 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The anti-HER2 antibody trastuzumab has been proven to be an effective targeting ligand for drug delivery. This study investigates the structural integrity of trastuzumab under different stress factors in formulation development and its long-term stability. A validated size exclusion high performance liquid chromatographic (SEC-HPLC) method was first developed. The stability of trastuzumab (0.21–21 mg/ml) under stress conditions (mechanical, freeze-and-thaw, pH and temperature) and long-term storage in the presence of formulation excipients were monitored for up to 12 months, using both the SEC-HPLC method and sodium-dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The anti-proliferation activity of the reconstituted antibody stored at 4 °C against HER2+ BT-474 breast cells was also monitored over 12 months. The developed SEC-HPLC method was sensitive and accurate. Solutions of trastuzumab were resistant to mechanical stress and repeated freeze-and-thaw; but were unstable under acidic (pH 2.0 and 4.0) and alkaline (pH 10.0 and 12.0) environments. The samples degraded over 5 days at 60 °C, and within 24 h at 75 °C. Low temperature (-80 °C or 4 °C) and low concentration (0.21 mg/ml) favoured the long-term stability. The anti-proliferation activity was conserved at 4 °C for at least 12 months. This study provided valuable stability information in developing trastuzumab involved nano-formulation as well as in clinical settings.
1. Introduction
Human epidermal growth factor receptor 2 (HER2) is an important member of the epidermal growth factor receptor (EGFR) family. Homo- and heterodimerisation of HER2 with other members of the EGFR family induces phosphorylation of the intracellular tyrosine residues, and activates a cascade of downstream pathways, resulting in cancer cell proliferation, invasion and migration (Hudis Citation2007; Schlam and Swain Citation2021). Amplification and over-expression of HER2 can be found in breast and gastric cancer, and many other types of solid tumours (Meric-Bernstam et al. Citation2019). Trastuzumab (Herceptin ®), an anti-HER2 humanised monoclonal antibody, is a revolutionary therapy that has clinically demonstrated 26% reduction of death rate in HER2 positive breast and gastric cancer patients (Meza-Junco et al. Citation2009; Cameron et al. Citation2017). Trastuzumab binds specifically to the extracellular domain of HER2 through the antigen binding fragments F(ab)’ (Maadi et al. Citation2018). Such specific binding has been utilised in the two marketed antibody-drug conjugates (ADCs), trastuzumab emtansine and trastuzumab deruxtecan, in which trastuzumab is used as part of the combination therapy as well as the guiding ligand of the chemotherapeutic agents, and demonstrated significantly improved overall survival of HER2 breast cancer patients (Kulhari et al. Citation2015; Ferraro et al. Citation2021). In addition, in the field of nanomedicine delivery, the intact trastuzumab or its F(ab)’ fragments has been researched extensively as a targeting ligand to decorate the surface of nanoparticles in the tumour-targeted drug delivery systems (), and have shown superior targeting ability and cytotoxicity against breast cancer in vitro (Kulhari et al. Citation2015; Rodallec et al. Citation2018; Niza et al. Citation2019; Nieto et al. Citation2020). For example, trastuzumab engrafted docetaxel immunoliposomes showed enhanced accumulation and 44% reduction in the half maximal inhibitory concentration (IC50) in HER2 positive cells than free docetaxel (Rodallec et al. Citation2018). Compared with ADCs, the antibody functionalised nanoparticles carry a much higher dose of chemotherapeutic agent(s), with the possibility to combine multiple drugs in the same system (Gao et al. Citation2020).
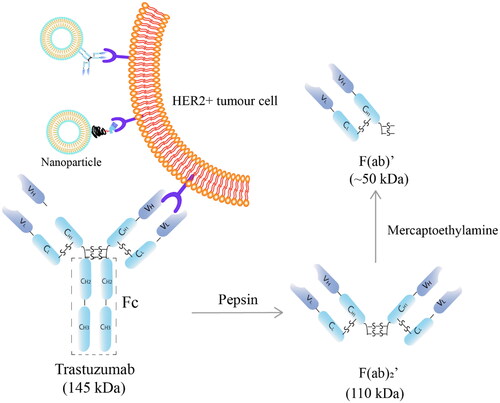
Figure 1. Schematic structure of trastuzumab (MW 145 kDa) and its fragments. F(ab)’, antigen binding fragment (MW ∼50 kDa); F(ab)2’, two F(ab)’ linked by disulphide bonds (MW ∼110 kDa); Fc: crystallisable fragment; C: constant domain; V: variable domain; H: heavy chains; L: light chains.
