?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of the research was to demonstrate the plasticization properties of divalproex sodium, due to its component—valproic acid, on ethyl cellulose, which could prove beneficial for film fabrications or hot melt extrusion based formulations. Films containing 10–50% w/w (DVS/EC) as dry weight were prepared using solvent evaporation method and characterized using texture analyzer, hybrid rheometer, differential scanning calorimetry, thermogravimetry, X-ray diffractometry, polarized microscopy, FTIR, and Raman spectroscopy. It was found that there was a decrease in average peak load, melt viscosity, and glass transition temperature (Tg) while increase in elongation, with increase in concentration of DVS in the films. These results demonstrate the plasticization tendency of DVS on EC, which was attributed to the presence of valproic acid (fatty acid) in DVS. XRD studies showed amorphous nature of the films; however, polarized microscopy revealed the presence of scattered undissolved sodium valproate crystals. The presence of a single Tg established complete miscibility between valproic acid and EC. Films showed reasonable physical stability (similar Tg) at 45 °C/75% RH for 2 weeks (open condition), attributable to the similar solubility parameters of DVS and EC. FTIR and Raman spectroscopy results proved the presence of hydrogen bonding between DVS and EC.
Introduction
Plasticizers are low molecular weight resins/polymers or small molecules, which are generally used to facilitate the device development processes by reducing the glass transition temperatures of the polymers. The majority of plasticizers are in the liquid state and form secondary bonds to polymer chains and spread them apart; thereby reducing the polymer-polymer secondary bonding and providing greater mobility for the macromolecules. Such phenomenon plasticization, results in a soft and deformable polymer mass (Rahman and Brazel Citation2004). In pharmaceuticals, this attribute allows it to be used extensively for film-coating and hot melt extrusion applications.
Plasticizers are incorporated into the amorphous parts of polymers leaving the crystalline part unaffected. It is expected that a plasticizer will reduce the modulus, tensile strength, hardness, density, melt viscosity, glass transition temperature, electrostatic chargeability and a volume resistivity of a polymer, while simultaneously increasing its flexibility, elongation at break, toughness, dielectric constant and power factor (Rahman and Brazel Citation2004).
Traditional plasticizers such as triethyl citrate, dibutyl sebacate, and triacetin have been used widely, additionally certain active pharmaceutical ingredients (API) or excipients have been also shown to act as plasticizers. For instance, methylparaben (Wu and McGinity Citation1999), ibuprofen, chlorpheniramine maleate (CPM) (Wu and McGinity Citation1999; Siepmann et al. Citation2006) and metoprolol tartarate (Siepmann et al. Citation2006) were found to decrease the glass transition temperature of Eudragit RS films.
Plasticization tendency of APIs on polymers can be advantageous for hot melt extrusion, and film coating application. During hot melt extrusion, the plasticization of the polymer by API lowers the processing temperature, which in turn helps in reducing thermal exposure on the thermally sensitive excipients such as antioxidants. API acting as a plasticizer helps in increasing the API loading since additional plasticizer is not required. Due to the inherent plasticizing property of API, it can be mixed uniformly with other polymers using a V-blender or high shear mixer without the use of additional organic solvents during the formulation process. During the coating process, a coating system containing the API, acting as a plasticizer, and polymer can be coated on nonpareil beads or tablets without the need of a traditional plasticizer. Addition of plasticizer imparts smoothness and flexibility to the coat, which provides a smooth substrate for subsequent enteric or sustained release coating. Such nonpareil beads may be compressed to form tablets without causing the rupture of the film coat due to the enhanced flexibility imparted by the plasticization effect of the API. On the contrary traditional liquid plasticizers require them to be sprayed as a solution on to the API-polymer mixture in a high shear mixer followed by drying the granules. For example, to use lipophilic plasticizers like dibutyl sebacate, organic solvents will be required to form a solution, which can cause safety concerns. Therefore, identification of APIs acting as a plasticizer aids in formulation development.
