Abstract
Background
Upper limb (UL) impairment-related functional limitation is a frequent post-stroke consequence, potentially negatively impacting health-related quality of life (HRQoL). Significant debate surrounds optimal approach to UL stroke rehabilitation. Task-oriented rehabilitation is recommended to improve UL performance, however despite the motor relearning program (MRP) being a task-oriented approach widely utilized in stroke rehabilitation, there has been insufficient appraisal on its specific effectiveness. This review therefore aimed to review evidence pertaining to the effectiveness of MRP on UL function post-stroke.
Objective
A systematized review on MRP effectiveness on post-stroke UL function in adults aged 18 years and above, also considering UL impairment and HRQoL.
Methods
A search of AMED, CINAHL, MEDLINE, CENTRAL and PEDro was conducted, accompanied by examination of individual study citations. Only full text English language randomized controlled trials (RCTs) were included. The methodological quality of each included study was rated using PEDro.
Results
Of 721 articles identified, 10 met analysis criteria. There was moderate evidence that MRP, as an individual approach, improved UL function post-stroke. Limited evidence was obtained for positive effect of MRP combined with adjunctive therapies on UL function and impairment. Evidence was lacking regarding the relationship of MRP and HRQoL.
Conclusion
There is inadequate evidence on the supremacy of the MRP in comparison to other interventions in improving UL function post-stroke. The inclusion of the MRP into rehabilitation shows some evidence for UL function and impairment improvements. Methodological flaws have been identified within the RCTs reviewed, warranting further high-quality RCTs.
Keywords:
Introduction
Whilst two-thirds of stroke survivors resume walking independently [Citation1], about half will not regain adequate upper limb (UL) function [Citation2]. Loss of UL function has been reported by patients as the most afflicting consequence of stroke. Patients with severe UL impairment are less likely to regain functional independence, impacting health-related quality of life (HRQoL) [Citation3]. Low HRQoL is a frequent occurrence post-stroke, predominantly in people dependent on support with their activities of daily living (ADLs) [Citation4].
Early rehabilitative care, within three months post-stroke, is vital to capitalize on spontaneous recovery [Citation5]. Physiotherapy can influence this motor recovery [Citation6] through neuroplasticity, which is learning dependent [Citation7], and can be influenced through task-specific training (TST) [Citation8] to facilitate return to ADLs. TST is closely linked to a widely utilized approach in stroke rehabilitation, namely the motor relearning program (MRP) [Citation9] as it is a key component of the approach [Citation10].
French et al. [Citation11] found repetitive task training (RTT) improved UL and lower limb (LL) function. However, there was a lack of assurance in the findings as quality of evidence was rated as low. Urton et al. [Citation12] examined TST, through reach-to-grasp training, and classified findings as discretionary according to Sackett’s level of evidence. Again, methodological quality was rated poor, making it difficult to extract valid conclusions [Citation13]. Furthermore, a randomized controlled trial (RCT) discovered that intensive RTT, compared with the equivalent dose of occupational therapy (OT) and routine OT, did not significantly enhance UL recovery or motor function 12 months post-stroke [Citation14].
Pollock et al. [Citation15] conducted a Cochrane review to establish the effectiveness of physical rehabilitation interventions in recovery of mobility and function post-stroke, and to compare the efficacy of each intervention. Pollock et al. [Citation15] concluded that physical rehabilitation is effective for improving mobility and function post-stroke, but no individual physical rehabilitative therapy is any more efficient than the other. Similarly, a Cochrane overview of systematic reviews, that synthesized systematic reviews of approaches provided to promote UL function post-stroke, established that high-quality evidence for intervention supremacy is deficient [Citation13]. Unlike Pollock et al. [Citation13], findings from Pollock et al. [Citation15] were not applicable to UL rehabilitation. The exclusion of UL recovery is surprising as UL hemiparesis is the most frequently occurring deficit post-stroke, with greater than 40% of stroke survivors encountering this condition chronically and more than 80% acutely [Citation16].
As comparison to Pollock et al. [Citation15] who included the whole MRP approach into their investigation, Pollock et al. [Citation13] - like French et al. [Citation11] - focused on a component part, namely the TST and RTT. With inconsistent findings regarding RTT and TST, it can be argued that the approach task-specific rehabilitation originating from the MRP [Citation17] should be reconsidered. Whilst the chief focus of French et al. [Citation11] was to analyze the efficiency of RTT, trials were investigated whereby the whole MRP approach was used if the amount of task practice was identifiable. One trial was incorporated into the review that observed the MRP, accounting for 3% of the total studies included. Such reporting does not enable the reader to obtain evidence regarding the effects of the whole MRP approach post-stroke. Only utilizing specific components of the MRP rather than the whole approach may deprive the patient of other additional MRP benefits, such as experience-dependent plasticity achieved through repetitive functional performance and deemed advantageous through cortical reorganization [Citation7], as opposed to repetitive performance of insignificant tasks [Citation18].
