Abstract
The bioavailability of drugs is dependent on several factors such as solubility and the administration route. A drug with poor aqueous solubility, therefore, poses challenges with regards to its pharmaceutical advance and ultimately its biological usage. Lipid nanoparticles have been used in pharmaceutical science due to their importance in green chemistry. Their biochemical properties as ‘green’ materials and biochemical processes as ‘green’ processes mean they can be environmentally sustainable. Generally, lipid nanoparticles can be employed as carriers for both lipophilic and hydrophilic drugs. The proposed administration route for nanoparticles can present advantages and disadvantages which should be considered by a formulator. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are attractive delivery systems because of their ease of manufacture, biocompatibility, biodegradability, and scale-up capacity of formulation constituents. The easy and simple scalability of novel SLNs and nano lipid carriers, along with their various processing procedures, recent developments, limitation and toxicity, formulation optimization and approaches for the manufacture of lipid nanoparticles, lyophilization and drug release are comprehensively discussed in this review. This review also summarizes the research data related to the various preparation methods and excipients used for SLNs and NLCs in recent years.
1. Introduction
Various drug-delivery technologies have achieved research interest in the last few decades and an exciting section of this has been the improvement of nanomedicine and nano-delivery systems (Patra et al. Citation2018). Many nanoparticulate drug deliveries can be used to improve drug bioavailability via different mechanisms such as enhancing the permeation of drug or dominating the first-pass effect and, or the efflux of the P-glycoprotein (P-gp) (Ye et al. Citation2020; Halder et al. Citation2021). Lipid-based nanoparticles are however non-biotoxic for in vitro/in vivo usages and as such dynamic advancement has been focused in the field of lipid-based DNA/RNA nanocarriers (Duan et al. Citation2020). Most lipids applied to synthesize lipid nanoparticles are biodegradable and biocompatible with few adverse side effects and chronic toxicity (Xu et al. Citation2022). Some polymeric nanoparticles have shown toxic effects during in vivo degradation (Cheng et al. Citation2021). The biocompatibility and physiochemical diversity properties of lipids and their ability to improve drugs bioavailability have offered a promising drug delivery system. Furthermore, lipid-based formulations can improve drug absorption in a variety of different ways such as enhancing membrane permeability, enhancing solubilization capacity, increasing intestinal drug dilution, inhibiting the P-gp efflux transporters, reducing cytochrome P450s (CYPs) enzymatic activity, increasing chylomicron biosynthesis and lymphatic transport rate (Ulldemolins et al. Citation2021). Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are two major kinds of lipid-based nanoparticles.
SLN was presented as the first generation of solid lipid carrier systems in the nanometer dimension, while NLC is often considered the second generation of SLN (Fonseca-Santos et al. Citation2020). SLNs possess the combinational benefits of different carrier systems which include nanoemulsions, polymeric nanoparticles, and liposomes. SLNs are made from physiologically accepted biocompatible excipients (similar to liposomes and nanoemulsions). Moreover, their solid matrix can successfully keep the loaded active pharmaceutical drugs contrary to enzymatic degradation and can deliver the maximum plasticity to achieve modified release profiles (Kelidari et al. Citation2015; Khezri et al. Citation2020). Besides, large industrial-scale production of SLN is possible by several methods such as high-pressure homogenization (HPH) and emulsion (Souto EB et al. Citation2020).
2. The impacts of green chemistry on the pharmaceutical industry
Pharmaceutical production is the supreme solvent-intensive and the least efficient of all chemical industries causing waste prepared per unit of produce (Rajagopal R Citation2014). To circumvent this challenge, ‘green’ methods have been developed as a way of decreasing the ecological influence the pharmaceutical industry has. Over the past few decades, the field of green technology, also called sustainable chemistry has expanded. Many independent activities have been carried out in research, science, and industry (Sanjay Citation2019). Green chemistry has a good plan, production, and use of chemical harvests and facilities which are environmentally safe. Nevertheless, while green chemistry works mainly on technical issues and engineering, sustainable chemistry includes all life cycle phases. Therefore, the physiological lipid component in the fabrication process of SLNs and NLCs makes this drug delivery system one of the most favorable delivery systems. The goal of this review is thus to explicitly describe and highlight the two kinds of novel and simple scaled-up SLN and NLC with their accompanying recent developments, limitation and toxicities, formulation optimization and approaches for the manufacturing of lipid nanoparticles, lyophilization, and pharmaceutical application. This review also presents the research studies reported on the various technique of production and their essential outcomes.
3. Recent progress in SLNs
SLNs are the type of nanosphere that consists of solid lipids with a photon correlation spectroscopy (PCS) between 50 and 1000 nm. These lipidic components can comprise complex mixtures of glyceride, purified triglycerides, or even waxes, that are solid at both the ambient temperature (25 °C) and the human body (around 37 °C), and are dispersed in suitable surfactant(s) (Kanojia et al. Citation2022). Compared to traditional carriers such as polymeric micro- and nanoparticles, liposomes or emulsions, SLN purports itself as an alternative drug delivery system. SLNs are remarkable lipid-based drug carriers because of many factors which include (I) materials used consisting of biodegradable, low toxicity, and biocompatible components; (II) having an average size from 50 to 1000 nm after drug encapsulation; (III) the lower cost and quick scalability of the particle production process (Mishra V et al. Citation2018).
As evident in the consequences of current research studies, they have the capacity to carry anti-tumor drugs with contrast agents and deliver simultaneous diagnosis and treatment. Kuang et al. revealed that SLNs with c(RGDyK) were considered as effective carriers to develop the targeted delivery of IR-780 to cancers cell (Kuang et al. Citation2017). The cRGD-IR-780 SLN considerably enhanced the photothermal therapy and tumor-specific targeting ability along with the ability of imaging of in vivo fate of the SLN combined IR-780 iodide photosensitizer (Kuang et al. Citation2017).
SLNs have been examined for incorporating various contrasting agents, such as iron oxide (Świętek et al. Citation2020), technetium-99 (99mTc) (Ghazizadeh et al. Citation2018), and carbon dots (Arduino et al. Citation2020). Current treatment possibilities for cancer were achieved by encapsulating an SLN with a quantum dot as a contrast agent (Mussi and Torchilin Citation2013). Both biomacromolecules and small-sized drug molecules comprising peptides and proteins can be loaded into SLNs for specific purposes. For instance, insulin as proteins/peptides typically suffers challenges in oral delivery as they are degraded during passage through the gastrointestinal tract (GIT) medium. An endosomal escaping agent was thus loaded into an SLNs lipidic core for attaining an endosomal escape for insulin drug delivery (Xu et al. Citation2018). It was reported that hemagglutinin2 peptide within SLN enhanced the insulin endosomal escape which protected the biological activity of insulin through the intracellular transport following oral administration. The most beneficial chemotherapeutic agent for colorectal carcinoma is the combination of siRNA and paclitaxel. The solid lipid matrix was integrated with a quantum dot and paclitaxel whereas siRNA was electrostatically attached to the exterior surface of SLN. To achieve the synergistic anticancer activity, dual therapeutic agent siRNA and paclitaxel loaded in SLN effectively accumulated in lung carcinoma. Sun et al. exhibited the effective integration of gadolinium(III) and fluorescein isothiocyanate complexes in SLN, which could be used as a tumor-absorbable contrast agent for the detection of colorectal tumors (Sun et al. Citation2016).
SLNs suffer from different shortcomings, for example, poor loading efficiency, drug leakage following polymorphic transformation and comparatively large water amount of the dispersions (Jacob et al. Citation2022). The drug with low entrapment efficiency and loading capacity of the conventional SLN was as a result of a tight packing of the lipid crystal network, the drug solubility in the melted lipid, the polymorphic state of the lipid matrix, and a physical and chemical structure constituent of the solid lipid matrix. The addition of hydrophilic ingredients to the surface of SLNs can modulate the preparation of the ‘protein corona’, i.e. the addition of plasma proteins to the surface of SLNs when they absorb into the bloodstream (Chen et al. Citation2019). This protein corona preparation exists in various lipid nanoparticles (including SLNs) (Nishihira et al. Citation2019) and has been confirmed to adjust nanoparticle biodistribution (Chen D et al. Citation2017). It has been proven that the absorption of polyethylene glycol (PEG) molecules results in a significant reduction in the amount of protein bonds (Partikel et al. Citation2019). Additionally, incorporating cytotoxic biocides into the SLNs can diminish cytotoxicity in eukaryotic cells while enhancing antimicrobial defense (Sandri et al. Citation2013). For example, it has been revealed that tilmicosin-loaded castor oil-SLNs can decrease drug side effects, for instance, cardiotoxicity (Xie et al. Citation2011). Other researchers have attempted to attain selective drug delivery in advanced pH-sensitive SLNs, which can adjust their release kinetics depending on the pH of the environment (Chuang et al. Citation2017). Mhule et al. incorporated antibiotic drugs into SLNs to improve drug delivery to the infection site (target tissue) (Mhule et al. Citation2018). Cetyl palmitate-based PEGylated was loaded with sorafenib and paclitaxel through a microfluidic technique. The anti-cancer activity of the SLNs formulation was estimated with 2D and 3D-cell cultures of U87-MG cell line and 2D-cell model of the human alveolar adenocarcinoma cells line (A549) (Arduino et al. Citation2021). In comparison with a 2D cell model, the 3D spheroid model indicated higher cell viability in terms of both drugs and SLNs. This consequence may be as a result of the minor cell nanoparticle interactions of the cells in the 3D cell cultures versus the traditional 2D cell culture, leading to a reduction of anti-proliferative activities of the cytotoxicity compounds which accomplish more potently on the proliferating (Fröhlich Citation2018; Figueiredo et al. Citation2019). The SLN prepared using microfluidics shown here can open a remarkable route for the scale-up of the production and future standardization of these nanosystems within the biomedical field.
Ramasamy et al. (Citation2014) prepared a layer-by-layer doxorubicin–dextran sulfate–SLNs to kill tumor cells. The anionic doxorubicin–dextran sulfate–SLNs were covered with cationic chitosan followed by decorating successively with anionic hyaluronic acid (HA). In this study, a novel approach of polyelectrolyte multilayer was used to facilitate EPR properties and prolong the blood circulation time of the hydrophilic drug. Transferrin (Tf) conjugated with SLNs was prepared to load curcumin for active targeting of prostate cancer cells. In comparison with curcumin-SLNs, prepared Tf-SLNs demonstrated minimal cytotoxicity while Tf-CRC-SLNs showed remarkable in vitro anti-proliferative properties. Cellular uptake of Tf-curcumin-SLNs was reported to be remarkably higher in comparison with pure drug or unconjugated SLNs (Akanda et al. Citation2021).