Divalproex sodium is a coordination complex of sodium valproate and valproic acid. It has a pKa of 4.6, with a solubility of 1 mg/ml at pH 1.0 and 200 mg/ml at pH 6.8 (Rao et al. Citation2003). Valproic acid is clear, colorless to pale yellow, slightly viscous liquid, and sparingly soluble in water, whereas sodium valproate is a white crystalline, hygroscopic, and highly soluble in water and alcohol (Alsarra et al. Citation2005). It was noticed that on the addition of divalproex sodium to water, the coordination complex readily dissociates into sodium valproate and valproic acid. Since sodium valproate is highly soluble in water, it dissolves in the aqueous phase, while valproic acid due to its poor solubility forms an oily phase over the aqueous phase. This shows that divalproex sodium cannot be used with aqueous granulation or coating processes. Therefore, only nonaqueous granulation or coating is feasible to avoid potential content uniformity issues segregation of valproic acid and sodium valproate in presence of water. Melt extrusion of divalproex sodium with ethylcellulose can achieve sustained release without using nonaqueous solvents and without additional plasticizer.
Divalproex sodium has been used as an anticonvulsant to treat seizures, bipolar disorder, and migraines. It is available in tablet, capsule and syrup to treat these conditions. Hence its use as a plasticizer as well as a API along with highly acceptable polymers such as various cellulose derivatives to develop novel formulations will potentially benefit the pharmaceutical field.
During preformulation studies of divalproex sodium with different polymers, it was found that films composed of divalproex sodium and ethyl cellulose were smooth and flexible in nature. This observation led to this investigation of divalproex sodium as a nontraditional plasticizer of ethylcellulose.
Materials and methodology
Materials
Divalproex sodium (manufactured by Sci Pharmatech, Inc, Taiwan) was gifted by G&W PA Laboratories (Sellersville, PA). Ethylcellulose (Ethocel Standard 10 Premium) with viscosity 9–11 mPa.s and ethyoxyl content of 48–49.5% wt was gifted by Colorcon, Inc (West Point, PA). Ethyl alcohol 200 proof (ACS/USP grade) was purchased from PHARMACO-AAPER (Brookfield, CT).
Preparation of DVS/EC films
Films of DVS and EC were prepared using solvent evaporation method. DVS and EC stock solutions at 5% w/w concentration (DVS to EC ratio) were prepared by dissolving them separately in ethanol. From the respective stock solutions, mixtures of API and polymer solutions with 10, 20, 30, 40, and 50% w/w DVS of the dry weight of EC were prepared, while mixing. After mixing for 10 min, the solution was poured in an aluminum plate and dried overnight at 35 °C to completely evaporate the solvent and achieve the constant weight to form films. The solid content of the mixture solution was kept similar in order to obtain films with similar thickness. Films were stored in polyethylene bags at ambient conditions until further used. The thickness of the rectangular films for texture analysis was measured with a digital thickness gauge (Mitutoya, IL) in mm up to two decimal places.
API content analysis
Approximately 10–30 mg of sample was dissolved in the ethanol. Aliquots were analyzed using HPLC, which was adapted from USP method for DVS Delayed-Release Capsule monograph. Briefly, samples of different dilutions (1.6–240 μg/mL) were prepared in acetonitrile: deionized water (1:1 v/v). The column used was Symmetry 4.6 × 75 mm, C-18, 3.5 μm. The mobile phase consisted of buffer: acetonitrile (2:3 v/v), where the buffer was 6.8 g/L of monobasic potassium phosphate adjusted to pH 3 with phosphoric acid. The flow rate was kept at 1.2 ml/min. The column temperature was maintained at 30 °C, while the sample temperature was maintained at 27 °C. The injection volume was 80 μL. UV detection was performed at a wavelength of 210 nm. The run time was kept at 7 min. The analysis was performed on a Waters HPLC system using Breeze data integration software. Percent API content were determined by using EquationEquation (1)(1)
(1) .
(1)
(1)
Mechanical properties of film
Brookfield Texture Analyzer attached to 4500g load cell with tension module was used to test the peak load and % elongation of films. Rectangular films 10 mm × 50 mm were cut and clamped on both sides with a flat faced grip with the target test distance of 38 mm. Films were extended at the rate of 0.1 mm/s and were extended until they break. Percentage Elongation was calculated from the elongation length using the initial length of the film (distance between the clamps), that is, 38 mm. Other parameters such as peak load, elongation length, and the load versus time (s) profile are reported. The test was carried out in duplicate for each film.