Through searching, the lack of appraisal and synthesis of RCTs investigating the effectiveness of the MRP on UL function was apparent. The primary aim of this systematized review was to evaluate the current evidence base to establish the effectiveness of the MRP on UL function in adults post-stroke. The subsidiary aim was to evaluate the effectiveness of the MRP on UL impairment and HRQoL in adults post-stroke.
Methods
To lessen the possibility of bias the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist was employed [Citation19].
Search strategy
An electronic search was conducted on the 13th of March 2020 within the following databases: AMED, CINAHL, MEDLINE via EBSCOhost, CENTRAL and PEDro.
A preliminary exploratory search was conducted within the Cochrane Library to identify relevant search terms. The search terms were associated with stroke, MRP, UL and UL function. In accordance with Farrugia et al. [Citation20], the researcher utilized the primary aim as the focus of the review, therefore, UL function was the outcome of interest within this search. The search terms were inputted into each database in diverse combinations, modelling Paltridge and Traves [Citation21]. The search strategy used within each database can be found in Supplementary Appendix A. No date restriction was placed on the search.
Eligibility criteria
Studies in this systematized review were restricted to RCTs for which full text English language reports were available. Participants had to be aged 18 years and above with a clinical stroke diagnosis. RCTs were sought that investigated the MRP, as an individual approach, with adjunctive therapy, or if an intervention - according to the authors of the RCTs - was based upon MRP principles. The primary outcome was UL function. Secondary outcomes included HRQoL and UL impairment. Studies were only included if they contained a significant focus on UL activity.
Study selection
One researcher retrieved all studies obtained from the search. Copies of all searches were combined together to eliminate duplicates. The remaining studies were evaluated for eligibility based on title and abstract. The full text of each remaining study was reviewed in detail. Selection of studies that met criteria occurred and any studies that did not satisfy criteria were omitted. The reference list of each included study was reviewed.
Data extraction
One researcher operated data extraction. Extracted data included citation details, study design, quantity of participants, gender ratio and age. Also included were lesion type, stroke phase and duration since stroke. Eligibility criteria, intervention details, relevant outcome measures (OMs) and results were also extracted.
Intervention reporting
The Template for Intervention Description and Replication checklist, as per Hoffman et al. [Citation22], was utilized to define the level of intervention reporting within the trials, in relation to the MRP.
Methodological quality
The PEDro scale was utilized to examine each study’s methodological quality. The PEDro scale comprises 11 items concerning external and internal validity along with statistical reporting [Citation23]. Corresponding to Díaz-Arribas et al. [Citation24], a study within this systematized review with a PEDro score of 6 or more was deemed high-quality and a score of 5 or less was deemed low-quality. There was no cut-off point for minimum score.
Data analysis
A narrative synthesis was executed to synthesize the results focused on the participants, intervention, OMs, statistical findings and methodological quality of each included study. To categorize strength of evidence, the rating method used by Challoumas et al. [Citation25], was applied within this systematized review ().
Table 1. Level of evidence with description (data from challoumas et al. [25]).
Results
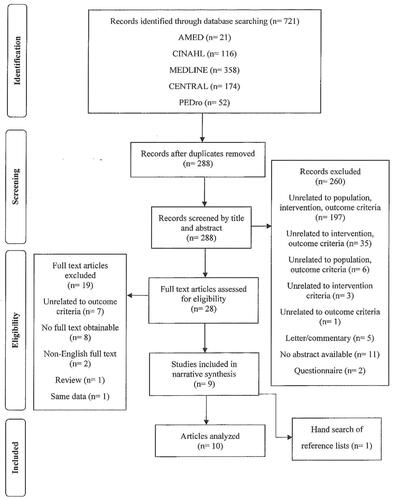
Study selection
From a total of 721 studies that were initially identified, after exclusion of duplicate studies, title and abstract screening, full text examination and reference list screening, 10 studies were found to fulfil the eligibility criteria (). Data from two articles obtained from the literature search originated from one study. Langhammer and Stanghelle [Citation26] conducted a follow-up study on the same participants from their earlier study [Citation27]. Both articles were still cited together in this review.
Participants
A total of 421 participants were included in this systematized review. Only one study did not report the quantity of females and males [Citation28]. The overall population from the remainder of the included studies was 361 and 62.05% were males.
Only five studies specified the number of strokes the participants had encountered [Citation26, Citation27, Citation29–32], with these all being first-time strokes. Six studies, totaling 194 participants, specified lesion type [Citation29–34]. Ischemic stroke occurred in 80.9% of the population in the aforementioned six studies, whilst 19.1% accounted for hemorrhagic strokes. Participant details can be seen in .