4. Recent progress in NLCs
To eliminate the shortcomings of SLN, NLC systems were introduced. The NLCs are formed from a combination of a diverse group of lipid molecules, typically the combination of both solid and liquid lipids as a core matrix. These cause defections in the matrix structure to accommodate other drug molecules as compared to SLNs. Despite the liquid lipid structure, the NLC matrix is solid at both body and room temperatures. NLCs remain in the solid state by adjusting the ratio of liquid lipid to solid lipid. Compared to emulsions, NLCs can effectively prevent particle coalescence through the solid matrix. NLCs have the benefits of SLNs such as sustained drug release capabilities, biodegradation, low toxicity potential, drug protection against harsh environments and prevention of organic solvents during manufacturing (Parvez et al. Citation2020; Rahman et al. Citation2020).
Recent research examinations show the significant role of NLC in the field of medicine. Multifunctional nanocarriers of IR 780 and Coumarin 6 fluorescent dye encapsulated CXCR4-targeted NLCs for breast cancer therapy using photodynamic therapy have been reported (Li H et al. Citation2017). The progressed system was demonstrated to be extremely effective in inhibiting cancer cell progression and metastasis and in parallel permitting imaging (Li H et al. Citation2017). Olerile et al. fabricated an NLC loaded with quantum dot and paclitaxel that was highly capable of monitoring and following cancer cell growth and simultaneously inhibiting tumor cells in the murine tumor model of hepatocellular cancer (Olerile et al. Citation2017).
This current study attempted to investigate the co-loaded NLC based on quantum dots and paclitaxel to provide an interpretation essential for a preclinical explanation of the formulation in part. In these experiments, the short-term stability and internalization of co-loaded NLC by HepG2 and MCF-7 cells were examined. It was theorized that the aforesaid investigations would prove that the co-loaded NLC was an effective clinical translation candidate for cancer theragnostic (Olerile Citation2020).
5. SLN/NLC applications in gene and peptide delivery systems
Nucleic acids and biological macromolecules are not able to diffuse through the mammalian cell membrane due to their highly anionic nature and hydrophilic properties. Their combination with cationic lipid nanoparticles can therefore enhance their intracellular delivery (Algarni et al. Citation2022). SLNs/NLCs have been recognized as non-toxic and effective lipophilic colloidal carriers for the delivery of drugs and various biological macromolecules such as peptides, DNA, etc. NLCs have significant nanostructures to accommodate drugs as well as genes and therefore attain higher loading capacities. The decorating of NLCs with Tf was prepared for enhanced co-delivery of a green fluorescence protein plasmid. Paclitaxel-loaded Tf-DNA-NLC showed high-efficiency gene transfection, low cytotoxicity, and improved anti-cancer efficacy in both in vitro and in vivo models (Shao et al. Citation2015). In this self-same research, two different types of nanocarriers (pEGFP and doxorubicin) were loaded on NLCs and SLNs. Tf-decorated doxorubicin and pEGFP-loaded NLCs (T-NLC) showed significantly higher gene transfection activity and improved in vivo anti-cancer efficacy than that of the modified SLNs. T-NLC also indicated a surface charge of +19 mV and an average particle size of 198 nm (Han et al. Citation2014).
Nanocarriers modified with various ligands were applied for lung cancer gene therapy. The goal of this investigation was to progress dual ligand-modified nanocarriers, which could specifically target cancer cells and increase cellular uptake to enhance the uptake of genetic material. HA and Tf decorating PEG-di-stearoyl phosphatidylethanolamine (HA-PEG-DSPE and Tf-PEG-DSPE) ligands were produced. Unique HA and Tf ligand-modified, plasmid-improved green fluorescent protein decorated NLC (Tf/HA-pDNA NLC) were prepared. The novel fabricated NLCs could effectively load HA, Tf, and gene operated as targeting ligands to enhance the cell-targeting activity (Zhang et al. Citation2017). Furthermore, cationic SLNs have been commonly applied in gene delivery because of the electrostatic interaction among the positive charges of the lipid and the negative charges of the DNA which cause the preparation of a complex (Dolatabadi et al. Citation2015). Jin et al. prepared siRNA-PEG/SLN, which can not only cross the blood–brain barrier (BBB) but also be delivered to the tumor environment without obvious systemic toxicity (Jin et al. Citation2011). SLN decorated with insulin through the micelle-double-emulsion technique was fabricated by Liu et al. In addition to greater stability during nebulization, SLNs presented a higher bioavailability of insulin in the lungs because of the deposition of insulin-SLNs (Liu et al. Citation2008). The advantages/disadvantages of lipid nanoparticles applied in gene/peptide delivery are presented in .
Table 1. Advantages/disadvantages of lipid nanoparticles as gene and peptide delivery systems.
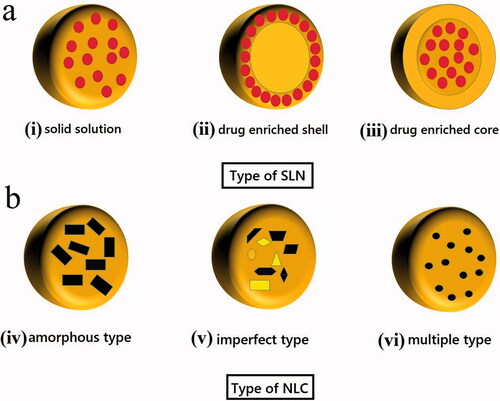
6. Different types of SLN/NLC
Different types of SLNs and NLCs are depicted in . It is crucial to note that the localization of the drug in the SLN/NLC relies on the lipophilicity and the structure of the target molecule (Borges et al. Citation2020). For example, in SLN type I, in the homogeneous matrix of solid solution, the drug is dispersed in the lipid core whereas in SLN type II, in the drug-enriched shell, a drug-free lipid core is prepared, and an external solid shell with both drug and lipid is prepared. The HPH method is used to produce both types of SLNs. The drug-enriched core model, SLN type III, is achieved when the drug concentration is near to its saturation solubility in the lipid, which results in its precipitation in the core and lipid with extended coverage prepared. It is notable that minor variations in some relevant variables can have an impact on SLN and NLC structure thus changing their applicability. For example, SLN type III is suitable for reaching an extended drug release profile, whereas SLN type I can display a controlled release profile, while SLN type II is not appropriate for this aim (Souto E et al. Citation2007). Moreover, imperfect crystal, type I NLC, is achieved by using solid lipids and liquid lipids, producing a matrix with many voids and spaces which can carry a payload of drug molecules in amorphous clusters. NLC type II as an amorphous model is achieved when using special lipids (i.e. hydroxyl octacosanyl, isopropyl myristate, dibutyl adipate, or hydroxyl stearate) which do not recrystallize after homogenization and cooling, producing a homogenous amorphous matrix that reduces drug leakage. NLC type III as a multiple model is obtained from liquid lipids moieties which are effectively dispersed in the solid lipid matrix because of the phase separation and it is achieved by mixing higher amounts of liquid lipids with solid lipids where the liquid lipids solubility in the solid lipid is increased. Type III model is acceptable to gain a high drug entrapment efficiency and controlled drug release enhancement that are commonly more soluble in liquid lipids (Ganesan and Narayanasamy Citation2017).
7. Limitations and toxicity of SLNs and NLCs
The main restriction in lipidic nanoparticles is their affinity to fuse, particularly when the average size of synthesized nanoformulation is less than 100 nm. The fusion causes the escape of encapsulated contents from the lipid vesicles and improves dispersity (Carregal-Romero et al. Citation2018). Nevertheless, this concern can be resolved by covering the lipid nanoparticles surface with PEG coating. Several lipid-based nanocarriers applied for the delivery of chemotherapeutic agents through the parenteral route may have cationic components for the attachment of targeting ligands to specific locations which can lead to an immune response. Similar to all nanoparticles, the evaluation of NLCs toxicity includes the impact of the surface charge, particle size, and other physicochemical features on the product safety (Haider et al. Citation2020). Other studies have reported that maximum cell lines could tolerate up to 1 mg/mL of lipid doses of drug-free NLCs (Haider et al. Citation2020). Numerous publications have reported sufficient cellular tolerability for lipid-based nanocarriers synthesized with positively charged cationic surfactants such as cetyltrimethylammonium bromide (CTAB). It was shown that SLNs fabricated with CTAB indicated low toxicity at concentrations above 1 mg/mL (Almeida et al. Citation2017). It is however important to note that the main risks are related to the usage of CTAB in the fabrication of SLNs as it increases the release of calcium from neutrophils and causes their damage (Hwang, Aljuffali, Hung, et al. Citation2015). On the other hand, NLCs synthesized using other surfactants such as poloxamer 188 and polysorbate 80, displayed low toxicity and sufficient biocompatibility. It has also been reported that applying a mixture of surfactants to enhance the stability of the product could increase the toxicity risk (Souto EB et al. Citation2020).
One of the challenges about the safety of NLCs is their affinity to induce oxidative stress. Hyperactivation of cellular defense mechanism in HepG2 liver tumor cells is the mark of oxidative stress activation which is detected after cure with CTAB modified lipid-based nanoparticles (Doktorovova et al. Citation2014). To reduce the risk of oxidative stress, curcumin or a combination of harmless ingredients such as ethoxylated medium-chain glycerides and hard fats have been used (Kyadarkunte et al. Citation2015).
Some researchers have also considered the side effects of NLCs on animal health (Carvalho et al. Citation2020). Indications show that chemotherapeutics agents incorporated into lipid-based nanoparticles are usually harmless after oral, parenteral, dermal, and ocular administration. Most of the detected side effects after administration were mainly attributed to the drug (del Pozo-Rodríguez et al. Citation2013). Some toxic properties, such as neurovascular injury, neuroinflammation, and microglia activation were injected with SLNs in mice (Hwang, Aljuffali, Hung, et al. Citation2015). The observed impacts were because of the joining of cationic ingredients or aggregation of the particles when presented to a medium with high ionic strength or proteins. The use of PEG in enhancing SLN’s stability was adequate to inhibit the informed inflammatory reaction (Hwang, Aljuffali, Lin, et al. Citation2015).
8. Quality by design method to optimize formulations of lipid nanoparticles
Traditional pharmaceutical improvement methodologies depending on quality through testing is outdated. In these techniques, the product quality can be controlled by adjusting the crude materials (e.g. excipients and drugs) and reproducing the procedures. Finalized products must satisfy the regulatory agencies’ specifications or reasons for failure must be established to allow for the process to be restarted. Quality by design (QbD) method has been presented as a way of overcoming these quality control challenges. QbD develops assembling routes and guarantees the safety and ultimately the quality of products (Zhang and Mao Citation2017; Cunha et al. Citation2020).
Various statistical tests and mathematical models have been applied in regards to parameters that impact the physical elements of the resulting nanosystems. These consist of differences in composition and manufacturing factors (e.g. homogenization pressure cycles, sonication amplitude time, and emulsification time). These are basic factors that impact nanoparticle/globule polydispersity index, mean size, drug encapsulation efficiency, zeta potential, and in vitro drug release.