Rheological studies
Rheological studies were conducted to demonstrate the plasticization effect of DVS on films as a result of change in melt viscosity. A hybrid rheometer with an oven heating assembly (Discovery DHR-3, TA instruments, DE) was utilized for the analysis. Films were chosen for melt viscosity studies since powdered sample or compressed sample may not be uniform. Furthermore, air bubbles from the powder may get entrapped between the plates leading to aberrant results. The films were placed between the parallel plates and a dynamic oscillation temperature sweep was performed from 150 to 200 °C at 5 °C/min. The velocity was 0.02 rad/s. The shear rate, velocity, torque, and stress were 1.25 1/s, 0.02 rad/s, 3.25946 Pa, and 10.0 µN.m, respectively. Since the glass transition temperature of ethylcellulose is 133 °C, a higher temperature was chosen to initiate the study to allow for melting of the polymer.
Differential scanning calorimetry (DSC)
DSC analysis of DVS, EC, and prepared films was performed using Q200 DSC differential scanning calorimeter (TA Instrument, New Castle, DE). Weighed samples (5–10 mg) were placed in Tzero aluminum pans and crimped with a Tzero lid. DSC thermograms were obtained at the heating rate of 10 °C/min from 30 to 160 °C comparing with the similar blank pan as a reference and continuous nitrogen flow was maintained to obtain inert atmospheres. Indium was used as a reference standard and Universal analysis software (TA Instrument, New Castle, DE) was used for the data analysis.
Polarized microscopy
DVS-EC films were observed under a Nikon microscope Eclipse 80I attached to DS-Fi1 camera using NIS-Element-BR (Melville, NY) using a polarizer under 4 × magnification to determine the physical nature of DVS in the films. Films were observed for presence of undissolved crystals of sodium valproate or other impurities.
Powder X-ray diffraction
DVS-EC films were characterized using Shimadzu X-Ray Diffractometer (Cu-Kα, Shimadzu, Columbia, MD) with accelerating voltage and anode current set as 40 kV and 30 mA, respectively. XRD patterns were recorded over the range 10–40° 2θ using a step size of 0.02°, counting time of 1 s/step, and the scanning rate of 2° 2θ/min.
Thermogravimetric analysis
A Q500 TGA (TA Instrument, New Castle, DE) instrument was used to determine the percentage weight loss of the DVS, EC, and 50% DVS (dry polymer weight) in ethylcellulose. Samples (3–10 mg) were placed into open aluminum crucibles. The samples were heated from 65 to 450 °C with a heating rate of 20 °C/min and a nitrogen gas purge of 60 ml/min.
Fourier transformed infrared (FTIR) spectroscopy analysis
FTIR spectra (4000–650 cm−1) were acquired with a Perkin Elmer Spectrum 100 FTIR spectrometer equipped with Universal ATR (Perkin Elmer Inc., Wellesley, MA) to understand the chemical interactions between DVS and EC. Samples were placed on the crystal surface and tightened using the screw of the pressure arm. IR Spectrums of films without DVS and with DVS were obtained by running 32/64 scans and compared using Spectrum E5. For analyzing DVS and EC, individual tablets were compressed on Carver Press to form a solid die that could be placed between the crystal surface and pressure arm.
Stability studies
Stability studies were performed at 45 °C/75% RH in humidity chamber for 2 weeks by placing samples separately in aluminum bags and covering with perforated Parafilm. Humidity condition (75%RH) was created manually using a saturated solution of sodium chloride. After 2 weeks, samples were characterized using XRD and DSC and observed for crystal formations and glass transition temperature change, respectively. Results of samples stored at 45 °C/75% RH and with 45 °C/75% RH were compared. A paired t-test was used to compare the experimental Tg values before and after stability using Microsoft Excel 2010 (α = 0.05).
Raman spectroscopy
The Raman spectra were collected to study the polymorphic form of the film. The Raman spectra were collected using Raman Rxn System Model # RXN1-785, Kaiser Optical Systems that was equipped with an excitation laser operating at 785 nm with a laser power setting of 400 mW. Data acquisition was done using an exposure time of 5 s for three accumulations.