Despite the emerging application of trastuzumab in designing a targeted delivery systems, its ability to withstand various stresses during formulation development and manufacturing is understudied. So far, most of the trastuzumab stability studies simulate a clinical setting and have investigated the in-use stability of the reconstituted solutions (usually below 4 mg/ml) (Kaiser and Kramer Citation2011; Paul et al. Citation2013; Nalenz et al. Citation2018). In addition, the shelf life recommended by the manufacturer is likely based on the risk of bacterial contamination rather than the decline of physicochemical stability (FDA Citation1998; Paul et al. Citation2013). However, the stress conditions such as mechanical stress and pH during nanoformulation development are different from those in clinical use. The physicochemical stability of trastuzumab during formulation manipulation and long term storage plays a vital role in determining its biological activity. A loss of the structural integrity of trastuzumab could lead to a compromised binding specificity and affinity, thus a compromised targeting ability in the drug delivery system (Ma et al. Citation2020).
As an antibody, trastuzumab consists of two identical heavy chains and two light chains, linked by disulphide bonds, forming two F(ab)’ and a crystallisable fragment (Fc) (). It can be subject to physical or chemical instabilities that are intertwined with each other. Physical instability is related to a conformational disruption of the antibody (Bana et al. Citation2022). Aggregation, which involves the reversible or irreversible association of monomers to oligomers or multimers, is the major form of physical instability (Le Basle et al. Citation2020; Thorat et al. Citation2020). Aggregation can cause detrimental consequences, such as a complete loss of function due to a loss of the structure; or hypersensitivity or anaphylaxis resulted from the cross-reaction with other endogenous particles (Bana et al. Citation2022). Chemical degradation includes oxidation, deamidation or amidation of the amino acids in the sequence, or cleavage of disulphide bonds (-S-S-) (Ma et al. Citation2020) (). Multiple factors can influence the stability of an antibody and they can be both intrinsic and extrinsic, including the concentration of the protein, external mechanical stress, pH and temperature, as well as the excipients in the formulation and light (Le Basle et al. Citation2020).
As a result of the complicated and intertwined degradation pathways, a variety of analytical tools are required to provide complementary information on the physical, chemical and biological stability of an antibody (Le Basle et al. Citation2020). Size exclusion chromatography (SEC) is one of the most widely used technique, since it provides qualitative and quantitative information on protein aggregation as well as the amount of chemical degradation (Arakawa et al. Citation2010; Goyon et al. Citation2018; Le Basle et al. Citation2020). SEC has the advantages of fast speed, high reproducibility and non-denaturing nature (Hong et al. Citation2012; Goyon et al. Citation2018). However, this method is usually valid only over a limited molecular mass range and the antibody aggregates can be removed by the column or dissociated upon sample dilution (Goyon et al. Citation2018). Therefore, the SEC measured results are usually interpreted complementarily with an orthogonal test. One example of such tests is sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) (Den Engelsman et al. Citation2011). SDS-PAGE provides information on the covalent aggregation and fragmentation of the proteins based on the molecular weight while non-covalent aggregates can be dissociated by SDS (Den Engelsman et al. Citation2011). The SDS-PAGE can be carried out with or without prior reduction to the disulphide bonds of the antibody. The non-reducing SDS-PAGE is the preferred condition used in stability testing since it preserves the structure of the intact antibody (Liu et al. Citation2007; Kirley and Norman Citation2018). Routine monitoring of the visual appearance and pH of the antibody in a solution, and biological tests with immunological or cytotoxic assays are also recommended in the stability tests (Le Basle et al. Citation2020; Bana et al. Citation2022). Other analytical tools to monitor antibody stability include peptide mapping, mass spectrometry, circular dichroism, nuclear magnetic resonance (NMR), dynamic light scattering and differential scanning calorimetry (Den Engelsman et al. Citation2011; Bana et al. Citation2022).