Bhatt et al. utilized a QbD approach for the improvement of curcumin loaded in SLNs for controlling breast cancer (Bhatt et al. Citation2018). Glyceryl monostearate was applied as a core material. The small lipid particles enhanced cellular uptake and instigated superior apoptosis in human breast adenocarcinoma cells compared to its free curcumin counterpart.
9. Regulatory status, commercialization plan and safety of lipidic nanoparticles
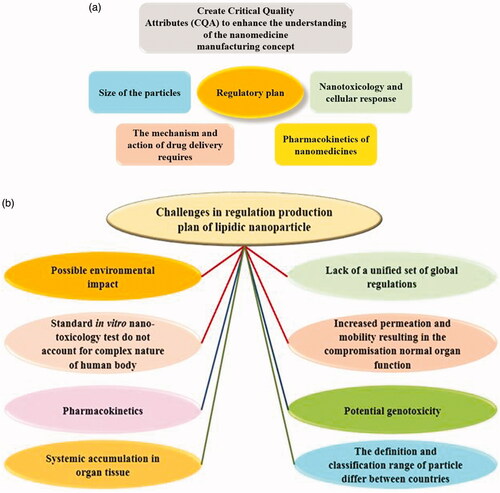
Nanomaterials have gained several advantages which make them suitable candidates for a wide range of clinical applications. Though there has been much hype around the emerging field of nanomedicine, there are currently very little official regulations in this field. Many nanomedicines study through direct interaction with biomolecules (e.g. protein, gene, compound, and chemical) are vital for cell division and normal genome function (Zhang et al. Citation2012), all of which can lead to mutagenicity and genotoxicity. Currently, the specific interactions of biological systems with several nanolipidic are not completely realized thus making the deduction about the toxicological and physicochemical properties of nanomedicines are challenging. The absence therefore of official regulation of nanolipidic production and nanomedicines for health-related applications is a global problem. Some concern is thus imperative in the regulatory plan and is as such presented in . The main issues faced in the regulation of SLN are described in . Nevertheless, this over-cautious approach seems to be demonstrating great inertia within the field. However, the standard checks needed for approval are still vague and align with the regulation for small drug molecules () which do not correctly reflect the nanomaterials potential. If the regulation was therefore appropriate and bespoke, it would enable better filtration at preclinical studies, decreasing failure rate either later in the clinical trials or indeed after clinical use and marketing.
Figure 2. (a) Regulatory status of lipidic nanoparticles production. (b) Diagram highlighting the major challenges faced in the regulation of lipidic nanoparticle. (c) Approval process for commercialization plan of small drug molecules. Imaged adapted from Foulkes et al. (Citation2020).
9.1. Commercially available products from lipid-based nanoparticles in market
Most of the commercial lipid nanoparticles products are cosmetic products such as Intensive Serum Nano Repair Q10, NanoLipid Q10 CLR, extra moist softener and extra moist emulsion, IOPE SuperVital cream, serum, eye cream, NLC Deep Effect Eye Serum, NLC Deep Effect Reconstruction Cream, Swiss Cellular White Intensive Ampoules, Olivenöl Augenpflegebalsam Olivenöl Anti Falten Pflegekonzentrat (Pardeike et al. Citation2009).
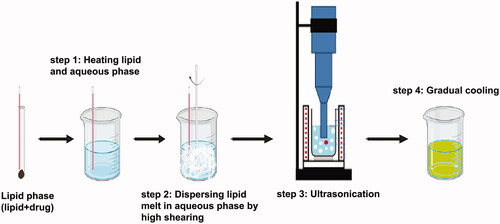
10. Approaches of preparation
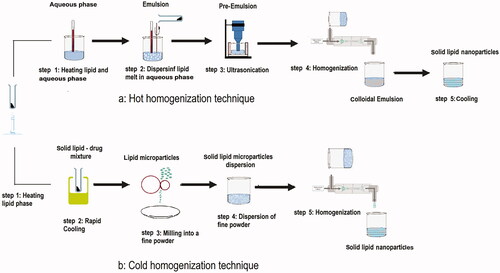
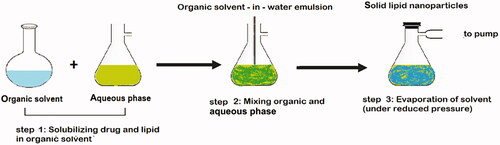
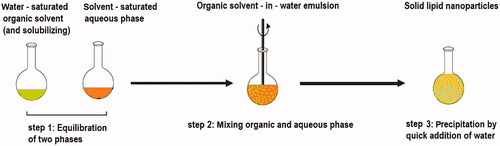
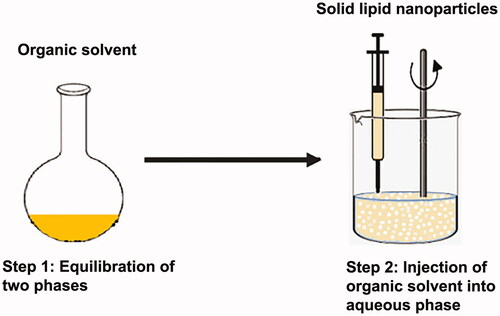
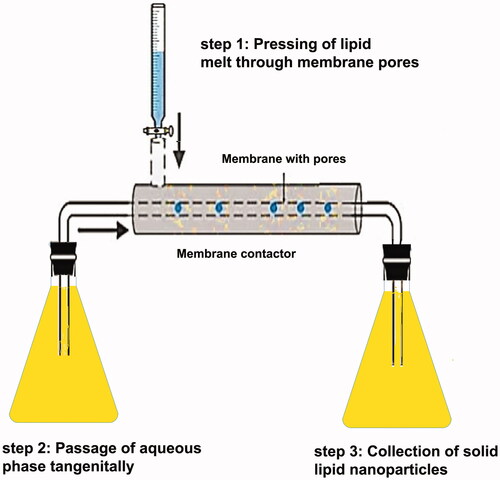
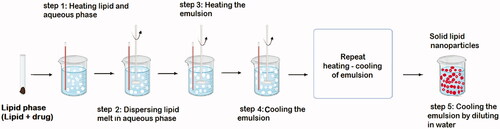
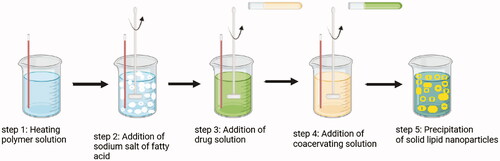
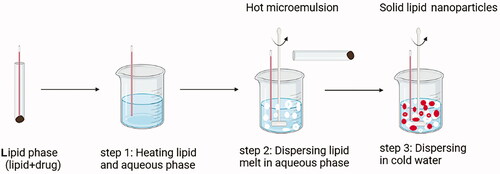
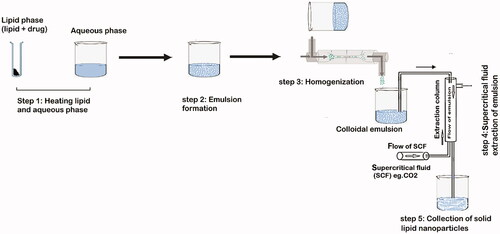
Many traditional physical methods have been considered for the controlled preparation of nanoparticles. The advantages of these approaches are limited by their energy supplies, lower level of hazardous products and their potential for high performance (Malik et al. Citation2014). Recently, some lipid-based nanoparticles (SLNs/NLCs) have been prepared via green procedures such as ultra-sonication (Aghazadeh et al. Citation2021; Boskabadi et al. Citation2021; Saeedi et al. Citation2021), supercritical fluid method (Kanwar et al. Citation2021), ultrafiltration (Passos et al. Citation2020), flocculation with surfactants (Malik et al. Citation2014; Li et al. Citation2021), and HPH (Vinchhi et al. Citation2021). The mechanisms, advantages, and disadvantages of each method are considered in .
Table 2. Comparison of different routes applied for the production of SLNs/NLCs.
11. Lyophilization of lipid nanoparticles
Lyophilization is a favorable method to enhance the physical and chemical stability of SLNs. Moreover, conversion into a solid form avoids hydrolysis reactions and Ostwald’s ripening (Mishra DK et al. Citation2012; Swathi et al. Citation2012). Stresses that destabilize the colloidal nanoparticulate suspension are probably produced throughout the freeze-drying process. Irreversible fusion/aggregation of nanoparticles might destabilize the colloidal system of the nanoparticulate. Observably, two additional formulations between the transformations are needed that might be the cause of additional stability challenges. Under vacuum, the primary change is obtained from an aqueous dispersion to a powder containing the water evaporation and the sample freezing. Sample freezing may lead to stability challenges due to the freezing out impact which leads to conversions in the pH and the osmolarity. Resolubilization includes, at least during its primary periods, a condition that considers the aggregation of particles.
To mitigate against this issue, specific excipients must be previously combined before freezing to keep the suspension of the nanoparticulate. Certain excipients with cryoprotectant properties can be added to protect the system from freezing stress and lyoprotectants are added to protect from the drying stress. Adding cryoprotection is essential to reducing the aggregation of SLN and enhancing the redispersion of the dry produce (Campos JR et al. Citation2019; Amis et al. Citation2020). Mannose, glucose, sorbitol, polyvinylpyrrolidone, and trehalose have cryoprotective properties. They reduce the crystallization, water osmotic pressure, and the vitreous state of the frozen paradigm (De Jesus and Zuhorn Citation2015).
12. Patent perspective of SLNs and NLCs
SLNs and NLCs have been examined for numerous usages in topical applications, cosmetics, non-invasive methods, food chemistry as a preservative, targeted delivery of anticancer drugs and the co-delivery of multiple medications (Dhiman et al. Citation2021). Various approaches of SLNs and NLCs fabrications and or encapsulating therapeutic agents into NLCs or SLNs have been patented. The summary of the patents according to SLN and NLC-based carriers has been indicated in .
Table 3. List of patents related to pharmaceutical, biomedical, and other applications of SLN and NLC.
13. Applications of lipid nanoparticles and various routes of administration
summarizes various publications which consider the numerous routes of administration such as topical, parenteral, ocular, oral, and brain delivery that have been utilized in the manufacture of lipid nanoparticles.
Table 4. Various loaded active compounds and routes of lipid nanoparticle administration.
14. The topical route of administration
Skin correlation is a common illness all over the world (Seth et al. Citation2017). The main challenge for curing this illness is the low efficiency of drugs to permeate through the skin. The stratum corneum is the main skin barrier. This can however be bypassed by changing the permeation from follicles or transcellular to paracellular. SLNs and NLCs have been fabricated to enhance permeation or penetration in the skin. Topical amphotericin B SLNs were prepared by a novel solvent diffusion technique with slight modification and lyophilized with and without cryoprotectants to determine their stability. It was reported that the SLNs particle size considerably enlarged in the SLN formulations lyophilized without cryoprotectants. Lipid nanoparticles have several benefits for manipulating drug delivery profiles () (Butani et al. Citation2016; Chen et al. Citation2017).