Results and discussion
API content
API content was found to be between 95–105% of the theoretical amount. The optimum API loading efficiencies are attributed to the no loss of API or polymer during film fabrications. These results also indicate that API was stable during film fabrication. Results of API content analysis are summarized in .
Table 1. %DVS content of films.
Mechanical properties
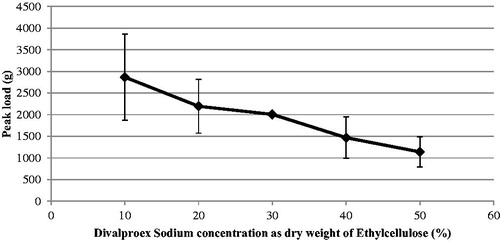
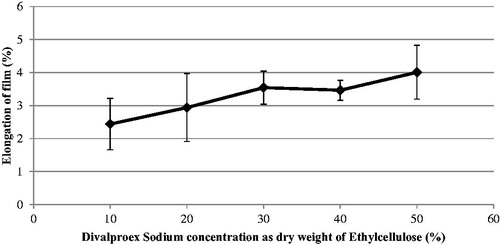
Rectangular films of 0.11–0.12 mm were used to study mechanical properties. The average peak load and average elongation of ethylcellulose films containing different concentrations of the DVS are as shown in and , respectively. It can be seen that the peak load decreases whereas the average elongation increases, with an increase in API concentration. This could be due to the increase in elasticity of ethylcellulose film upon addition of divalproex sodium. Similarly, increase in elasticity of ethylcellulose was also noticed upon addition of traditional plasticizers like triethyl citrate and dibutyl sebacate. However, at 20% plasticizer concentration, the elongation (%) of the films containing divalproex sodium was found to be 3.5%, which was much higher than that found with traditional plasticizers like triethyl citrate, dibutyl sebacate, triacetin, myvacet, and diethyl phthalate (Hyppölä et al. Citation1996).
Rheological studies
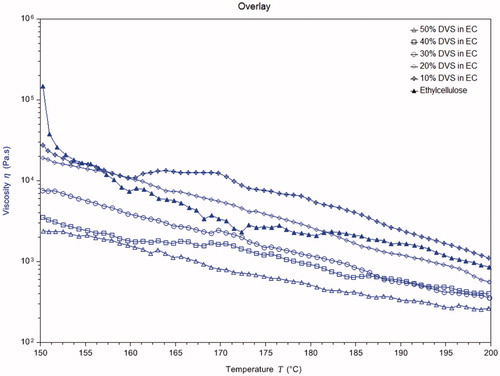
Rheological profiles of different films plotted as viscosity as a function of temperature are presented in . Plasticization of polymer leads to decrease in the melt viscosity of the polymer. It could be noticed that for all the films, the melt viscosity was found to decrease progressively with increase in temperature. The initial melt viscosity at 150 °C was found to decrease with increase in the concentration of divalproex sodium in the films. This provides further evidence of the plasticization of ethylcellulose by divalproex sodium. Earlier studies performed also indicate the decrease in melt temperatures of the films (Wu and McGinity Citation2003), which supports our conclusions.