Table 2. Participant characteristics from each included study.
Langhammer and Stanghelle [Citation26, Citation27], El-Bahrawy and El-Wishy [Citation29] and Paul [Citation34] reported location of stroke lesion. From a total of 121 participants in these studies, the right side of the brain was most afflicted, accounting for 57.9% of participants. Hesse et al. [Citation30], Pandian et al. [Citation33] and Jan et al. [Citation35] reported hemiplegic side. With a total of 146 participants from these three studies, left and right paretic side accounted for 50.68% and 49.32%, respectively.
Intervention
Five studies tested the MRP and comparative interventions as individual modalities [Citation28, Citation31, Citation34–36]. Bobath [Citation26, Citation27, Citation29], mirror therapy (MT) [Citation32, Citation35] and constraint-induced movement therapy (CIMT) [Citation31, Citation36] were the most common comparators to the MRP. Intervention details can be found in .
Table 3. Intervention characteristics from each included study.
Intervention reporting
Each study offered a name for the MRP intervention. All studies, except Hesse et al. [Citation30] and Jan et al. [Citation35], provided a theory or a goal underpinning the MRP. El-Bahrawy and El-Wishy [Citation29], Batool et al. [Citation31], Pandian et al. [Citation33] and Paul [Citation34] were the only studies that stated the exact objects used. Langhammer and Stanghelle [Citation26, Citation27] produced a manual describing the philosophy behind the intervention.
Six studies specified the task conducted by the group undertaking the MRP [Citation28, Citation29, Citation31–33, Citation35]. Shah et al. [Citation36] did not specify the exact task performed but advised training involved all UL functions. Five studies [Citation26, Citation27, Citation29–31, Citation33] specified intervention provider but only Hesse et al. [Citation30] and Pandian et al. [Citation33] specified the provider’s level of experience.
Hesse et al. [Citation30] and Shah et al. [Citation36] were the only studies that did not offer methodological detail regarding the country of study location. India was the most reported study location [Citation28, Citation32–34]. Hesse et al. [Citation30], Paul [Citation34] and Jan et al. [Citation35] carried out their trials in rehabilitation centers whilst Rehani et al. [Citation32] and Pandian et al. [Citation33] conducted their trials in an outpatient department. Langhammer and Stanghelle [Citation26, Citation27] initially administered MRP in a hospital, followed by continued therapy in their homes, outpatient departments or rehabilitation centers.
Despite some RCTs detailing location of intervention delivery and intervention provider, the specific mode of intervention delivery was not clearly stated. However, Immadi et al. [Citation28] and Pandian et al. [Citation33] advised manual guidance was used within the MRP so it may be assumed that the MRP within these RCTs was delivered face-to-face. No study, apart from Hesse et al. [Citation30], specified if intervention was given as a group or individually. Hesse et al. [Citation30] carried out the MRP based intervention individually. All studies, except Langhammer and Stanghelle [Citation26, Citation27], provided sufficient details regarding period of intervention delivery, fluctuating from two [Citation36] to eight weeks [Citation28]. Timing of the MRP sessions fluctuated from 30 [Citation30,Citation34,Citation36] to 120 min [Citation31, Citation35]. Langhammer and Stanghelle [Citation26, Citation27] stated the minimum duration of sessions whilst patients were in hospital, but no further duration details were offered after discharge from hospital. Hesse et al. [Citation30] advised intervention was tailored in the most severely affected patients by enriching intervention with impairment-oriented arm ability training (IOAAT). No study clearly stated that intervention was modified or if intervention adherence or fidelity was assessed and no study gave adequate details of strategies implemented to maintain or improve fidelity.
Statistical analysis
The statistical tests used were stated in all 10 studies except Pandian et al. [Citation33]. All included studies carried out between group analysis on all relevant OMs (). All included studies that reported data on UL function and impairment conducted within group analysis. Furthermore, Langhammer and Stanghelle [Citation26, Citation27] did not report within group analysis on HRQoL.
Table 4. Summary of measures used to test outcomes of interest along with results from each included study.
Additionally, no study reported effect size (ES). For this systematized review, ES was calculated by the standard mean difference which serves as a simple way to assess the magnitude of the effect through Cohen’s general guidelines [Citation37]. Furthermore, despite confidence interval (CI) denoting the accuracy of the estimate of an intervention effect [Citation38] only Immadi et al. [Citation28] and Hesse et al. [Citation30] mentioned the 95% CI.