Table 5. Advantages/disadvantages of lipid nanoparticles as topical drug delivery systems.
14.1. Oral administration
High hepatic first-pass effect and, or partial drug solubility leads to low oral bioavailability which is the most significant challenge in the oral drug delivery system. Enhanced oral bioavailability is obtained by incorporating nanoparticle-based drug delivery systems. The surface modification of nanoparticles with chitosan improved the oral absorption of drugs (Enayatifard et al. Citation2018; Tan and Billa Citation2021). NLCs and SLNs indicate the benefit of extended drug release ability to keep a constant plasma concentration. P-glycoprotein efflux pumps and enzymatic or chemical degradation are other central issues in the oral drug delivery system. Lipid nanoparticles could improve lymphatic transport and inhibit the first-pass hepatic effect. For instance, a baicalin-NLC carrier system for oral delivery to improve bioavailability. The NLC was synthesized through emulsion-evaporation and low temperature-solidification technique. The drug loading and entrapment efficiency were 3.54% and 59.51%, respectively (Cirri et al. Citation2017). Lipid nanoparticles have several advantages/disadvantages when used for the oral route and have been tabulated ().
Table 6. Advantages/disadvantages of lipid nanoparticles as oral drug delivery systems.
14.2. Ocular administration
Ocular drug delivery has several drawbacks due to the physiological and anatomical characteristics of the eyes. Drug delivery is often to the eye’s frontal segment. Conjunctival blood flow, ocular blood barrier, corneal epithelium, and tear drainage are often challenges that have to be overcome. Lipid nanoparticles can cross the ocular blood barrier, control drug release, and protect drugs from lacrimal enzymes. In gene therapy, non-viral gene delivery containing SLNs and NLCs have been used for retinal targeting in retinal diseases. Indomethacin (IN)-SLNs and NLCs were formulated to examine their potential usage in topical ocular delivery. IN-loaded SLNs were fabricated through a hot homogenization technique. The surface modification of the SLNs with chitosan improved the ocular penetration of IN. IN SLNs (0.1% w/v) and NLCs (0.8% w/v) were successfully achieved (Chetoni et al. Citation2016). A summary list of advantages/disadvantages of this administration method is detailed in .
Table 7. Advantages/disadvantages of lipid nanoparticles as ocular drug delivery systems.
14.3. Parenteral administration
Lipid nanoparticles’ advantages/disadvantages are summarized in . Drug-loaded lipid nanoparticles can be injected intramuscularly, subcutaneously, intravenously, and directly adjacent to the target organs. In this manner, NLCs are suitable alternatives whereas SLNs are not appropriate carriers because of their inadequate drug loading. Carvacrol NLCs were prepared using a warm microemulsion technique and considered the effect of lipid matrix and concentration of components on the NLCs formation. The NLCs formulation with the lowest particle size (98 nm), highest encapsulation efficiency and narrowest size distribution, was optimized by using surfactant and beeswax (HLB = 9) as solid lipid and 5% of lipids (Galvão et al. Citation2020).
Table 8. Advantages/disadvantages of lipid nanoparticles as parenteral drug delivery systems.
14.4. Pulmonary delivery
For both systemic and local management, the pulmonary drug delivery system is a non-invasive method of drug delivery. Through this direct delivery profile, drug amount may be reduced, subsequently decreasing the drug adverse effects. Among delivery systems, lipid nanoparticles containing SLNs and NLCs for the pulmonary delivery systems have been examined. For instance, phosphodiesterase type 5 inhibitors – among which sildenafil citrate – show a key role in the cure of pulmonary hypertension. SLNs were prepared using a modified melt emulsification method. A sustained release and high encapsulation efficiency (88–100%) of the cargo above 24 h was observed. Besides, sterilization via autoclaving and nebulization with a jet nebulizer affected the drug entrapment and the colloidal stability of SLNs, demonstrating their potential as a pulmonary delivery system (Makled et al. Citation2017). A summary list of the advantages/disadvantages of this method can be found in .
Table 9. Advantages/disadvantages of lipid nanoparticles as pulmonary drug delivery systems.
14.5. Brain delivery
Drug delivery to the brain is one of the significant challenges due to the BBB. Nanoparticles are appropriate as brain drug delivery candidates because they can subsequently pass the reticuloendothelial system (RES). Inadequate permeation of drugs across the BBB and transported drugs efflux from the brain to blood circulation are two main challenges in brain drug delivery. To address these concerns, SLNs and NLCs as colloidal drug delivery systems have been employed. Novel levofloxacin/doxycycline (LEVO/DOX)-loaded SLNs were synthesized via an emulsification method through high-speed homogenization followed by ultrasonication. The results of the pharmacokinetic study of the optimized SLN-HPMC gel in plasma and the brain showed a considerable increase in the brain peak concentration and the AUC0–360 min in comparison to the intranasal LEVO/DOX free solution (Hady et al. Citation2020). The advantages/disadvantages of lipid nanoparticles used in brain drug delivery systems are documented in (Tosi et al. Citation2016).
Table 10. Advantages/disadvantages of lipid nanoparticles as brain drug delivery systems.
15. Nanomedicines approved for clinical use
A variety of nanotechnologies and nanomaterials have already achieved regulatory approval and some are in the clinical setting for further investigation. These include anticancer drugs such as doxorubicin, cemiplimab, paclitaxel (Kemp and Kwon Citation2021) and antifungals such as amphotericin B (Patra et al. Citation2018), ambisome (Foulkes et al. Citation2020), and natamycin (Chandasana et al. Citation2014). At the time of writing this review, a search was performed on the website www.clinicaltrials.gov, and a number of relevant consequences corresponding to the keywords ‘nanoparticles’ and ‘lipid’ were determined and some of them are presented in .
Table 11. Lipid nanoparticle drug delivery systems on currently active clinical trials.
16. Conclusions
Compared to other colloidal and polymeric nanocarriers, lipid nanoparticles are novel drug delivery systems that present several benefits. Ease of scalability, biodegradability, biocompatibility, and ability to achieve controlled release profiles are some of the most advantageous qualities that these lipid carriers present. An understanding of the two kinds of lipid nanoparticles (SLN and NLC) allows for targeted drug delivery using various methods of administration. Cytotoxicity of SLN/NLC has been considered in several publications via using various combinations of surfactants and lipids on different cell lines. In many examinations, the consequences demonstrate low levels of cytotoxicity. Furthermore, there is limited information associated with unwanted side-effects linked to the administration of lipid particles. It is therefore essential to consider the issues related to the physical instability of the nanoparticles, chiefly the formation of unexpected gelation or aggregates during administration, which can be hazardous at the time of usage. In a few cases, the reason for the observed side effect could perhaps be related to the choice of excipients. An appropriate selection of SLN/NLC dose and selection of excipients are therefore the most critical items for SLN and NLC safety. The concept of surface modification is also very promising in a bid to moderate these challenges. PEG coatings on nanoparticles shield the surface from aggregation, RES and prolonging systemic circulation time in the bloodstream. Additionally, formulations attributed to the stabilization of particles via the use of surfactants have been reported to demonstrate less toxicity in comparison to their lipid alone nanoparticles. For example, for poloxamer 188 and polysorbate 80, two commonly used surfactants in SLN and NLC formulations, sufficient evidence of their safety has been confirmed. Possible deficiency for SLN and NLC is that they have the potential to induce oxidative stress, hence induction of inflammatory response. Nevertheless, the evidence is still restricted. SLN and NLC are attractive candidates that will permit effective and targeted delivery of hydrophilic and hydrophobic drug compounds. Intracellular entry of drugs and its extended-release profile are the main properties of SLN/NLC drug delivery mechanisms that can diminish side effects and provide treatment of the root cause of the illness rather than the symptoms of the illness. Lipid nanoparticles are therefore considerable drug delivery systems for the delivery of different types of pharmaceutically active ingredients from small molecules to proteins and potentially genes.
| Abbreviations | ||
| 99mTc | = | technetium-99 |
| AUC | = | area under the curve |
| BBB | = | blood–brain barrier |
| CTAB | = | cetyltrimethylammonium bromide |
| CYPs | = | cytochrome P450s |
| DTX | = | docetaxel |
| EPR | = | enhanced permeability and retention |
| HPH | = | high-pressure homogenization |
| IN | = | indomethacin |
| LEVO/DOX | = | levofloxacin/doxycycline |
| MRT | = | mean residence time |
| NLCs | = | nanostructured lipid carriers |
| PCS | = | photon correlation spectroscopy |
| PEG | = | polyethylene glycol |
| P-gp | = | P-glycoprotein |
| QbD | = | quality by design |
| RES | = | reticuloendothelial system |
| SLNs | = | solid lipid nanoparticles |
| Tf | = | transferrin |
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Attama AA. 2011. SLN, NLC, LDC: state of the art in drug and active delivery. Recent Pat Drug Deliv Formul. 5(3):178–187.
- Abdelaziz HM, Freag MS, Elzoghby AO. 2019. Chapter 5 - Solid lipid nanoparticle-based drug delivery for lung cancer. In: Kesharwani P, editor. Nanotechnology-based targeted drug delivery systems for lung cancer. London: Academic Press; p. 95–121.
- Aghazadeh T, Bakhtiari N, Rad IA, Ramezani F. 2021. Formulation of kaempferol in nanostructured lipid carriers (NLCs): a delivery platform to sensitization of MDA-MB468 breast cancer cells to paclitaxel. Biointerface Res Appl Chem. 11(6):14591–14601.
- Ahmad N, Pandey M, Mohamad N, Chen XY, Amin MCIM. 2020. Chapter 21 - Hydrogels for pulmonary drug delivery. In: Dua K, Hansbro PM, Wadhwa R, et al., editors. Targeting chronic inflammatory lung diseases using advanced drug delivery systems. London: Academic Press; p. 441–474.
- Ahmed MM, Fatima F, Anwer MK, Aldawsari MF, Alsaidan YSM, Alfaiz SA, Haque A, Alanazi A, Alhazzani K. 2020. Development and characterization of brigatinib loaded solid lipid nanoparticles: in-vitro cytotoxicity against human carcinoma A549 lung cell lines. Chem Phys Lipids. 233:105003.
- Ajorlou E, Khosroushahi AY. 2017. Trends on polymer- and lipid-based nanostructures for parenteral drug delivery to tumors. Cancer Chemother Pharmacol. 79(2):251–265.
- Akanda M, Getti G, Nandi U, Mithu MS, Douroumis D. 2021. Bioconjugated solid lipid nanoparticles (SLNs) for targeted prostate cancer therapy. Int J Pharm. 599:120416.