The aim of this study was to assess the stability and degradation pathways of trastuzumab under various stress conditions including mechanical stress, repeated freeze-and-thaw, pH and temperature as well as measuring its long term storage stability. First, a simple and sensitive SEC high performance liquid chromatography (SEC-HPLC) method was developed and validated. F(ab)’ of trastuzumab was obtained by pepsin digestion followed by reduction with 2-mercaptoethylamine, and used to investigate the specificity of the SEC-HPLC method. Reconstituted lyophilised Herceptin (21 mg/ml in Water for Injection) and its dilutions (0.21 and 2.1 mg/ml) for clinical use were subjected to commonly applied experimental procedures during nanoparticle development; and the structural integrity after the stress conditions were investigated using the validated SEC-HPLC method along with SDS-PAGE. Lastly, the physicochemical stability of trastuzumab at −80 °C, 4 °C, 25 °C and 37 °C were assessed for up to 12 months using the two analytical methods; and the anti-proliferation stability of the solution stored at 4 °C based on the manufacturer’s recommendation for clinical settings was evaluated on a HER2 positive BT-474 breast cancer cell line. Compared with the existing reports (Kaiser and Kramer Citation2011; Pabari et al. Citation2011; Nalenz et al. Citation2018), trastuzumab was challenged to different pH, and covered a broader concentration range for an extended period of time in this study
2. Materials and methods
2.1. Materials and cell culture
Each vial of 150-mg trastuzumab (Herceptin ®, Roche) powder (containing 3.36 mg L-histidine HCl, 2.16 mg L-histidine, 136.2 mg trehalose dihydrate, and 0.6 mg polysorbate 20) was reconstituted with 7.2 ml of Bacteriostatic Water for Injection to yield a solution of 21 mg/ml trastuzumab as recommended prior to clinical use. The samples were used immediately or stored at 4 °C overnight before further studies.
Dodecylsulphate sodium (SDS) was obtained from Serva (Heidelberg, Germany). Precision Plus Protein ™ dual colour standards, 30% acrylamide/bis solution, 2-amino-2-(hydroxymethyl)-1,3-propanediol (Tris base) and tetramethylethylenediamine (TEMED) were purchased from Bio-Rad Laboratories (California, USA). UltraPure™ Glycine, Coomassie Brilliant Blue, 2-aminoethanethiol hydrochloride (2-mercaptoethylamine, 2-MEA) were purchased from Thermofisher Scientific (Auckland, New Zealand). Ethylenediaminetetraacetic acid (EDTA) was purchased from Sigma (Auckland, New Zealand).
The HER2 positive breast cancer BT-474 cells was from American Type Culture Collection (ATCC® HTB-20TM) and tested negative for mycoplasma contamination before undertaking a biological stability test. Dulbecco’s modified eagle’s medium (DMEM), fetal bovine serum (FBS) and penicillin-streptomycin (PS) were obtained from Thermofisher Scientific (Auckland, New Zealand). Thiazolyl blue tetrazolium bromide (MTT) was purchased from AK scientific (California, USA). Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (Auckland, New Zealand). Cells were grown in DMEDM medium with 10% FBS and 1% PS at 37 °C, 5% CO2.
All other materials used were analytical grade.
2.2. SEC-HPLC method development
2.2.1. SEC-HPLC instruments and chromatographic conditions
An Agilent 1260 series LC system (Agilent Co., California, United States) equipped with a quaternary pump, a diode array detector (DAD), and an autosampler were used. The signal was analysed using Chemstation software (Agilent Technologies, Australia, version B. 03). A BioZen SEC-2 LC Column (150 × 4.6 mm) with a particle size of 1.8 μm, a pore size of 150 Å and molecular weight separation range of 1 – 450 kDa (Phenomenex, California, USA) was used and kept at 20 °C.
2.2.2. Optimisation of SEC-HPLC wavelength and mobile phase
To maximise the detection sensitivity, the UV-Vis spectrum of trastuzumab solution (100 μg/ml) was scanned from 190 nm to 400 nm with the DAD during the HPLC. The wavelength that gave the highest reading of the area under the curve was determined as the optimum, and used as the detection wavelength in the subsequent SEC-HPLC method.
The retention time and the peak symmetry of the trastuzumab was optimised by comparing the chromatography profiles between different buffer strengths (100 mM and 250 mM KH2PO4) and pH (pH 6.8 and 7.4). The use of co-solvent NaCl (150 mM and 300 mM) and acetonitrile (ACN) at 2, 5, and 10% to suppress the electrostatic and hydrophobic interactions between the analyte and the column respectively, were also investigated. The final mobile phase consisted of 100 mM KH2PO4, pH 6.8 with a flow rate of 0.2 ml/min, and the signal was detected at 278 nm.
