poorer face recognition in men but not women. Neuropsychologia, 51(13), 2702–2708. https://doi.org/10.1016/j.neuropsychologia.2013.08.016
- Rupp, C. I., Derntl, B., Osthaus, F., Kemmler, G., & Fleischhacker, W. W. (2017). Impact of social cognition on alcohol dependence treatment outcome: Poorer facial emotion recognition predicts relapse/dropout. Alcoholism, Clinical and Experimental Research, 41(12), 2197–2206. https://doi.org/10.1111/acer.13522
- Saunders, J. B., Aasland, O. G., Babor, T. F., Fuente, J. R., & Grant, M. (1993). Development of the Alcohol use Disorders identification Test (AUDIT): WHO Collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction, 88(6), 791–804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x
- Shah, P., Gaule, A., Sowden, S., Bird, G., & Cook, R. (2015). The 20-Item Prosopagnosia Index (PI20): A self-report instrument for identifying developmental prosopagnosia. Royal Society Open Science, 2(6), 140343. https://doi.org/10.1098/rsos.140343
- Stantic, M., Hearne, B., Catmur, C., & Geoffrey, B. (2021). Use of the Oxford Face Matching Test reveals an effect of aging on face perception but not face memory. Cortex, 145, 226–235. https://doi.org/10.1016/j.cortx.2021.08.016
- Stavro, K., Pelletier, J., & Potvin, S. (2013). Widespread and sustained cognitive deficits in alcoholism: A meta-analysis. Addiction Biology, 18(2), 203–213. https://doi.org/10.1111/j.1369-1600.2011.00418.x
- Sunday, M. A., Patel, P. A., Dodd, M. D., & Gauthier, I. (2019). Gender and hometown population density interact to predict face recognition ability. Vision Research, 163, 14–23. https://doi.org/10.1016/j.visres.2019.08.006
- Tindall, I. K., Curtis, G. J., & Locke, V. (2021). Can anxiety and race interact to influence face recognition accuracy? A systematic review. PloS One, 16(8), e0254477. https://doi.org/10.1371/journal.pone.0254477
- Todorov, A., Baron, S. G., & Oosterhof, N. N. (2008). Evaluating face trustworthiness: A model based approach. Social Cognitive and Affective Neuroscience, 3(2), 119–127. https://doi.org/10.1093/scan/nsn009
- Topiwala, A., Allan, C., Valkanova, V., Zsoldos, E., Filippini, N., Sexton, C., Mahmood, A., Fooks, P., Singh-Manoux, A., Mackay, C. E., Kivimaki, M., & Ebmeier, K. P. (2017). Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ (Clinical Research ed.), 357, j2353. https://doi.org/10.1136/bmj.j2353
- Tsao, D. Y., & Livingstone, M. S. (2008). Mechanisms of face perception. Annual Review of Neuroscience, 31, 411–437. https://doi.org/10.1146/annurev.neuro.30.051606.094238
- Tsuchiya, N., Kawasaki, H., Oya, H., Howard, M. A., & Adolphs, R. (2008). Decoding face information in time, frequency and space from direct intracranial recordings of the human brain. PLoS One, 3(12), e3892. https://doi.org/10.1371/journal.pone.0003892
- Van den Stock, J., Van de Riet, W., Righart, R., & de Gelder, B. (2008). Neural correlates of perceiving emotional faces and bodies in developmental prosopagnosia: An event-related fMRI-study. PloS One, 3(9), e3195-e3195. (https://doi.org/10.1371/journal.pone.0003195
- Villarosa-Hurlocker, M. C., Bravo, A. J., & Pearson, M. R. (2019). The relationship between social anxiety and alcohol and marijuana use outcomes among concurrent users: A motivational model of substance use. Alcoholism, Clinical and Experimental Research, 43(4), 732–740. https://doi.org/10.111/acer.13966
- Wilcockson, T. D., Burns, E. J., Xia, B., Tree, J., & Crawford, T. J. (2020). Atypically heterogeneous vertical first fixations to faces in a case series of people with developmental prosopagnosia. Visual Cognition, 28(4), 311–323. https://doi.org/10.1080/13506285.2020.1797968
- Wilson, J. P., & Rule, N. O. (2015). Facial trustworthiness predicts extreme criminal-sentencing outcomes. Psychological Science, 26(8), 1325–1331. https://doi.org/10.1177/0956797615590992
- Woods, A., Porges, E., Bryant, V., Seider, T., Gongvatana, A., Kahler, C., de la Monte, S., Monti, P. M., & Cohen, R. A. (2016). Current heavy alcohol consumption is associated with greater cognitive impairment in older adults. Alcoholism, Clinical and Experimental Research, 40(11), 2435–2444. https://doi.org/10.1111/acer.13211
- Yardley, L., McDermott, L., Pisarski, S., Duchaine, B., & Nakayama, K. (2008). Psychosocial consequences of developmental prosopagnosia: A problem of recognition. Journal of Psychosomatic Research, 65(5), 445–451. https://doi.org/10.1016/j.psychores.2008.03.013
- Ying, H., Burns, E. J., Choo, A. M., & Xu, H. (2020). Temporal and spatial ensemble statistics are formed by distinct mechanisms. Cognition, 195, 104128. https://doi.org/10.1016/j.cognition.2019.104128
- Ying, H., Burns, E., Lin, X., & Xu, H. (2019). Ensemble statistics shape face adaptation and the cheerleader effect. Journal of Experimental Psychology. General, 148(3), 421–436. https://doi.org/10.1037/xge0000564