- Algarni A, Pilkington EH, Suys EJ, Al-Wassiti H, Pouton CW, Truong NP. 2022. In vivo delivery of plasmid DNA by lipid nanoparticles: the influence of ionizable cationic lipids on organ-selective gene expression. Biomater Sci.
- Almeida H, Lobão P, Frigerio C, Fonseca J, Silva R, Sousa Lobo JM, Amaral MH. 2017. Preparation, characterization and biocompatibility studies of thermoresponsive eyedrops based on the combination of nanostructured lipid carriers (NLC) and the polymer Pluronic F-127 for controlled delivery of ibuprofen. Pharm Dev Technol. 22(3):336–349.
- Amis TM, Renukuntla J, Bolla PK, Clark BA. 2020. Selection of cryoprotectant in lyophilization of progesterone-loaded stearic acid solid lipid nanoparticles. Pharmaceutics. 12(9):892.
- Ansari MA, Almatroudi A, Alzohairy MA, AlYahya S, Alomary MN, Al-Dossary HA, Alghamdi S. 2020. Lipid-based nano delivery of Tat-peptide conjugated drug or vaccine-promising therapeutic strategy for SARS-CoV-2 treatment. Expert Opin Drug Deliv. 17(12):1671–1674.
- Arduino I, Depalo N, Re F, Dal Magro R, Panniello A, Margiotta N, Fanizza E, Lopalco A, Laquintana V, Cutrignelli A, et al. 2020. PEGylated solid lipid nanoparticles for brain delivery of lipophilic kiteplatin Pt(IV) prodrugs: an in vitro study. Int J Pharm. 583:119351.
- Arduino I, Liu Z, Rahikkala A, Figueiredo P, Correia A, Cutrignelli A, Denora N, Santos HA. 2021. Preparation of cetyl palmitate-based PEGylated solid lipid nanoparticles by microfluidic technique. Acta Biomater. 121:566–578.
- Asif AH, Desu PK, Alavala RR, Rao GSNK, Sreeharsha N, Meravanige G. 2022. Development, statistical optimization and characterization of fluvastatin loaded solid lipid nanoparticles: a 32 factorial design approach. Pharmaceutics. 14(3):584.
- Attama AA, Reichl S, Müller-Goymann CC. 2008. Diclofenac sodium delivery to the eye: in vitro evaluation of novel solid lipid nanoparticle formulation using human cornea construct. Int J Pharm. 355(1–2):307–313.
- Başaran E, Demirel M, Sırmagül B, Yazan Y. 2010. Cyclosporine-A incorporated cationic solid lipid nanoparticles for ocular delivery. J Microencapsul. 27(1):37–47.
- Beloqui A, Solinís MÁ, Delgado A, Évora C, Isla A, Rodríguez-Gascón A. 2014. Fate of nanostructured lipid carriers (NLCs) following the oral route: design, pharmacokinetics and biodistribution. J Microencapsul. 31(1):1–8.
- Beloqui A, Solinís MÁ, Rodríguez-Gascón A, Almeida AJ, Préat V. 2016. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine. 12(1):143–161.
- Bhatt H, Rompicharla SV, Komanduri N, Aashma S, Paradkar S, Ghosh B, Biswas S. 2018. Development of curcumin-loaded solid lipid nanoparticles utilizing glyceryl monostearate as single lipid using QbD approach: characterization and evaluation of anticancer activity against human breast cancer cell line. Curr Drug Deliv. 15(9):1271–1283.
- Bhatt S, Sharma J, Singh M, Saini V. 2018. Solid lipid nanoparticles: a promising technology for delivery of poorly water-soluble drugs. Acta Pharm Sci. 56(3):27.
- Bhise K, Kashaw SK, Sau S, Iyer AK. 2017. Nanostructured lipid carriers employing polyphenols as promising anticancer agents: quality by design (QbD) approach. Int J Pharm. 526(1–2):506–515.
- Blasi P, Giovagnoli S, Schoubben A, Puglia C, Bonina F, Rossi C, Ricci M. 2011. Lipid nanoparticles for brain targeting. I. Formulation optimization. Int J Pharm. 419(1–2):287–295.
- Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. 2007. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 59(6):454–477.
- Bonilla L, Espina M, Severino P, Cano A, Ettcheto M, Camins A, García ML, Souto EB, Sánchez-López E. 2021. Lipid nanoparticles for the posterior eye segment. Pharmaceutics. 14(1):90.
- Borges A, de Freitas V, Mateus N, Fernandes I, Oliveira J. 2020. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants. 9(10):998.
- Boskabadi M, Saeedi M, Akbari J, Morteza-Semnani K, Hashemi SMH, Babaei A. 2021. Topical gel of vitamin A solid lipid nanoparticles: a hopeful promise as a dermal delivery system. Adv Pharm Bull. 11(4):663–674.
- Butani D, Yewale C, Misra A. 2016. Topical amphotericin B solid lipid nanoparticles: design and development. Colloids Surf B Biointerfaces. 139:17–24.
- Campos J, Severino P, Santini A, Silva A, Shegokar R, Souto S, Souto EB. 2020. Chapter 1 - Solid lipid nanoparticles (SLN): prediction of toxicity, metabolism, fate and physicochemical properties. In: Shegokar R, editor. Nanopharmaceuticals. Zimmern: Elsevier; p. 1–15.
- Campos JR, Fernandes AR, Sousa R, Fangueiro JF, Boonme P, Garcia ML, Silva AM, Naveros BC, Souto EB. 2019. Optimization of nimesulide-loaded solid lipid nanoparticles (SLN) by factorial design, release profile and cytotoxicity in human colon adenocarcinoma cell line. Pharm Dev Technol. 24(5):616–622.
- Carregal-Romero S, Plaza-García S, Piñol R, Murillo JL, Ruiz-Cabello J, Padro D, Millán A, Ramos-Cabrer P. 2018. MRI study of the influence of surface coating aging on the in vivo biodistribution of iron oxide nanoparticles. Biosensors. 8(4):127.
- Carvalho SG, Araujo VHS, Dos Santos AM, Duarte JL, Silvestre ALP, Fonseca-Santos B, Villanova JCO, Gremião MPD, Chorilli M. 2020. Advances and challenges in nanocarriers and nanomedicines for veterinary application. Int J Pharm. 580:119214.
- Chandasana H, Prasad YD, Chhonker YS, Chaitanya TK, Mishra NN, Mitra K, Shukla PK, Bhatta RS. 2014. Corneal targeted nanoparticles for sustained natamycin delivery and their PK/PD indices: an approach to reduce dose and dosing frequency. Int J Pharm. 477(1–2):317–325.
- Chattopadhyay P, Shekunov BY, Yim D, Cipolla D, Boyd B, Farr S. 2007. Production of solid lipid nanoparticle suspensions using supercritical fluid extraction of emulsions (SFEE) for pulmonary delivery using the AERx system. Adv Drug Deliv Rev. 59(6):444–453.
- Chen D, Ganesh S, Wang W, Amiji M. 2017. Plasma protein adsorption and biological identity of systemically administered nanoparticles. Nanomedicine. 12(17):2113–2135.
- Chen D, Ganesh S, Wang W, Amiji M. 2019. The role of surface chemistry in serum protein corona-mediated cellular delivery and gene silencing with lipid nanoparticles. Nanoscale. 11(18):8760–8775.
- Chen J, Wei N, Lopez-Garcia M, Ambrose D, Lee J, Annelin C, Peterson T. 2017. Development and evaluation of resveratrol, vitamin E, and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur J Pharm Biopharm. 117:286–291.
- Cheng Z, Li M, Dey R, Chen Y. 2021. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 14(1):85.
- Chetoni P, Burgalassi S, Monti D, Tampucci S, Tullio V, Cuffini AM, Muntoni E, Spagnolo R, Zara GP, Cavalli R. 2016. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: pharmacokinetic studies on rabbits. Eur J Pharm Biopharm. 109:214–223.
- Chuang C-H, Wu P-C, Tsai T-H, Fang Y-P, Tsai Y-H, Cheng T-C, Huang C-C, Huang M-Y, Chen F-M, Hsieh Y-C, et al. 2017. Development of pH-sensitive cationic PEGylated solid lipid nanoparticles for selective cancer-targeted therapy. J Biomed Nanotechnol. 13(2):192–203.
- Cipolla D, Shekunov B, Blanchard J, Hickey A. 2014. Lipid-based carriers for pulmonary products: preclinical development and case studies in humans. Adv Drug Deliv Rev. 75:53–80.
- Cirri M, Mennini N, Maestrelli F, Mura P, Ghelardini C, Mannelli LDC. 2017. Development and in vivo evaluation of an innovative “hydrochlorothiazide-in cyclodextrins-in solid lipid nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int J Pharm. 521(1–2):73–83.
- Cunha S, Costa CP, Loureiro JA, Alves J, Peixoto AF, Forbes B, Sousa Lobo JM, Silva AC. 2020. Double optimization of rivastigmine-loaded nanostructured lipid carriers (NLC) for nose-to-brain delivery using the quality by design (QbD) approach: formulation variables and instrumental parameters. Pharmaceutics. 12(7):599.
- Dal Magro R, Ornaghi F, Cambianica I, Beretta S, Re F, Musicanti C, Rigolio R, Donzelli E, Canta A, Ballarini E, et al. 2017. ApoE-modified solid lipid nanoparticles: a feasible strategy to cross the blood–brain barrier. J Control Release. 249:103–110.
- Dang H, Dong C, Zhang L. 2022. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm Dev Technol. 27(2):127.
- De Jesus MB, Zuhorn IS. 2015. Solid lipid nanoparticles as nucleic acid delivery system: properties and molecular mechanisms. J Control Release. 201:1–13.
- del Pozo-Rodríguez A, Delgado D, Gascón AR, Solinís MÁ. 2013. Lipid nanoparticles as drug/gene delivery systems to the retina. J Ocul Pharmacol Ther. 29(2):173–188.
- Dhiman N, Awasthi R, Sharma B, Kharkwal H, Kulkarni GT. 2021. Lipid nanoparticles as carriers for bioactive delivery. Front Chem. 9:580118.
- Diorio C, Lokhnauth J. 2019. Curcumin solid lipid particles and methods for their preparation and use. US 10166187B2.
- Dissanayake T, Sun X, Abbey L, Bandara N. 2022. Recent advances in lipid–protein conjugate-based delivery systems in nutraceutical, drug, and gene delivery. Food Hydrocolloids Health. 2:100054.
- Doktorovova S, Souto EB, Silva AM. 2014. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers – a systematic review of in vitro data. Eur J Pharm Biopharm. 87(1):1–18.
- Dolatabadi JEN, Valizadeh H, Hamishehkar H. 2015. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv Pharm Bull. 5(2):151–159.
- Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, Kumar NS, Vekariya RL. 2020. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv. 10(45):26777–26791.