2.3. SEC-HPLC method validation
Following the International Conference on Harmonisation (ICH) guidelines (EMA Citation2005), the SEC-HPLC method was validated for characteristics including specificity, linearity, range, accuracy, precision, limit of detection (LOD) and limit of quantification (LOQ).
The specificity of the assay was analysed by the distinct peak separation of trastuzumab and one of its degraded products F(ab)’ (). F(ab)’ was generated from trastuzumab in two steps (Manjappa et al. Citation2011). First, F(ab)2′ was obtained by incubating trastuzumab with immobilised pepsin (Thermofisher, Auckland) at 37 °C for 9 h. F(ab)’ was then generated by incubating F(ab)2′ with 50 mM 2-MEA in the presence of 3 mM EDTA at 37 °C for 5 h. The 2-MEA in the mixture was placed in cellulose acetate dialysis bag (MWCO 14 kDa) and removed by dialysis against 3 mM EDTA in MilliQ water at room temperature.
The range of linearity was validated from 5 µg/ml to 120 µg/ml. Six standard solutions at concentrations of 5, 10, 30, 60, 80 and 120 µg/ml were prepared by diluting the freshly reconstituted solution of trastuzumab (21 mg/ml) with appropriate volumes of MilliQ water. The peak area against the trastuzumab concentration was plotted, and a standard curve was constructed using a linear regression analysis. The experiments were run in triplicates.
To determine the accuracy and precision, trastuzumab solutions at 20, 50 and 90 µg/ml were prepared independently by diluting the reconstituted trastuzumab solution with MilliQ water. The concentration of each sample was calculated using the linear equation generated from the calibration curve. The accuracy which measures the closeness of agreement between the calculated concentrations and the nominal concentrations (EMA Citation2005) was expressed as the percentage of the former compared to the latter. The precision which measures the closeness of agreement between multiple measurements (EMA Citation2005) was expressed as the relative standard deviation (RSD). The intra-day and inter-day accuracy and precision were determined by analysing the three samples of each concentration at three different time points in one day, and on three consecutive days, respectively.
The LOD and LOQ which measure the sensitivity of the method were determined based on the signal-to-noise ratios of 3:1 and 10:1, respectively (n = 3), using the areas under the curve in the chromatograms.
2.4. Stability of trastuzumab under stress conditions
Two concentrations of trastuzumab solutions were tested under stress conditions. The high concentration (21 mg/ml) samples used were the clinically reconstituted trastuzumab (Herceptin ®) solution, and a low concentration solution of 0.21 mg/ml was obtained by diluting the abovementioned solution with MilliQ water.
2.4.1. Stress conditions
Various stress conditions potentially incurred in a nanoparticle formulation process were investigated: 1) mechanical stress: the trastuzumab solutions were sonicated in the ultrasonic water bath (Elmasonic S 100, Elma) at 37 kHz for 60 min, or vortexed on a vortex mixer for 10 min; 2) freeze-and-thaw cycle of freezing at −80 °C for 3 min, and thawing at 45 °C for 7 min; and the cycles were repeated 7 times; 3) pH: the trastuzumab solutions were adjusted to pH 2, 4, 10 and 12 with 0.1 M HCl and 0.1 M NaOH and stored at 4 °C 16 h; 4) short-term high temperature: the solutions were stored at 60 °C and 75 °C, and the stability was assessed after 24 h and 5 days.
All samples were analysed in triplicates. After they underwent stressed conditions, samples were visually inspected for clearness, colour change and precipitation.
2.4.2. SEC-HPLC analysis
After exposure of 0.21 mg/ml and 21 mg/ml trastuzumab solution to aforementioned stress conditions, samples were taken immediately and diluted 2 and 200 times respectively with MilliQ water to a concentration in the range of the standard curve, and measured by the SEC-HPLC method. The actual trastuzumab concentration remaining was calculated from the linear regression equation, and expressed as the percentage of the nominal concentration.