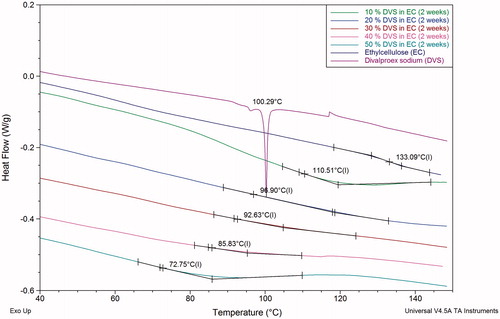
Differential scanning calorimetry (DSC)
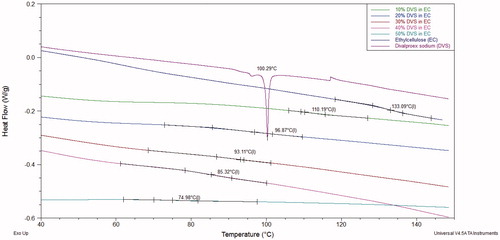
DSC thermograms indicating glass transition temperature of ethyl cellulose films with and without divalproex sodium are as shown in . It was observed that the glass transition temperature of ethylcellulose decreases progressively with increase in the concentration of divalproex sodium in the films. The glass transition temperature decreased from 133 °C of pure ethylcellulose to 110 °C at 10% DVS concentration. The plasticization effect of divalproex sodium can be attributed to the presence of valproic acid, which is a medium chain fatty acid. The small chemical structure of valproic acid allows it to penetrate the ethylcellulose chain and plasticize it efficiently leading to decrease in glass transition temperature. Ethylcellulose is an uncharged moiety, which does not cause flocculation with charged sodium valproate, whereas we observed that Eudragit L100-55, due to its charged nature, precipitates on the addition of divalproex sodium due to the presence of sodium valproate (unpublished data). At 20% concentration, the glass transition temperature of ethylcellulose films containing divalproex sodium showed glass transition temperature of 97 °C, which is comparable to that obtained with triacetin (Hyppölä et al. Citation1996). At 50% DVS concentration, the Tg of ethyl cellulose was found to be 75 °C, which is comparable with the plasticization efficiency of traditional plasticizers like triethyl citrate, assuming that the effective concentration of valproic acid available for plasticization is approximately half of divalproex sodium added, that is, 25% if 50% of divalproex sodium is added (Vesey et al. Citation2005). Since no melting peak was observed in the thermograms, the physical nature of API can be assumed to be amorphous; however, crystallinities of under 2% cannot generally be detected with DSC (Kreuter Citation1999). These studies support that DVS has similar plasticizing effect as other well-known plasticizers.
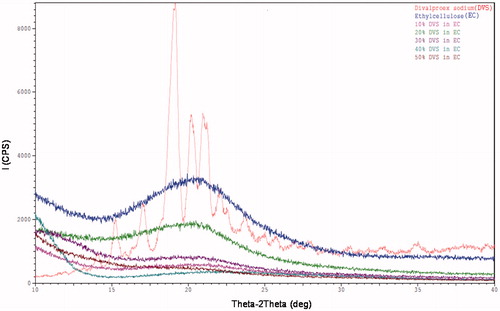
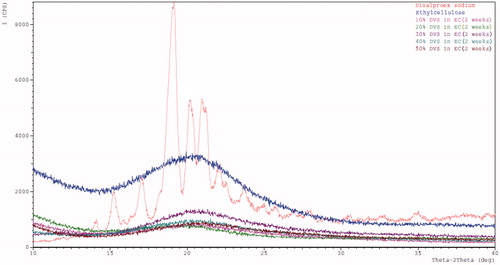
Powder x-ray diffraction (XRD)
shows no sharp peaks in the X-ray diffractograms of the films that indicate their amorphous nature of API and polymer. This is in agreement with the absence of melting point peak in DSC thermograms of the films. However, crystallinity less than 5–10% is difficult to be detected using XRD (Leuner and Dressman Citation2000) but it may be based on the sensitivity of the instrument too. Therefore, techniques such as polarized microscopy can be employed to confirm the amorphous nature of the API.
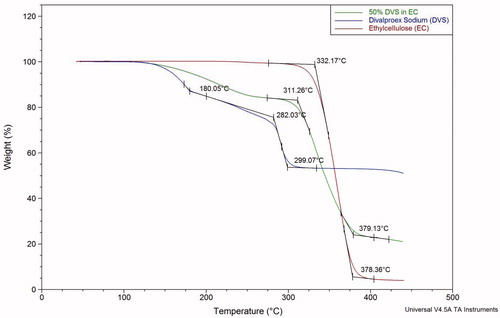
Thermogravimetric analysis
shows the thermogravimetric profile of divalproex sodium, ethylcellulose, and 50% divalproex sodium (by dry weight of polymer) in ethylcellulose film. Divalproex sodium is a coordination complex of valproic acid and sodium valproate. Valproic acid inherently exists in a liquid form and is volatile, however, along with sodium valproate, it forms a coordination complex that melts at approximately 100 °C. It was observed that around 53% of the weight of DVS is left at 406 °C, that is, at plateau phase. This is in close agreement with the theoretical weight (46% w/w) of valproic acid in DVS. The evaporation of valproic acid was observed to be in three phases. On heating to 100 °C, a saturated solution of sodium valproate in valproic acid is formed and any additional sodium valproate will precipitate out (Sherman Citation2000). During the first phase, valproic acid evaporates at a faster rate while sodium valproate precipitates due to the decrease in the solvent (valproic acid). At the end of the first phase, approximately 15% of the total weight, that is, approximately 30% of the valproic acid is evaporated. During the second phase, the evaporation rate decreases; this can be attributed to the dissolution of sodium valproate in valproic acid as the solubility of sodium valproate might increase with temperature, which increases the viscosity of the solution, or