- Elbrink K, Van Hees S, Chamanza R, Roelant D, Loomans T, Holm R, Kiekens F. 2021. Application of solid lipid nanoparticles as a long-term drug delivery platform for intramuscular and subcutaneous administration: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 163:158–170.
- El-Emam GA, Girgis GN, Hamed MF, Soliman O-A, Abd El AEGH. 2021. Formulation and pathohistological study of mizolastine-solid lipid nanoparticles-loaded ocular hydrogels. Int J Nanomedicine. 16:7775–7799.
- Enayatifard R, Akbari J, Saeedi M, Morteza-Semnani K, Parvin S, Hashemi MH, Babaei A, Rostamkalaei S. 2018. Investigating the effect of coated lipid nano particles of spironolactone with chitosan on their properties. J Mazandaran Univ Med Sci. 28(162):25–36.
- Eskandani M, Eskandani M, Vandghanooni S, Navidshad B, Aghjehgheshlagh FM, Nobakht A. 2022. Protective effect of l-carnitine-loaded solid lipid nanoparticles against H2O2-induced genotoxicity and apoptosis. Colloids Surf B Biointerfaces. 212:112365.
- Figueiredo P, Sipponen MH, Lintinen K, Correia A, Kiriazis A, Yli-Kauhaluoma J, Österberg M, George A, Hirvonen J, Kostiainen MA, et al. 2019. Preparation and characterization of dentin phosphophoryn-derived peptide-functionalized lignin nanoparticles for enhanced cellular uptake. Small. 15(24):1901427.
- Fonseca-Santos B, Silva PB, Rigon RB, Sato MR, Chorilli M. 2020. Formulating SLN and NLC as innovative drug delivery systems for non-invasive routes of drug administration. Curr Med Chem. 27(22):3623–3656.
- Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C. 2020. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci. 8(17):4653–4664.
- Fröhlich E. 2018. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif Cells Nanomed Biotechnol. 46(Suppl. 2):1091–1107.
- Galvão JG, Santos RL, Silva AR, Santos JS, Costa AM, Chandasana H, Andrade-Neto VV, Torres-Santos EC, Lira AAM, Dolabella S, et al. 2020. Carvacrol loaded nanostructured lipid carriers as a promising parenteral formulation for leishmaniasis treatment. Eur J Pharm Sci. 150:105335.
- Ganesan P, Kim B, Ramalingam P, Karthivashan G, Revuri V, Park S, Kim JS, Ko YT, Choi D-K. 2019. Antineuroinflammatory activities and neurotoxicological assessment of curcumin loaded solid lipid nanoparticles on LPS-stimulated BV-2 microglia cell models. Molecules. 24(6):1170.
- Ganesan P, Narayanasamy D. 2017. Lipid nanoparticles: different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm. 6:37–56.
- Garcês A, Amaral M, Lobo JS, Silva AC. 2018. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: a review. Eur J Pharm Sci. 112:159–167.
- Gautam M, Verma M, Chauhan I, Yasir M, Singh AP, Saraswat PK. 2020. Solid lipid nanoparticles for oral drug delivery: a review. Curr Nanomed. 10(3):208–224.
- Ghasemiyeh P, Mohammadi-Samani S. 2018. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 13(4):288–303.
- Ghazizadeh F, Ghaffari S, Mirshojaei SF, Mazidid M, Azarmi S. 2018. Biodistribution of Tc-99m labeled isoniazid solid lipid nanoparticles in Wistar rats. Iran J Pharm Res. 17(4):1209–1216.
- Gordillo-Galeano A, Ospina-Giraldo LF, Mora-Huertas CE. 2021. Lipid nanoparticles with improved biopharmaceutical attributes for tuberculosis treatment. Int J Pharm. 596:120321.
- Hady MA, Sayed OM, Akl MA. 2020. Brain uptake and accumulation of new levofloxacin–doxycycline combination through the use of solid lipid nanoparticles: formulation; optimization and in-vivo evaluation. Colloids Surf B Biointerfaces. 193:111076.
- Haider M, Abdin SM, Kamal L, Orive G. 2020. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics. 12(3):288.
- Halder J, Pradhan D, Kar B, Ghosh G, Rath G. 2021. Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomedicine. 40:102494.
- Han Y, Zhang Y, Li D, Chen Y, Sun J, Kong F. 2014. Transferrin-modified nanostructured lipid carriers as multifunctional nanomedicine for codelivery of DNA and doxorubicin. Int J Nanomedicine. 9:4107–4116.
- Haque S, Whittaker M, McIntosh MP, Pouton CW, Phipps S, Kaminskas LM. 2018. A comparison of the lung clearance kinetics of solid lipid nanoparticles and liposomes by following the 3H-labelled structural lipids after pulmonary delivery in rats. Eur J Pharm Biopharm. 125:1–12.
- He H, Yao J, Zhang Y, Chen Y, Wang K, Lee RJ, Yu B, Zhang X. 2019. Solid lipid nanoparticles as a drug delivery system to across the blood–brain barrier. Biochem Biophys Res Commun. 519(2):385–390.
- Hidalgo A, Cruz A, Pérez-Gil J. 2015. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur J Pharm Biopharm. 95(Pt A):117–127.
- Hosseini M, Haji-Fatahaliha M, Jadidi-Niaragh F, Majidi J, Yousefi M. 2016. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif Cells Nanomed Biotechnol. 44(4):1051–1061.
- Hwang T-L, Aljuffali IA, Hung C-F, Chen C-H, Fang J-Y. 2015. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs). Chem Biol Interact. 235:106–114.
- Hwang T-L, Aljuffali IA, Lin C-F, Chang Y-T, Fang J-Y. 2015. Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: lipid nanoparticles versus polymeric nanoparticles. Int J Nanomedicine. 10:371–385.
- Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. 2012. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target. 20(10):813–830.
- Jacob S, Nair AB, Shah J, Gupta S, Boddu SH, Sreeharsha N, Joseph A, Shinu P, Morsy MA. 2022. Lipid nanoparticles as a promising drug delivery carrier for topical ocular therapy—an overview on recent advances. Pharmaceutics. 14(3):533.
- Jesudian G, Gnana J, Vijaya C, Kaur I. 2014. Topical tazarotene solid lipid nanoparticles. IN149/CHE/2014.
- Jin J, Bae KH, Yang H, Lee SJ, Kim H, Kim Y, Joo KM, Seo SW, Park TG, Nam D-H. 2011. In vivo specific delivery of c-Met siRNA to glioblastoma using cationic solid lipid nanoparticles. Bioconjug Chem. 22(12):2568–2572.
- Joshi MD, Müller RH. 2009. Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Biopharm. 71(2):161–172.
- Kanojia N, Sharma N, Gupta N, Singh S. 2022. Applications of nanostructured lipid carriers: recent advancements and patent review. Biointerface Res Appl Chem. 12(1):638–652.
- Kanwar R, Uppal S, Mehta SK. 2021. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): fabrication and functionalization for impending therapeutic applications. In: Kumar V, Guleria P, Dasgupta N, et al., editors. Functionalized nanomaterials II: applications. Boca Raton: CRC Press; p. 57–70.
- Kaufman RC. 2018. Lipid nanoparticle compositions and methods as carriers of cannabinoids in standardized precision-metered dosage forms. US 10028919B2.
- Kaur IP, Bhandari R, Bhandari S, Kakkar V. 2008. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 127(2):97–109.
- Kelidari H, Saeedi M, Akbari J, Morteza-Semnani K, Gill P, Valizadeh H, Nokhodchi A. 2015. Formulation optimization and in vitro skin penetration of spironolactone loaded solid lipid nanoparticles. Colloids Surf B Biointerfaces. 128:473–479.
- Kemp JA, Kwon YJ. 2021. Cancer nanotechnology: current status and perspectives. Nano Converg. 8(1):1–38.
- Khames A, Khaleel MA, El-Badawy MF, El-Nezhawy AO. 2019. Natamycin solid lipid nanoparticles – sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: preparation and optimization. Int J Nanomedicine. 14:2515–2531.
- Khandhar AP, Van Hoeven N, Jesse H, Lin SS. 2020. Nanostructured lipid carriers and stable emulsions and uses thereof. WO2018232257A9.
- Khater D, Nsairat H, Odeh F, Saleh M, Jaber A, Alshaer W, Al Bawab A, Mubarak MS. 2021. Design, preparation, and characterization of effective dermal and transdermal lipid nanoparticles: a review. Cosmetics. 8(2):39.
- Khezri K, Saeedi M, Morteza-Semnani K, Akbari J, Rostamkalaei SS. 2020. An emerging technology in lipid research for targeting hydrophilic drugs to the skin in the treatment of hyperpigmentation disorders: kojic acid-solid lipid nanoparticles. Artif Cells Nanomed Biotechnol. 48(1):841–853.
- Koziara JM, Lockman PR, Allen DD, Mumper RJ. 2004. Paclitaxel nanoparticles for the potential treatment of brain tumors. J Control Release. 99(2):259–269.
- Kraisit P, Hirun N, Mahadlek J, Limmatvapirat S. 2021. Fluconazole-loaded solid lipid nanoparticles (SLNs) as a potential carrier for buccal drug delivery of oral candidiasis treatment using the Box–Behnken design. J Drug Deliv Sci Technol. 63:102437.
- Krysztof C, Wosicka H. 2015. Solid lipid nanoparticles of roxithromycin for hair loss or acne. EP2919756B1.
- Kuang Y, Zhang K, Cao Y, Chen X, Wang K, Liu M, Pei R. 2017. Hydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl Mater Interfaces. 9(14):12217–12226.
- Kumar K, Chawla R. 2021. Nanocarriers-mediated therapeutics as a promising approach for treatment and diagnosis of lung cancer. J Drug Deliv Sci Technol. 65:102677.
- Kyadarkunte AY, Patole MS, Pokharkar VB. 2015. Cellular interactions and photoprotective effects of idebenone-loaded nanostructured lipid carriers stabilized using PEG-free surfactant. Int J Pharm. 479(1):77–87.
- Lauterbach A, Müller-Goymann CC. 2015. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur J Pharm Biopharm. 97(Pt A):152–163.
- Li G, Lee WJ, Tan CP, Lai OM, Wang Y, Qiu C. 2021. Tailored rigidity of W/O pickering emulsions using diacylglycerol-based surface-active solid lipid nanoparticles. Food Funct. 12(23):11732–11746.
- Li H, Wang K, Yang X, Zhou Y, Ping Q, Oupicky D, Sun M. 2017. Dual-function nanostructured lipid carriers to deliver IR780 for breast cancer treatment: anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 53:399–413.
- Li S, Chen G. 2020. Agricultural waste-derived superabsorbent hydrogels: preparation, performance, and socioeconomic impacts. J Clean Prod. 251:119669.