Methodological quality
The methodological quality of the studies ranged from three to eight on the PEDro scale. Five studies were deemed high-quality [Citation26, Citation27, Citation29–31, Citation33] and five studies were deemed low-quality [Citation28, Citation32, Citation34–36] using the PEDro threshold limits discussed by Díaz-Arribas et al. [Citation24]. All participants were randomized in the 10 included studies. Only El-Bahrawy and El-Wishy [Citation29], Batool et al. [Citation31] and Jan et al. [Citation35] conducted adequate concealed allocation. All studies, expect Immadi et al. [Citation28], provided details showing baseline group similarities. Langhammer and Stanghelle [Citation26, Citation27], El-Bahrawy and El-Wishy [Citation29] and Hesse et al. [Citation30] reduced detection bias through outcome assessor blinding. No study carried out therapist or participant blinding. Langhammer and Stanghelle [Citation26, Citation27], El-Bahrawy and El-Wishy [Citation29], Hesse et al. [Citation30], Batool et al. [Citation31] and Pandian et al. [Citation33] measured OMs in greater than 85% of participants assigned to groups. El-Bahrawy and El-Wishy [Citation29] alongside Pandian et al. [Citation33] reported participants received allocated treatment. Also, Hesse et al. [Citation30] used intention-to-treat (ITT) analysis. All 10 included studies reported results of between group statistical comparisons and provided point measurements and measurements of variability for at least one key outcome.
Strength of evidence
Strength of evidence was employed to the MRP and its comparison to other interventions in the RCTs.
Primary outcome
There was moderate evidence, supported by one high-quality [Citation31] and three low-quality RCTs [Citation28, Citation34, Citation35], that found the MRP as an individual approach can effectively improve UL function post-stroke. Limited evidence was found showing the MRP had a positive effect on self-rated UL function [Citation36]. Limited evidence was found showing the MRP with conventional physiotherapy (CPT) did not influence UL function [Citation32]. Limited evidence was found to support the positive effect of the MRP alongside either multidisciplinary treatment [MDT] [Citation26, Citation27], neuromuscular electrical stimulation (NMES) [Citation29] or conventional OT (COT) [Citation33] on UL function. Limited evidence was found indicating the group that received a double session of MRP based IAT, enriched with IOAAT as needed, and the group that received a single session of MRP based IAT, enriched with IOAAT as needed, with RAGT improved UL function. Both groups also participated in a rehabilitation program and as needed neuropsychological and speech therapies.
Limited evidence was found showing no supremacy between the IAT and RAGT groups [Citation30]. Limited evidence was discovered that neither the MRP nor Bobath coupled with MDT [Citation26, Citation27] or NMES [Citation29] was superior over the other at improving UL function. Limited evidence was discovered that neither the MRP nor MT coupled with CPT was superior over the other at improving UL function [Citation32]. However, limited evidence was available showing the MRP was superior over MT at improving UL function [Citation35]. Limited evidence was found showing Brunnstrom hand manipulation (BHM), with COT, was superior over the MRP, with COT, at improving UL function [Citation33]. Limited evidence was found for the superiority of the MRP over CPT [Citation28] and thermal stimulation [Citation34] at enhancing UL function. With two RCTs [Citation31, Citation36] reporting dissimilar results concerning the superiority of CIMT over the MRP at improving UL function, evidence was conflicting. Furthermore, limited evidence was found showing CIMT was superior over MRP in patient reporting of UL function [Citation36].
Secondary outcomes
With two RCTs [Citation28, Citation36] reporting dissimilar results concerning the effects of the MRP, as an individual approach, at improving UL impairment, evidence was conflicting. Limited evidence was found to support the positive effect of the MRP alongside either NMES [Citation29] or COT [Citation33] on UL impairment. Limited evidence was found indicating the group that received a double session of MRP based IAT, enriched with IOAAT as needed, and the group that received a single session of MRP based IAT, enriched with IOAAT as needed, with RAGT improved UL impairment.
Limited evidence was found showing no supremacy between the IAT and RAGT groups [Citation30]. Limited evidence was found for neither Bobath nor the MRP, coupled with NMES, having superiority over the other for decreasing wrist flexor spasticity [Citation29]. The same was true for the MRP versus CIMT at improving UL impairment [Citation36]. Limited evidence was discovered showing the MRP, with NMES, was greater than Bobath, with NMES, at enhancing ulnar deviation resting angle and hand grip strength [Citation29]. Limited evidence was found for the supremacy of BHM with COT over the MRP with COT [Citation33] and the MRP over CPT [Citation28] at improving UL impairment. Furthermore, limited evidence was discovered for neither the MRP nor Bobath, coupled with MDT, having supremacy over the other for enhancing HRQoL post-stroke [Citation26, Citation27].
Discussion
There is inadequate evidence to ascertain supremacy of the MRP over other interventions at improving UL function post-stroke. The qualitative synthesis results advocated that the MRP, as an individual intervention or with certain adjunctive therapies, proved effective at enhancing UL function in the short-term post-stroke. The MRP, with certain adjunctive therapies, was effective at enhancing UL impairment in the short-term post-stroke. Evidence was lacking overall to explain the MRP’s efficacy on HRQoL. Several methodological issues mean these findings must be considered with some caution.