2.4.3. SDS-PAGE analysis
All SDS-PAGE analyses were performed using 8% SDS-PAGE gels prepared from 30% acrylamide solution. After they underwent the stressed conditions, trastuzumab samples of 0.21 mg/ml and 21 mg/ml were diluted 2 times and 200 times respectively with MilliQ water, and 20 µL of each sample was mixed with 5 µL non-reducing laemmli buffer (312 mM Tris pH 6.8, 10% SDS w/v, 50% glycerol v/v and 0.005% Bromophenol Blue). The non-reducing samples, along with the Precision Plus Protein™ standards (10–250 kDa), were run at a voltage of 150 V for 55 min in the running buffer that consisted of 25 mM Tris base, 192 mM glycine and 0.1% (w/v) SDS. The gel was then fixed in a mixture of 50% (v/v) ethanol and 3% (v/v) of 85% phosphoric acid for 3 h before washed in distilled water. Coomassie blue (0.05% w/v) in 34% (v/v) methanol, 3% (v/v) of 85% phosphoric acid and 17% (w/v) ammonium sulphate was used to stain the gels. The gels were de-stained by washing in distilled water.
2.5. Stability of trastuzumab after long term storage
2.5.1. Storage conditions
The Bacteriostatic Water for Injection reconstituted trastuzumab solutions (21 mg/ml), and those MilliQ water diluted medium (2.1 mg/ml) and low (0.21 mg/ml) concentration solutions were stored at −80 °C, 4 °C, 25 °C, and 37 °C to assess the long term storage stability. Samples were taken at predetermined intervals of 1, 4, 6 and 12 months and were visually inspected for colour change, clearness and presence of particulates before being investigated in triplicates using the validated SEC-HPLC assay along with SDS-PAGE. In addition, samples stored at 4 °C as recommended by the manufacturer, were evaluated for their anti-proliferation activity.
2.5.2. Stability by SEC-HPLC and SDS-PAGE analysis
All trastuzumab samples stored as 0.21 mg/ml, 2.1 mg/ml and 21 mg/ml solution were diluted 2, 20 and 200 times respectively with MilliQ water before analysed by the validated SEC-HPLC method directly; in addition, a portion (20 µl) of the diluted sample was mixed with 5 µl of non-reducing laemmli buffer and analysed by SDS-PAGE.
2.5.3. Biological stability of trastuzumab using cell viability assay
Following the storage for 6 and 12 months, trastuzumab solutions (0.21 mg/ml and 21 mg/ml) stored at 4 °C, which is the recommended storage temperature by the manufacture, were assessed for the anti-proliferation activity using the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay (MTT) cell viability assay. The HER2 breast cancer BT-474 cells were seeded at a density of 3,000 cells per well in a 96-well plate, and incubated at 37 °C, 5% CO2 for 24 h to allow cells to attach. Cells were then treated with trastuzumab solutions at a final concentration of 0.07 nM to 1 µM for 5 days. At the end of treatment, 20 µL of 5 mg/ml MTT was added into each well and incubated for 4 h. DMSO (150 µL) was added to dissolve the formazan crystals after removing the cell medium, and the absorbance was read at 570 nm in a microplate plate reader (Varioskan Lux, Massachusetts, USA). IC50 of the samples were calculated using GraphPad Prism 8 software, and compared with that of the freshly-made trastuzumab solution to assess any changes in the anti-proliferation activity.
2.6. Statistical analysis
All samples were analysed from three independent experiments, and data are presented as mean ± standard deviation (SD). Data were analysed by student T test or one-way analysis of variance (ANOVA) using Prism 8 software (GraphPad). A p-value < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Optimised SEC-HPLC conditions
SEC-HPLC is a convenient and non-invasive technique to quantify the size of proteins and polymers. In the present study, a BioZen SEC-2 LC column with fully porous particles as the stationary phase was used. The antibody samples were injected into the column, and the molecules are separated based on their hydrodynamic diameter. Large molecules cannot penetrate the pores, and are eluted first, whereas small molecules can penetrate the pores and take longer to be eluted (Hong et al. Citation2012). The column used in the current study has a particle size of 1.8 µm. Smaller particle size, especially those between 1 to 2 µm, are believed to have improved separation resolution and higher efficiency compared with previously used 3.5 or 5 µm particle sizes (Hong et al. Citation2012).