- Lili Q, Jialei C, Songhui Y. 2018. Sports fatigue-resistant resveratrol solid lipid nanoparticles and preparation method thereof. CN108420801A.
- Liu D, Li J, Cheng B, Wu Q, Pan H. 2017. Ex vivo and in vivo evaluation of the effect of coating a coumarin-6-labeled nanostructured lipid carrier with chitosan-N-acetylcysteine on rabbit ocular distribution. Mol Pharm. 14(8):2639–2648.
- Liu J, Gong T, Fu H, Wang C, Wang X, Chen Q, Zhang Q, He Q, Zhang Z. 2008. Solid lipid nanoparticles for pulmonary delivery of insulin. Int J Pharm. 356(1–2):333–344.
- Makled S, Nafee N, Boraie N. 2017. Nebulized solid lipid nanoparticles for the potential treatment of pulmonary hypertension via targeted delivery of phosphodiesterase-5-inhibitor. Int J Pharm. 517(1–2):312–321.
- Malik P, Shankar R, Malik V, Sharma N, Mukherjee TK. 2014. Green chemistry based benign routes for nanoparticle synthesis. J Nanopart. 2014:1–14.
- Maretti E, Pavan B, Rustichelli C, Montanari M, Dalpiaz A, Iannuccelli V, Leo E. 2021. Chitosan/heparin polyelectrolyte complexes as ion-paring approach to encapsulate heparin in orally administrable SLN: in vitro evaluation. Colloids Surf A. 608:125606.
- Mei Z, Li X, Wu Q, Hu S, Yang X. 2005. The research on the anti-inflammatory activity and hepatotoxicity of triptolide-loaded solid lipid nanoparticle. Pharmacol Res. 51(4):345–351.
- Mendoza-Muñoz N, Urbán-Morlán Z, Leyva-Gómez G, de la L, Zambrano-Zaragoza M, Quintanar-Guerrero D. 2021. Solid lipid nanoparticles: an approach to improve oral drug delivery. J Pharm Pharm Sci. 24:509–532.
- Mhule D, Kalhapure RS, Jadhav M, Omolo CA, Rambharose S, Mocktar C, Singh S, Waddad AY, Ndesendo VM, Govender T. 2018. Synthesis of an oleic acid based pH-responsive lipid and its application in nanodelivery of vancomycin. Int J Pharm. 550(1–2):149–159.
- Mishra DK, Dhote V, Bhatnagar P, Mishra PK. 2012. Engineering solid lipid nanoparticles for improved drug delivery: promises and challenges of translational research. Drug Deliv Transl Res. 2(4):238–253.
- Mishra V, Bansal KK, Verma A, Yadav N, Thakur S, Sudhakar K, Rosenholm JM. 2018. Solid lipid nanoparticles: emerging colloidal nano drug delivery systems. Pharmaceutics. 10(4):191.
- Mohammad Y, Prentice RN, Boyd BJ, Rizwan SB. 2022. Comparison of cubosomes and hexosomes for the delivery of phenytoin to the brain. J Colloid Interface Sci. 605:146–154.
- Mudshinge SR, Deore AB, Patil S, Bhalgat CM. 2011. Nanoparticles: emerging carriers for drug delivery. Saudi Pharm J. 19(3):129–141.
- Mura P, Maestrelli F, D’Ambrosio M, Luceri C, Cirri M. 2021. Evaluation and comparison of solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as vectors to develop hydrochlorothiazide effective and safe pediatric oral liquid formulations. Pharmaceutics. 13(4):437.
- Mussi SV, Torchilin VP. 2013. Recent trends in the use of lipidic nanoparticles as pharmaceutical carriers for cancer therapy and diagnostics. J Mater Chem B. 1(39):5201–5209.
- Nair A, Shah J, Al-Dhubiab B, Jacob S, Patel S, Venugopala K, Morsy M, Gupta S, Attimarad M, Sreeharsha N, et al. 2021. Clarithromycin solid lipid nanoparticles for topical ocular therapy: optimization, evaluation and in vivo studies. Pharmaceutics. 13(4):523.
- Nasirizadeh S, Malaekeh-Nikouei B. 2020. Solid lipid nanoparticles and nanostructured lipid carriers in oral cancer drug delivery. J Drug Deliv Sci Technol. 55:101458.
- Nishihira VSK, Rubim AM, Brondani M, Dos Santos JT, Pohl AR, Friedrich JF, de Lara JD, Nunes CM, Feksa LR, Simão E, et al. 2019. In vitro and in silico protein corona formation evaluation of curcumin and capsaicin loaded-solid lipid nanoparticles. Toxicol In Vitro. 61:104598.
- Olerile LD. 2020. Further development of near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carrier system for cancer theragnostic. Technol Cancer Res Treat. 19:1533033820914308.
- Olerile LD, Liu Y, Zhang B, Wang T, Mu S, Zhang J, Selotlegeng L, Zhang N. 2017. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf B Biointerfaces. 150:121–130.
- Pandita D, Kumar S, Poonia N, Lather V. 2014. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res Int. 62:1165–1174.
- Paranjpe M, Müller-Goymann CC. 2014. Nanoparticle-mediated pulmonary drug delivery: a review. Int J Mol Sci. 15(4):5852–5873.
- Pardeike J, Hommoss A, Müller RH. 2009. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 366(1–2):170–184.
- Pardeike J, Weber S, Haber T, Wagner J, Zarfl H, Plank H, Zimmer A. 2011. Development of an itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int J Pharm. 419(1–2):329–338.
- Partikel K, Korte R, Stein NC, Mulac D, Herrmann FC, Humpf H-U, Langer K. 2019. Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur J Pharm Biopharm. 141:70–80.
- Parvez S, Yadagiri G, Gedda MR, Singh A, Singh OP, Verma A, Sundar S, Mudavath SL. 2020. Modified solid lipid nanoparticles encapsulated with amphotericin B and paromomycin: an effective oral combination against experimental murine visceral leishmaniasis. Sci Rep. 10(1):1–14.
- Passos JS, de Martino LC, Dartora VFC, de Araujo GL, Ishida K, Lopes LB. 2020. Development, skin targeting and antifungal efficacy of topical lipid nanoparticles containing itraconazole. Eur J Pharm Sci. 149:105296.
- Patel DK, Kesharwani R, Kumar V. 2019. Lipid nanoparticle topical and transdermal delivery: a review on production, penetration mechanism to skin. Int J Pharm Investig. 9(4):148–153.
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MdP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, et al. 2018. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 16(1):1–33.
- Pindiprolu SKS, Krishnamurthy PT, Dev C, Chintamaneni PK. 2021. DR5 antibody conjugated lipid-based nanocarriers of gamma-secretase inhibitor for the treatment of triple negative breast cancer. Chem Phys Lipids. 235:105033.
- Poovi G, Damodharan N. 2018. Lipid nanoparticles: a challenging approach for oral delivery of BCS class-II drugs. Future J Pharm Sci. 4(2):191–205.
- Popova EV, Tikhomirova VE, Beznos OV, Chesnokova NB, Grigoriev YV, Klyachko NL, Kost OA. 2022. Chitosan-covered calcium phosphate particles as a drug vehicle for delivery to the eye. Nanomed Nanotechnol Biol Med. 40:102493.
- Prasad D, Chauhan H. 2014. Nanotoxicity of polymeric and solid lipid nanoparticles. In: Sutarriya VB, Pathak Y, editors. Biointeractions of nanomaterials; p. 141–158.
- Rahman HS, Othman HH, Hammadi NI, Yeap SK, Amin KM, Samad NA, Alitheen NB. 2020. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int J Nanomedicine. 15:2439–2483.
- Rajagopal R. 2014. Sustainable value creation in the fine and speciality chemicals industry. New York: John Wiley & Sons.
- Rajagopal V, Rajathurai S, Parthasarathy K, Gimbun J, Ramakrishnan P, Ramakrishnan RP, Ranganathan B. 2022. Review of nano-chitosan based drug delivery of plant extracts for the treatment of breast cancer. Trends Biomater Artif Organs. 36(S1):83–87.
- Ramasamy T, Tran TH, Choi JY, Cho HJ, Kim JH, Yong CS, Choi H-G, Kim JO. 2014. Layer-by-layer coated lipid–polymer hybrid nanoparticles designed for use in anticancer drug delivery. Carbohydr Polym. 102:653–661.
- Rao KK. 2008. Polymerized solid lipid nanoparticles for oral or mucosal delivery of therapeutic proteins and peptides. US 20080311214A1.
- Rao S, Prestidge CA. 2016. Polymer–lipid hybrid systems: merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opin Drug Deliv. 13(5):691–707.
- Rasouliyan F, Eskandani M, Jaymand M, Nakhjavani SA, Farahzadi R, Vandghanooni S, Eskandani M. 2021. Preparation, physicochemical characterization, and anti-proliferative properties of Lawsone-loaded solid lipid nanoparticles. Chem Phys Lipids. 239:105123.
- Repka MA, Patil HG, Majumdar S, Park J-B, Kulkarni VI. 2017. Systems and methods for preparing solid lipid nanoparticles. US 20170172937A1.
- Rita BA, Luisa BM, Cavallaro G, Letizia CGM, Craparo E, Giammona G, Licciardi M, Pitarresi G, Granata G, Saladino P. 2015. Nanostructured formulations for the delivery of silibinin and other active ingredients for treating ocular diseases. AU 2021202374A1.
- Rizvi SZH, Shah FA, Khan N, Muhammad I, Ali KH, Ansari MM, Din FU, Qureshi OS, Kim K-W, Choe Y-H, et al. 2019. Simvastatin-loaded solid lipid nanoparticles for enhanced anti-hyperlipidemic activity in hyperlipidemia animal model. Int J Pharm. 560:136–143.
- Rudhrabatla V, Sudhakar B, Reddy K. 2020. In vitro and in vivo assessment of designed melphalan loaded stealth solid lipid nanoparticles for parenteral delivery. BioNanoScience. 10(1):168–190.
- Saeedi M, Morteza-Semnani K, Akbari J, Siahposht-Khachaki A, Firouzi M, Goodarzi A, Abootorabi S, Babaei A, Hashemi SMH, Nokhodchi A. 2021. Brain targeting of venlafaxine HCl as a hydrophilic agent prepared through green lipid nanotechnology. J Drug Deliv Sci Technol. 66:102813.
- Sánchez-López E, Espina M, Doktorovova S, Souto E, García M. 2017. Lipid nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye. Part II. Ocular drug-loaded lipid nanoparticles. Eur J Pharm Biopharm. 110:58–69.
- Sandri G, Bonferoni MC, D'Autilia F, Rossi S, Ferrari F, Grisoli P, Sorrenti M, Catenacci L, Del Fante C, Perotti C, et al. 2013. Wound dressings based on silver sulfadiazine solid lipid nanoparticles for tissue repairing. Eur J Pharm Biopharm. 84(1):84–90.