due to the precipitation of sodium valproate that was dissolved in valproic acid, which forms a physical barrier for evaporation. During the third phase (around 290 °C), the evaporation rate of valproic acid increases since at such a high temperature, which greatly exceeds the boiling point of valproic acid (219.5 °C) (Alsarra et al. Citation2005), the valproic acid molecules acquire enough energy much to overcome any physical interaction with sodium valproate. The residue at the end of the thermogravimetric analysis was observed to dissolve rapidly in water indicating the presence of residual sodium valproate since it is readily soluble in water. The decrease in weight of ethylcellulose is due to thermal degradation at a higher temperature of around 320 °C. The aluminum crucible was found to contain charred residue that was 4% of the initial weight. This indicates that in films, valproic acid dissociates from DVS to plasticize ethylcellulose as well as to dissolve sodium valproate. The thermogravimetric profile of 50% divalproex sodium (by dry polymer weight) in ethylcellulose can also be subdivided into three phases—the first phase is characterized by the monophasic evaporation of valproic acid. The evaporation rate of valproic acid from the film is slower than that from divalproex sodium neat API due to its hydrogen bonding interaction with ethylcellulose. At 286 °C, 16% of the weight of the film is lost. This is in agreement with the theoretical quantity (15.48% w/w) of valproic acid in the film. In the next phase 310 °C, ethylcellulose starts degrading until around 400 °C. At 422 °C, the residual weight was found to be 22% of the initial weight. The residue is composed of sodium valproate and charred ethylcellulose. The quantity of residue of the film is in agreement with the predicted value based on the thermogravimetric analysis of divalproex sodium and ethylcellulose.
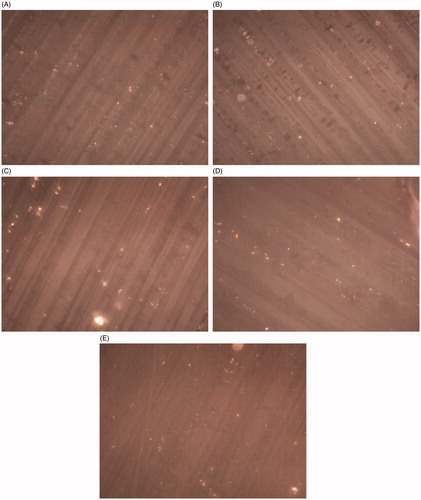
Polarized microscopy
Since X-ray diffraction cannot identify crystallinity below 5–10%, polarized microscopy provides visual qualitative identification of the presence of any crystalline material (). The presence of birefringence in the polarized microscopic image for all samples might be due to the presence of sodium valproate or divalproex sodium in crystalline form. However, since plasticization of ethylcellulose can be attributed to the presence of valproic acid as shown during DSC studies, dissociation of divalproex sodium into valproic acid and sodium valproate should occur as indicated by TGA studies. Therefore, the birefringence can be attributed to sodium valproate crystals only. The black background is due to the amorphous monophase composed of ethylcellulose and valproic acid. Interestingly, the occurrence of sodium valproate crystals in all samples is almost similar in concentration, visually. This can be explained in this manner. At 10% divalproex sodium concentration, the valproic acid is utilized in plasticizing ethylcellulose. As the concentration of divalproex sodium increases, the plasticization of ethylcellulose increases as seen from the decrease in the glass transition temperature. However, the plasticization efficiency (decrease in glass transition temperature with plasticizer concentration) decreases at higher API concentration (40 and 50% concentration). At 50% divalproex sodium concentration with respect to the dry polymer, the overall API concentration in the film is 33.3% w/w. Nearly half the weight of the API is sodium valproate, which implies approximately 17% of the area of the film should show birefringence due to the crystallinity of sodium valproate. However, the birefringence due to sodium valproate is much less and is scattered. This might be due to the solubility of sodium valproate in valproic acid leading to the formation of the solid solution, which is amorphous in nature. Literature shows that valproic acid can dissolve up to 50% of sodium valproate by weight at room temperature (Sherman Citation2000). This might explain the higher solubility of sodium valproate in valproic acid to form a solid solution.