Jan et al. [Citation35] reported that the MRP is commonly utilized to improve UL function post-stroke but that evidence regarding its efficacy is scarce. The frequent utilization of the MRP in UL rehabilitation post-stroke [Citation35] in the absence of high-level evidence through a systematic review [Citation39] is of some concern as clinicians rely on this to deliver evidence-based practice [Citation40]. To the best of the researcher’s awareness, having systemically reviewed the existing literature, this is the first review including only RCTs focused on assessing the effects of the MRP on UL function in adults post-stroke.
As not all studies were comparative studies due to the addition of various adjunctive therapies, it provided challenges in determining the MRP’s true efficacy. Pollock et al. [Citation15] reported that the combination of different approaches is effective for functional recovery. Even with many of the included studies combining approaches, only limited evidence was available for each combination, meaning it is difficult to derive robust conclusions concerning the selection of therapies with the greatest efficacy in improving UL function. The use of combined therapies is a problem when intending to assess the efficacy of a single modality [Citation41]. Therefore, it is important to interpret the findings regarding the adjunctive therapies cautiously as it is possible that findings are accredited to a collective treatment effect [Citation42], though such a collective approach is often typical within clinical practice. Furthermore, treatment preservation once treatment was discontinued could not be evaluated as the long-term influence of the MRP has yet to be satisfactorily assessed, therefore, carryover effects could not be truly evaluated.
Along with considering adjunctive therapy it is important to contemplate the time period since stroke onset. As spontaneous recovery most frequently arises within the preliminary three months post-stroke [Citation43] findings of the studies whereby the intervention was administered within this timeframe ought to be carefully interpreted. Cautious interpretation is necessary as spontaneous recovery may be a confounder in identifying the real intervention effect [Citation44], leading to a distortion of results [Citation45]. The UL function improvement noted by Langhammer and Stanghelle [Citation26, Citation27] along with Hesse et al. [Citation30] and Batool et al. [Citation31] who administered the interventions within three months post-stroke may be due to a possible interaction between spontaneous recovery and the intervention [Citation46]. Furthermore, Paul [Citation34] recruited participants six weeks to six months post-stroke so improvements in these trials due to spontaneous recovery could potentially act as extraneous variables [Citation47]. The findings of El-Bahrawy and El-Wishy [Citation29] and Pandian et al. [Citation33] whose trials are conducted on adults in the chronic phase of stroke may be exclusively due to treatment as the prospect of spontaneous recovery has lessened [Citation47].
Pollock and Beige [Citation48] reported the importance of considering the comparison between the intervention under investigation and other interventions to truly reflect the challenges faced by clinicians in deciphering which approach to utilize in practice. There is not enough evidence, from this review, to support the supremacy of any intervention. These results agree with Pollock et al. [Citation13] who stated that there is a deficit in evidence to enable adequate comparisons between interventions. Pollock et al. [Citation15] did, however, report that no single approach is any more efficient in enhancing functional recovery post-stroke. These results may differ from this systematized review as Pollock et al. [Citation15] investigated various interventions while this systematized review focused on a single intervention. Moreover, Pollock et al. [Citation15] investigated LL and Veerbeek et al. [Citation49] stated that a greater number of RCTs focus on LL post-stroke, which may suggest that Pollock et al. [Citation15] had a larger quantity of literature to extract conclusions from.
Reporting of the content of the MRP was deficient in this systematized review with only 30% of the RCTs outlining MRP content in sufficient detail. Inadequate intervention descriptions hamper intervention replicability by other researchers and reduces the translation of study findings into clinical practice [Citation50]. Kollen et al. [Citation42], who reviewed the efficacy of Bobath, reported the same weakness and Pollock et al. [Citation15] reported a tendency to name approaches whilst lacking detail or description of the specific methodology. The deficits in intervention reporting may possibly limit the strength of evidence found within this systematized review. It was clear in three studies [Citation28, Citation29, Citation33] that the MRP was conducted appropriately as they clearly reported that their intervention followed the MRP’s step sequence, but this was lacking in others. The lack of intention reporting identified within this systematized review causes uncertainty in reliable intervention execution [Citation22] and, as previously mentioned, limits transference of findings into clinical practice [Citation50]. Lately, there has been an upsurge in the amount of research investigating the amalgamation of various interventions alongside TST which is a component of the MRP approach [Citation10]. This may justify why few RCTs were obtained for this systematized review whilst further explaining the possible deficit in up-to-date literature on the MRP and more focus on components that comprise the approach.