The UV-Vis spectrum scanning of chromatographic peaks revealed that the both trastuzumab and F(ab)’ had steady absorption between 240 nm and 280 nm, with a peak absorbance at 278 nm (). Therefore, a wavelength of 278 nm was used in the next step to develop an SEC-HPLC method. Most proteins have peak absorbance near 280 nm and the optimal wavelength identified for trastuzumab (278 nm) is consistent with previous reports in the literature (Porterfield and Alotnick Citation2010; Chan et al. Citation2020). The absorbance recorded at this wavelength is believed to be caused by aromatic amino acids, in particular tyrosine and tryptophan in the trastuzumab structure (Chan et al. Citation2020).
Figure 2. UV-vis scan and typical SEC-HPLC chromatograms of trastuzumab and F(ab)’. (A) UV scan of trastuzumab (100 µg/ml) and F(ab)’ (generated from 10 mg/ml trastuzumab) peaks with a DAD on SEC-HPLC, indicating suitable detection of both compounds at the wavelength of 278 nm. (B) SEC-HPLC chromatograms of 100 µg/ml trastuzumab showing a pure peak at 6.3 ± 0.1 min, and its F(ab)’ fragment (confirmed by SDS-PAGE) at 7.7 ± 0.1 min. Solvent peaks appeared after 9 min and did not interfere the detection of the interests.
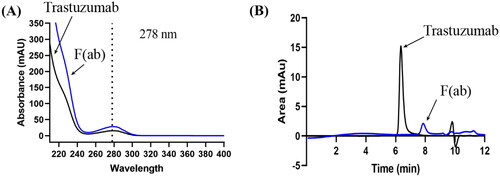
The use of 100 mM KH2PO4 (pH 6.8) as the starting mobile phase in the SEC-HPLC, enabled the antibody to be analysed in a non-denaturing environment (Hong et al. Citation2012). The resulting chromatograph showed an analytical peak at 6.3 min, which was in the ideal retention time range (5–8 min) of an HPLC assay () (Wu et al. Citation2005). The peak symmetry of trastuzumab was optimised by suppressing its interaction with the packing material of the stationary phase. Although increasing the ionic strength in the mobile phase could theoretically suppress the electrostatic interaction, increasing the concentration of KH2PO4 in the mobile buffer to 250 mM or addition of NaCl (150 mM and 300 mM) resulted in no change in the retention time nor peak shape in the current study. Similarly, addition of co-solvent ACN (2 - 10%, v/v) to suppress the hydrophobic interaction of the antibody with the column, had negligible effect on the chromatogram. The results possibly suggested that trastuzumab only had weak electrostatic and hydrophobic interaction with the column. Furthermore, increasing the mobile phase pH to 7.4 only delayed the retention time but did not improve the peak symmetry. Therefore, a final of 100 mM KH2PO4, pH 6.8 delivered at 0.2 ml/min was used.
3.2. Validation of SEC-HPLC method
The developed SEC-HPLC method was validated and complied with ICH guidelines (EMA Citation2005). The method was shown to be specific, with distinct separation of intact trastuzumab (retention time 6.3 ± 0.1 min) to F(ab)’ (retention time 7.7 ± 0.1 min) (). The identity of F(ab)’ was confirmed on SDS-PAGE with a clear band at ∼50 kDa (Data not shown). During the validation of the developed method, the peak area measured showed a significant linear relationship with the trastuzumab concentration in the range of 5 to 120 μg/ml (R2=0.9993).
The method was accurate with the calculated intra and inter-day concentrations between 99.0 to 102.8% of the actual values (). The intra- and inter-day measurements of the method expressed close agreement with an RSD less than 5% in all samples.
Table 1. Intra-day and inter-day accuracy and precision of the developed SEC-HPLC method. Data are mean ± SD, n = 3.
LOD and LOQ of the method were found to be 0.6 µg/ml and 1.8 µg/ml, respectively. This demonstrated that the assay is more sensitive than a method previously reported (15.6 µg/ml and 31.3 µg/ml for LOD and LOQ, respectively) by Sundaram Palaniswamy et al. (Sundaram Palaniswamy Citation2016). In their SEC-HPLC method, a column with a larger particle size (2.7 µm) and a mobile phase of phosphate buffered saline with 50 mM sodium phosphate containing 150 mM sodium chloride (pH 7.4) were used.
3.3. Stability of trastuzumab under stress conditions
The validated SEC-HPLC methods was applied to measure the percentage of trastuzumab remained after various stress conditions in 0.21 mg/ml and 21 mg/ml solutions, and the results were summarised in .