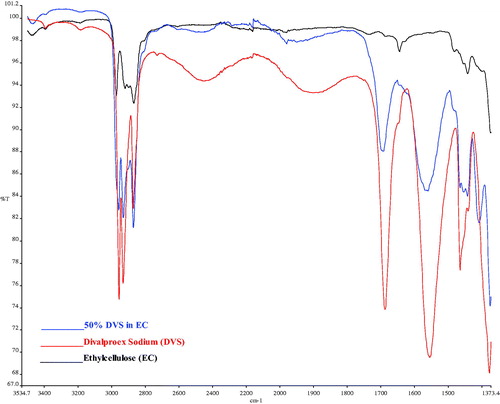
Fourier transformed infrared spectroscopy analysis
FTIR spectra of DVS () shows characteristic peaks due to stretching of carbonyl group (>C = O) at 1688.39 cm−1, peaks at 2956.76, and 2871.71 cm−1 due to methyl groups, peaks at 1555.28 and 1378.57 cm−1 are due, respectively, to antisymmetric and symmetric O-C-O stretching vibrations of the carboxyl salt, peaks at 2454.89 and 1899.26 cm−1 are due to –OH groups of carboxylic acid that are intramolecularly bounded. The peaks at 2932.47 and 1688.39 cm−1 are due to stretching of methylene groups and at 1465.48 cm−1 and 1378.57 cm−1 due to bending of C-H. Shifting of peaks towards higher wavenumber indicates intermolecular interaction. In the FTIR spectra of 50% w/w divalproex sodium in ethylcellulose, the peak due to stretching of carbonyl group was located at 1693.31 cm−1 in contrast to at 1688.39 cm−1 in the pure API. The peaks at 2454.89 and 1899.26 cm−1 were found to disappear indicating the extensive involvement of the hydroxyl group of carboxylic acid in the hydrogen bond formation. Considering the structure of DVS and EC and since EC has several proton donor/acceptor possibilities per monomeric unit, the shift of the carbonyl stretching frequency along with the disappearance of the stretching hydroxyl group peaks indicates the formation of strong hydrogen bond between the API and polymer. A new peak at 1980.41 cm−1 was found to appear. The peak at 1693.31 cm−1 was found to be broader, which indicates the presence of amorphous form. As per the mechanisms of plasticization, the plasticizer interacts with the polymer by breaking polymer-polymer interaction and forming plasticizer-polymer interaction (Marcilla and Beltran Citation2004). Plasticization of ethylcellulose by divalproex sodium can be explained by the formation of strong hydrogen bond as shown by the FTIR spectra. Also, strong interaction like hydrogen bonding leads to one phase system between API and polymer as indicated by single glass transition temperature. As a rule of thumb, the glass transition temperature should be 50° C above room temperature to avoid any phase separation. However, the formation of a strong interaction between API and polymer might help prevent any phase separation during storage.
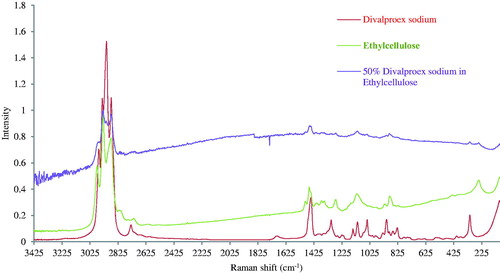
Raman spectroscopy
shows the Raman spectra of divalproex sodium, ethylcellulose, and films containing divalproex sodium and ethylcellulose. Divalproex sodium shows characteristics peak at 1691 cm−1 due to the stretching of the carbonyl group (>C = O). This peak is absent in the Raman spectra of the films. This might be due to the involvement of carbonyl group in the formation of a strong hydrogen bond with ethylcellulose. This further strengthens the results from FTIR spectra indicating the formation of a hydrogen bond with ethylcellulose.