Review process
As the researcher was unable to conduct a systematic review due to a limited timescale as part of an accelerated postgraduate program of study, this project was conducted as a systematized review. The exclusive inclusion of RCTs was a strength of this systematized review as such trials are less liable to offer biased estimations of treatment effects [Citation51]. Furthermore, this systematized review contained an extensive search process over five databases, which is beyond the quantity recommended [Citation52]. Finally, to the best of the researcher’s awareness, this is the first-time literature concerning the influence of the MRP on UL function post-stroke has been critically reviewed, appraised and synthesized.
Despite the thorough database and reference list search there is potential that research may have been missed. This review excluded any grey literature which may have increased the possibility of publication bias [Citation53] and led to exclusion of relevant data [Citation54]. The act of peer-reviewing was unachievable in this review due to the need for the researcher to carry it out independently as part of a program of study, potentially increasing the risk of researcher bias. Moreover, the review concentrated only on studies available in English which may have instigated a language bias and caused the formation of erroneous conclusions [Citation55]. Language restrictions can also decrease the overall applicability, generalizability and precision of findings [Citation56].
An additional literature search was carried out on 12th February 2024 using all five original databases to identify any additional papers for inclusion in the review. Forty additional papers were identified, of which 36 were excluded after screening of the title and abstract and four excluded after screening of the full-text articles, meaning that no additional papers met the criteria for inclusion in the review.
Clinical implications
There is inadequate evidence on the supremacy of the MRP in comparison to other interventions at promoting UL function post-stroke. This review found evidence that the MRP, as an individual approach, has positive effects on UL function in adults in the short-term post-stroke and that MRP, with adjunctive therapies, can enhance UL function and impairment in the short-term post-stroke. However, due to the heterogeneity relating to frequency and duration of the MRP within this systematized review, it is impossible to guide clinicians toward an optimal MRP dose, though the findings do demonstrate its benefits as part of a combined therapy approach, which is typical of contemporary neurological clinical practice.
Implications for research
Large, high-quality RCTs, utilizing rigorous methodologies, are necessitated which may offer more decisive evidence on the efficacy of the MRP on UL function in adults post-stroke. Future research should direct attention to blinding of outcome assessors and participants, concealed allocation and ITT analysis. Intervention reporting needs to be conducted in a manner that allows for transparency and reproducibility. Furthermore, study designs integrating analysis of data by repeated measurements, with longer follow-ups, should be conducted in future research. Finally, the duration and frequency of the MRP requires further examination due to lack of uniformity that was highlighted in this systematized review.
Conclusion
There is not enough evidence to verify the supremacy of the MRP over other interventions at promoting UL function post-stroke. The findings extracted from this systematized review highlight that the MRP, as an individual approach or with adjunctive therapy, can improve UL function, in the short-term, of adults post-stroke. Furthermore, the MRP with adjunctive therapy can improve UL impairment, in the short-term, of adults post-stroke. Evidence is inadequate to establish the utmost adjunctive therapy with the MRP to elicit positive effects on UL function and impairment post-stroke. Until further high-quality RCTs with adequate intervention reporting are conducted, the researcher suggests following evidence-based guidelines as opposed to clinician preference for a named approach.
Supplemental Material
Download MS Word (26.4 KB)Disclosure statement
Neither author has any conflict of interest with any external agencies and no external funding was sought.
Additional information
Funding
Notes on contributors
Claire O’Rourke
Claire O’Rourke graduated from Institute of Technology Carlow, Ireland with the award of BSc (Hons) in Sports Rehabilitation and Athletic Therapy with First Class Honours. She then graduated from Manchester Metropolitan University, UK with the award of MSc in Pre-Registration Physiotherapy with Distinction.
David Edwards
David Edwards is a Lecturer in Physiotherapy at The University of Liverpool, UK teaching on the undergraduate and postgraduate programs of study. He has a background in cardiorespiratory and neurological teaching and practice. At the time of this review he was working as a Senior Lecturer at Manchester Metropolitan University.
References
- Stockley R, Peel R, Jarvis K, et al. Current therapy for the upper limb after stroke: a cross-sectional survey of UK therapists. BMJ Open. 2019;9(9):e030262. doi:10.1136/bmjopen-2019-030262.
- Broeks JG, Lankhorst GJ, Rumping K, et al. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21(8):357–364. doi:10.1080/096382899297459.
- Rodgers H, Bosomworth H, Krebs HI, et al. Robot assisted training for upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet. 2019;394(10192):51–62. doi:10.1016/S0140-6736(19)31055-4.
- Sprigg N, Selby J, Fox L, et al. Very low quality of life after acute stroke: data from the efficacy of nitric oxide in stroke trial. Stroke. 2013;44(12):3458–3462. doi:10.1161/STROKEAHA.113.002201.
- Stinear C, Ackerley S, Byblow W. Rehabilitation is initiated early after stroke, but most motor rehabilitation trials are not a systematic review. Stroke. 2013;44(7):2039–2045. doi:10.1161/STROKEAHA.113.000968.