- Sanjay SS. 2019. Chapter 2 – safe nano is green nano. In: Shukla AK, Iravani S, editors. Green synthesis, characterization and applications of nanoparticles. Amsterdam: Elsevier; p. 27–36.
- Santonocito D, Puglia C. 2022. Applications of lipid-based nanocarriers for parenteral drug delivery. Curr Med Chem. 29.
- Seabra CL, Nunes C, De Freitas MDLS, Rodrigues FHRD, Da Costa MCTL, Martins PL. 2018. Nanostructured lipid carriers, methods and uses thereof. WO 2018002853A1.
- Selvamuthukumar S, Velmurugan R. 2012. Nanostructured lipid carriers: a potential drug carrier for cancer chemotherapy. Lipids Health Dis. 11(1):159.
- Seth D, Cheldize K, Brown D, Freeman EE. 2017. Global burden of skin disease: inequities and innovations. Curr Dermatol Rep. 6(3):204–210.
- Seyfoddin A, Shaw J, Al-Kassas R. 2010. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 17(7):467–489.
- Shadambikar G, Marathe S, Ji N, Almutairi M, Bandari S, Zhang F, Chougule M, Repka M. 2021. Formulation development of itraconazole PEGylated nano-lipid carriers for pulmonary aspergillosis using hot-melt extrusion technology. Int J Pharm X. 3:100074.
- Shah R, Eldridge D, Palombo E, Harding I. 2015. Lipid nanoparticles: production, characterization and stability. Vol. 1. New York: Springer.
- Shah S, Bhanderi B, Soniwala M, Chavda J. 2021. Lutein-loaded solid lipid nanoparticles for ocular delivery: statistical optimization and ex vivo evaluation. J Pharm Innov. 1–15.
- Shao Z, Shao J, Tan B, Guan S, Liu Z, Zhao Z, He F, Zhao J. 2015. Targeted lung cancer therapy: preparation and optimization of transferrin-decorated nanostructured lipid carriers as novel nanomedicine for co-delivery of anticancer drugs and DNA. Int J Nanomedicine. 10:1223–1233.
- Souto E, Almeida A, Müller R. 2007. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: structure, protection and skin effects. J Biomed Nanotechnol. 3(4):317–331.
- Souto EB, Baldim I, Oliveira WP, Rao R, Yadav N, Gama FM, Mahant S. 2020. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin Drug Deliv. 17(3):357–377.
- Sun J, Zhang S, Jiang S, Bai W, Liu F, Yuan H, Ji J, Luo J, Han G, Chen L, et al. 2016. Gadolinium-loaded solid lipid nanoparticles as a tumor-absorbable contrast agent for early diagnosis of colorectal tumors using magnetic resonance colonography. J Biomed Nanotechnol. 12(9):1709–1723.
- Swathi G, Prasanthi N, Manikiran S, Ramarao N. 2012. Solid lipid nanoparticles: colloidal carrier systems for drug delivery. ChemInform. 43(2).
- Świętek M, Panchuk R, Skorokhyd N, Černoch P, Finiuk N, Klyuchivska O, Hrubý M, Molčan M, Berger W, Trousil J, et al. 2020. Magnetic temperature-sensitive solid-lipid particles for targeting and killing tumor cells. Front Chem. 8:205.
- Tan F, Cui H, Bai C, Qin C, Xu L, Han J. 2021. Preparation, optimization, and transcorneal permeability study of lutein-loaded solid lipid nanoparticles. J Drug Deliv Sci Technol. 62:102362.
- Tan SLJ, Billa N. 2021. Improved bioavailability of poorly soluble drugs through gastrointestinal muco-adhesion of lipid nanoparticles. Pharmaceutics. 13(11):1817.
- Teixeira MI, Lopes CM, Gonçalves H, Catita J, Silva AM, Rodrigues F, Amaral MH, Costa PC. 2022. Formulation, characterization, and cytotoxicity evaluation of lactoferrin functionalized lipid nanoparticles for riluzole delivery to the brain. Pharmaceutics. 14(1):185.
- Tosi G, Musumeci T, Ruozi B, Carbone C, Belletti D, Pignatello R, Vandelli MA, Puglisi G. 2016. The “fate” of polymeric and lipid nanoparticles for brain delivery and targeting: strategies and mechanism of blood–brain barrier crossing and trafficking into the central nervous system. J Drug Deliv Sci Technol. 32:66–76.
- Tran TH, Ramasamy T, Truong DH, Choi H-G, Yong CS, Kim JO. 2014. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS PharmSciTech. 15(6):1509–1515.
- Trapani A, Guerra L, Corbo F, Castellani S, Sanna E, Capobianco L, Monteduro AG, Manno DE, Mandracchia D, Di Gioia S, et al. 2021. Cyto/biocompatibility of dopamine combined with the antioxidant grape seed-derived polyphenol compounds in solid lipid nanoparticles. Molecules. 26(4):916.
- Tretiakova D, Vodovozova E. 2022. Liposomes as adjuvants and vaccine delivery systems. Biochem (Mosc) Suppl Ser A Membr Cell Biol. 16(1):1–20.
- Tzanova MM, Hagesaether E, Tho I. 2021. Solid lipid nanoparticle-loaded mucoadhesive buccal films – critical quality attributes and in vitro safety & efficacy. Int J Pharm. 592:120100.
- Ulldemolins A, Seras-Franzoso J, Andrade F, Rafael D, Abasolo I, Gener P, Schwartz S Jr. 2021. Perspectives of nano-carrier drug delivery systems to overcome cancer drug resistance in the clinics. Cancer Drug Resist. 4(1):44–68.
- Vandghanooni S, Rasoulian F, Eskandani M, Akbari Nakhjavani S, Eskandani M. 2021. Acriflavine-loaded solid lipid nanoparticles: preparation, physicochemical characterization, and anti-proliferative properties. Pharm Dev Technol. 26(9):934–942.
- Vinchhi P, Patel JK, Patel MM. 2021. High-pressure homogenization techniques for nanoparticles. In: Patel JK, Pathak YV, editors. Emerging technologies for nanoparticle manufacturing. Cham: Springer International Publishing; p. 263–285.
- Wang J, Zhou T, Liu Y, Chen S, Yu Z. 2021. Application of nanoparticles in the treatment of lung cancer with emphasis on receptors. Front Pharmacol. 12:781425.
- Wang J-L, Hanafy MS, Xu H, Leal J, Zhai Y, Ghosh D, Williams Iii RO, Smyth HDC, Cui Z. 2021. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int J Pharm. 596:120215.
- Wanping Z, Xiaomei Z, Xiaozhi L, Haiyang Z. 2016. Nanostructured solid lipid carrier coating vitamin A palmitate and preparation method thereof. CN 105496801A.
- Weber S, Zimmer A, Pardeike J. 2014. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 86(1):7–22.
- Wissing S, Kayser O, Müller R. 2004. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 56(9):1257–1272.
- Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. 2007. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 59(6):491–504.
- Xiang Q-y, Wang M-t, Chen F, Gong T, Jian Y-l, Zhang Z-r, Huang Y. 2007. Lung-targeting delivery of dexamethasone acetate loaded solid lipid nanoparticles. Arch Pharm Res. 30(4):519–525.
- Xie S, Wang F, Wang Y, Zhu L, Dong Z, Wang X, Li X, Zhou W. 2011. Acute toxicity study of tilmicosin-loaded hydrogenated castor oil-solid lipid nanoparticles. Part Fibre Toxicol. 8(1):33.
- Xu L, Wang X, Liu Y, Yang G, Falconer RJ, Zhao C-X. 2022. Lipid nanoparticles for drug delivery. Adv NanoBiomed Res. 2(2):2100109.
- Xu Y, Zheng Y, Wu L, Zhu X, Zhang Z, Huang Y. 2018. Novel solid lipid nanoparticle with endosomal escape function for oral delivery of insulin. ACS Appl Mater Interfaces. 10(11):9315–9324.
- Yadav N, Khatak S, Sara US. 2013. Solid lipid nanoparticles—a review. Int J Appl Pharm. 5(2):8–18.
- Yadav P, Yadav AB. 2022. Preparation and optimization of insulin loaded solid lipid nanoparticles for the targeted delivery of insulin to the lungs for diabetes treatment via inhalation. In: Krishnapillai S, Ha SK, editors. Composite materials for extreme loading. Lecture Notes in Mechanical Engineering. Singapore: Springer; p. 73–86.
- Yasir M, Chauhan I, Zafar A, Verma M, Noorulla KM, Tura AJ, Alruwaili NK, Haji MJ, Puri D, Gobena WG, et al. 2021. Buspirone loaded solid lipid nanoparticles for amplification of nose to brain efficacy: formulation development, optimization by Box–Behnken design, in-vitro characterization and in-vivo biological evaluation. J Drug Deliv Sci Technol. 61:102164.
- Ye J-Y, Chen Z-Y, Huang C-L, Huang B, Zheng Y-R, Zhang Y-F, Lu B-Y, He L, Liu C-S, Long X-Y. 2020. A non-lipolysis nanoemulsion improved oral bioavailability by reducing the first-pass metabolism of raloxifene, and related absorption mechanisms being studied. Int J Nanomedicine. 15:6503–6518.
- Yue-Xing C, Fei-Fei Y, Han W, Tao-Tao F, Chun-Yu L, Li-Hui Q, Yong-Hong L. 2018. The effect of l-leucine on the stabilization and inhalability of spray-dried solid lipid nanoparticles for pulmonary drug delivery. J Drug Deliv Sci Technol. 46:474–481.
- Zancan LR, Bruinsmann FA, Paese K, Türck P, Bahr A, Zimmer A, Carraro CC, Schenkel PC, Belló-Klein A, Schwertz CI, et al. 2021. Oral delivery of ambrisentan-loaded lipid-core nanocapsules as a novel approach for the treatment of pulmonary arterial hypertension. Int J Pharm. 610:121181.
- Zhang B, Zhang Y, Yu D. 2017. Lung cancer gene therapy: transferrin and hyaluronic acid dual ligand-decorated novel lipid carriers for targeted gene delivery. Oncol Rep. 37(2):937–944.
- Zhang L, Mao S. 2017. Application of quality by design in the current drug development. Asian J Pharm Sci. 12(1):1–8.
- Zhang X-Q, Xu X, Bertrand N, Pridgen E, Swami A, Farokhzad OC. 2012. Interactions of nanomaterials and biological systems: implications to personalized nanomedicine. Adv Drug Deliv Rev. 64(13):1363–1384.
- Zhang Y, Li Z, Zhang K, Yang G, Wang Z, Zhao J, Hu R, Feng N. 2016. Ethyl oleate-containing nanostructured lipid carriers improve oral bioavailability of trans-ferulic acid as compared with conventional solid lipid nanoparticles. Int J Pharm. 511(1):57–64.