Figure 7. Polarized microscopic image(40×) of ethylcellulose film containing (A) 10% divalproex sodium of ethylcellulose by weight, (B) 20% divalproex sodium of ethylcellulose by weight, (C) 30% divalproex sodium of ethylcellulose by weight, (D) 40% divalproex sodium of ethylcellulose by weight, (E) 50% divalproex sodium of ethylcellulose by weight.
Stability studies
XRD diffractograms for DVS, EC, and stability samples of the DVS-EC films stored at 45 °C/75% RH for 2 weeks in open condition are as shown in . It can be seen that there is no crystallinity in the DVS-EC films on exposure to stability conditions. Also, DSC thermograms () of the stability samples indicated a single glass transition temperature, which did not change significantly after storing the samples at stability conditions (p = 0.4955 > 0.05). This indicates that the films are physically stable at the given storage condition similar to that of initial condition samples and there is no phase separation when exposed to stability conditions. The stability of the DVS-EC films might be due to the similar solubility parameter of DVS and EC. Therefore, choice of plasticizer based on solubility parameter of the polymer is recommended.
Conclusion
In the current study, the mechanical, thermal, and rheological characterizations clearly demonstrate that DVS was able to plasticize EC films. Plasticization was attributed to the presence of valproic acid in DVS. Scattered crystals of sodium valproate were found in the films; however, since the concentration of sodium valproate was too low, it could not be detected during DSC analysis. The presence of a single Tg established complete miscibility between valproic acid, a component of DVS, and EC. TGA study indicated that valproic acid may dissociate from the coordination complex of DVS in order to plasticize EC. The FT-IR and Raman spectroscopy results proved the presence of hydrogen bonding between DVS and EC. This study can prove to be useful for developing a sustained release melt extruded formulation of DVS since DVS can act as a processing aid to reduce the extrusion temperature owing its ability to plasticize ethylcellulose.
Acknowledgements
The authors express their heartfelt thanks to Dr. Abu Serajuddin, Dr. Suhas Gumaste, and St. John’s University to allow use of their lab facilities for this work. The authors acknowledge Rutgers University for providing research facilities and G&W PA Laboratories for providing financial assistance and research facilities to carry out this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alsarra IA, Al‐Omar M, Belal F. 2005. Valproic acid and sodium valproate: comprehensive profile. Profiles Drug Subst Excip Relat Methodol. 32:209–240.
- Hyppölä R, Husson I, Sundholm F. 1996. Evaluation of physical properties of plasticized ethyl cellulose films cast from ethanol solution part I. Int J Pharm. 133:161–170.
- Kreuter J. 1999. Feste dispersionen. [Fixed dispersions]. Grundlagen Der Arzneiformenlehre Galenik. [Basics Pharm Forms Galenics]. 2:262–274.
- Leuner C, Dressman J. 2000. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 50:47–60.
- Marcilla A, Beltran M. 2004. Mechanisms of plasticizers action. Handbook of plasticizers. Ontario (Canada): ChemTec Publishing.
- Rahman M, Brazel CS. 2004. The plasticizer market: an assessment of traditional plasticizers and research trends to meet new challenges. Prog Polym Sci. 29:1223–1248.
- Rao VM, Engh K, Qiu Y. 2003. Design of pH-independent controlled release matrix tablets for acidic drugs. Int J Pharm. 252:81–86.
- Sherman BC. 2000. Solid substances comprising valproic acid and sodium valproate. United States patent US 6,077,542.
- Siepmann F, Le Brun V, Siepmann J. 2006. Drugs acting as plasticizers in polymeric systems: a quantitative treatment. J Controlled Release 115:298–306.
- Vesey CF, Farrell T, Rajabi-Siahboomi AR. 2005. Evaluation of alternative plasticizers for surelease®, an aqueous ethylcellulose dispersion for modified release film-coating. Miami (FL): Controlled Released Society Annual Meeting and Exposition.
- Wu C, McGinity JW. 1999. Non-traditional plasticization of polymeric films. Int J Pharm. 177:15–27.
- Wu C, McGinity JW. 2003. Influence of methylparaben as a solid-state plasticizer on the physicochemical properties of eudragit® RS PO hot-melt extrudates. Eur J Pharm Biopharm. 56:95–100.