- Olaleye OA, Lawal ZI. Utilization of physiotherapy in the continuum of stroke care at a tertiary hospital in Ibadan, Nigeria. Afr Health Sci. 2017;17(1):79–87.
- Carey L, Walsh A, Adikari A, et al. Finding the intersection of neuroplasticity, stroke recovery, and learning: scope and contributions to stroke rehabilitation. Neural Plast. 2019;2019:5232374. doi:10.1155/2019/5232374.
- Hubbard IJ, Parsons MW, Neilson C, et al. Task-specific training: evidence for and translation to clinical practice. Occup Ther Int. 2009;16(3-4):175–189. doi:10.1002/oti.275.
- Tunney N. Is there a best approach to the rehabilitation of adult hemiplegia? Phys Ther Rev. 2018;23(6):348–354. doi:10.1080/10833196.2018.1539293.
- Valkenborghs SR, Callister R, Visser MM, et al. Interventions combined with task-specific training to improve upper limb motor recovery following stroke: a systematic review with meta-analyses. Phys Ther Rev. 2017;24(3-4):100–117.
- French B, Thomas LH, Coupe J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2016;11(11):CD006073. doi:10.1002/14651858.CD006073.pub3.
- Urton ML, Kohia M, Davis J, et al. Systemati literature review of treatment interventions for upper extremity hemiparesis following stroke. Occup Ther Int. 2007;14(1):11–27.
- Pollock A, Farmer SE, Brady MC, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. 2014;2014(11):CD010820. doi:10.1002/14651858.CD010820.pub2.
- Winstein CJ, Wolf SL, Dromerick AW, et al. Effect of a task-oriented rehabilitation program on upper extremity recovery following stroke. J Am Med Assoc. 2016;135(6):571–581.
- Pollock A, Baer G, Campbell P, et al. Physical rehabilitation approached for the recovery of function and mobility following stroke. Cochrane Database Syst Rev. 2014;2014(4):CD001920. doi:10.1002/14651858.CD001920.pub3.
- Hatem SM, Saussez G, Della Faille M, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci. 2016;10:442. doi:10.3389/fnhum.2016.00442.
- Plummer-D'Amato P, Kyvelidou A, Sternad D, et al. Training dual-task walking in community-dwelling adults within 1 year of stroke: a protocol for a single-blind randomized controlled trial. BMC Neurol. 2012;12(1):129. doi:10.1186/1471-2377-12-129.
- Maier M, Ballester BR, Verschure PFM. Principles of neurorehabilitation after stroke based on motor learning and brain plasticity mechanisms. Front Syst Neurosci. 2019;13:74. doi:10.3389/fnsys.2019.00074.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-34.
- Farrugia P, Petrisor BA, Farrokhyar F, et al. Research questions, hypotheses and objectives. Can J Surg. 2010;53(4):278–281.
- Paltridge M, Traves A. The health effects of strongyloidiasis on pregnant women and children: a systematic literature review. Trop Med Infet Dis. 2018;3(2):50.
- Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guidelines. BMJ. 2014;348(mar07 3):g1687. doi:10.1136/bmj.g1687.
- Cashin AG, McAuley JH. Clinimetrics: physiotherapy evidence database (PEDro) scale. J Physiother. 2020;66(1):59. doi:10.1016/j.jphys.2019.08.005.
- Díaz-Arribas MJ, Martín-Casas P, Cano-de-la-Cuerda R, et al. Effectiveness of Bobath concept in the treatment of stroke: a systematic review. Disabil Rehabil. 2019;42(12):1636–1649. doi:10.1080/09638288.2019.1590865.
- Challoumas D, Clifford C, Kirwan P, et al. How does surgery compare to sham surgery or physiotherapy as a treatment for tendinopathy? A systematic review of randomised trials. BMJ Open Sport Exerc Med. 2019;5(1):e000528. doi:10.1136/bmjsem-2019-000528.
- Langhammer B, Stanghelle JK. Bobath or motor relearning programme? A follow-up one and four years post stroke. Clin Rehabil. 2003;17(7):731–734. doi:10.1191/0269215503cr670oa.
- Langhammer B, Stanghelle JK. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in stroke rehabilitation: a randomized controlled trial. Clin Rehabil. 2000;14(4):361–369. doi:10.1191/0269215500cr338oa.
- Immadi SK, Achyutha KK, Reddy A, et al. Effectiveness of the motor relearning approach in promoting physical function of the upper limb after a stroke. Int J Physiother. 2015;2(1):386–390. doi:10.15621/ijphy/2015/v2i1/60047.
- El-Bahrawy MN, El-Wishy AAB. Efficacy of motor relearning approach on hand function in chronic stroke patients. A controlled randomized study. Ital J Physiother. 2012;2(4):121–127.
- Hesse S, Heß A, Werner C C, et al. Effect on arm function and cost of robot-assisted group therapy in subacute patients with stroke and a moderately to severely affected arm: a randomized controlled trial. Clin Rehabil. 2014;28(7):637–647. doi:10.1177/0269215513516967.
- Batool S, Soomro N, Amjad D, et al. To compare the effectiveness of constraint induced movement therapy versus motor relearning programme to improve motor function of hemiplegic upper extremity after stroke. Pak J Med Sci. 2015;31(5):1167–1171. doi:10.12669/pjms.315.7910.
- Rehani P, Kumari R, Midha D. Effectiveness of motor relearning programme and mirror therapy on hand functions in patients with stroke-a randomized clinical trial. Int J Ther Rehabil Res. 2015;4(3):20–24.
- Pandian S, Arya KN, Davidson EWR. Comparison of Brunnstrom movement therapy and motor relearning program in rehabilitation of post-stroke hemiparetic hand: a randomized trial. J Bodyw Mov Ther. 2012;16(3):330–337.
- Paul J. Comparative study on the effect of task oriented motor relearning program and thermal stimulation over upper limb motor function among stroke subjects. Int J Physiother. 2014;1(4):227–232. doi:10.15621/ijphy/2014/v1i4/54565.
- Jan S, Arsh A, Darian H, et al. A randomized control trial comparing the effects of motor relearning programme and mirror therapy for improving upper limb motor functions in stroke patients. J Pak Med Assoc. 2019;69(9):1242–1245.
- Shah MV, Kumar S, Muragod AR. Effect of constraint induced movement therapy v/s motor relearning program for upper extremity function in sub acute hemiparetic patients-a randomized clinical trial. Indian J Physiother and Occup Ther. 2016;10(1):71–75.
- Takeshima N, Sozu T, Tajika A, et al. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol. 2014;14(1):30. doi:10.1186/1471-2288-14-30.
- Freire APCF, Elkins MR, Ramos EMC, et al. Use of 95% confidence intervals in the reporting of between-group differences in randomized controlled trials: analysis of a representative sample of 200 physical therapy trials. Braz J Phys Ther. 2019;23(4):302–310. doi:10.1016/j.bjpt.2018.10.004.
- McNamara ER, Scales CD. Jr. Role of systematic reviews and meta-analysis in evidence-based clinical practice. Indian J Urol. 2016;27(4):520–524.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta-analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2(1):9–14.
- Damgaard P, Bartels EM, Ris I, et al. Evidence of physiotherapy interventions for patients with chronic neck pain: a systematic review of randomised controlled trials. ISRN Pain. 2013;2013:567175–567123. doi:10.1155/2013/567175.
- Kollen BJ, Lennon S, Lyons B, et al. The effectiveness of the Bobath concept in stroke rehabilitation: what is the evidence? Stroke. 2009;40(4):e89-97. doi:10.1161/STROKEAHA.108.533828.
- Wahl AS, Schwab ME. Finding an optimal rehabilitation paradigm after stroke: enhancing fiber growth of the brain at the right moment. Front Hum Neurosci. 2014;8:381. doi:10.3389/fnhum.2014.00381.
- Winters C, van Wegen EEH, Daffertshofer A, et al. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair. 2014;29(7):614–622. doi:10.1177/1545968314562115.
- Skelly AC, Dettori JR, Brodt ED. Assessing bias: the importance of considering confounding. Evid Based Spine Care J. 2012;3(1):9–12. doi:10.1055/s-0031-1298595.
- Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke. 2006;37(9):2348–2353. doi:10.1161/01.STR.0000238594.91938.1e.
- Wolf SL, Winstein C, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. J Am Med Assoc. 2006;296(17):2095–2014.
- Pollock A, Beige E. How to do a systematic review. Int J Stroke. 2018;13(2):138–156.
- Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9(2):e87987. doi:10.1371/journal.pone.0087987.
- Page P, Hoogenboom B, Voight M. Improving the reporting of therapeutic exercise interventions in rehabilitation research. Int J Sports Phys Ther. 2017;12(2):297–304.
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–754. doi:10.1016/S1474-4422(09)70150-4.
- Charrois TL. Systematic reviews: what do you need to know to get started? Can J Hosp Pharm. 2015;68(2):144–148.
- Adams J, Hillier-Brown FC, Moore HJ, et al. Searching and synthesising ‘grey literature’ and ‘grey information’ in public health: critical reflections on three case studies. Syst Rev. 2016;5(1):164. doi:10.1186/s13643-016-0337y.
- Robinson H, Williams V, Curtis F, et al. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19. doi:10.1038/s41533-018-0085-7.
- Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–144. doi:10.1017/S0266462312000086.
- Jüni P, Holenstein F, Sterne J, et al. Direction and impact of language in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31(1):115–123. doi:10.1093/ije/31.1.115.