Table 2. Percentage of trastuzumab remaining after different stress conditions measured by SEC-HPLC.
3.3.1. Effect of mechanical stress
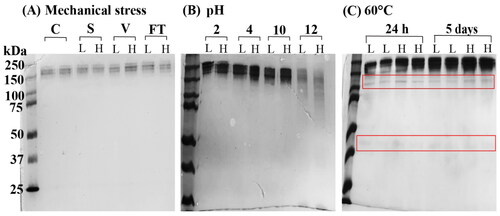
An antibody can be susceptible to a range of mechanical stress during formulation development, storage and transportation, such as shaking, sonication, mixing and vortexing (Bekard et al. Citation2011). Sonication is a common technique used to dissociate particles and improve dissolution rate (Pabari et al. Citation2011), or reduce the particle size of liposomes (Huang et al. Citation2010). In the present study, sonication of both high and low concentration trastuzumab samples at 37 kHz for 60 min did not change the retention time nor the peak shape on the SEC-HPLC analysis, and the calculated trastuzumab concentrations were close to 100% (). Similarly, there was no degradation or aggregation of the antibody detected, as only one band was shown on SDS-PAGE. This is consistent with the finding by Pabari et al.(Pabari et al. Citation2011), who demonstrated conformational integrity of trastuzumab after sonication at 1 and 3 Watts for 3 min. As stated in the data sheet of transtuzumab injection shaking should be avoided. However, vortexing of the samples (0.21 mg/ml and 21 mg/ml) for 10 min in this study did not cause any detrimental effect on trastuzumab structure as the concentrations after vortexing were 101.7 ± 1.0% and 100.1 ± 0.6% of the original, respectively. This was further confirmed by SDS-PAGE (). All samples tested under mechanical stress appeared clear and free of visible particles.
Figure 3. SDS-PAGE of trastuzumab solutions 0.21 mg/ml (L) and 21 mg/ml (H) after (A) mechanical stress. Trastuzumab control (C) of 21 mg/ml showed a single band at molecular weight of approximately 150 kDa. Sonication (S), vortexing (V) and repeated freeze-and-thaw (FT) did not show any aggregation or fragmentation; (B) solutions were adjusted to pH 2, 4, 10 and 12, and stored at 4 °C for 16 h or (C) incubated at 60 °C for 24 h or 5 days.
Mechanical stress is also known as hydrodynamic shear forces or agitation (shaking) stress (Mahler et al. Citation2009; Thomas and Geer Citation2011). Although the three terms are often used interchangeably, some authors point out that shearing and agitation are actually two different forms of mechanical stress (Mahler et al. Citation2009; Le Basle et al. Citation2020). The shearing force, which is a result of the fluid velocity gradient in moving liquid, does not cause aggregation (Thomas and Geer Citation2011; Le Basle et al. Citation2020). Instead, agitation introduces air bubbles in the liquid (cavitation) and creates new air-liquid interfaces, which leads to aggregation (Thomas and Geer Citation2011). When the antibody comes in contact with the air-liquid interface, it exposes its hydrophobic core to the air, causing unfolding of the structure and non-specific intermolecular interactions, thus the antibody becomes unstable (Le Basle et al. Citation2020). The mechanical resistance observed in the reconstituted trastuzumab solution (Herceptin) is largely due to the presence of the excipient polysorbate 20 in the original formulation (Patapoff and Esue Citation2009). Polysorbate 20 is a non-ionic surfactant and it protects trastuzumab against interface-induced aggregation by inhibiting the interaction between trastuzumab and the air-liquid interfaces (Singh et al. Citation2017).
In addition, the mechanical resistance of trastuzumab measured appeared to be concentration independent. Protein concentrations is generally considered to play an important and complicated role in the antibody stability (Le Basle et al. Citation2020). A high concentration of antibody can lead to enhanced molecular interactions, thus a higher propensity to aggregate. Similarly, low antibody concentration is less likely to show aggregation due to reduced protein-protein interaction, as well as the dissociation of weakly bonded aggregates via dilution. However, the dilution of the formulation can reduce the concentration of excipients and may compromise their protection effect. A lack of concentration effect observed with trastuzumab solution indicated that polysorbate 20 maintained its effective working concentration after imposed dilution.
3.3.2. Effect of freeze-and-thaw
In this study, the trastuzumab solutions remained clear and colourless after 7 cycles of freeze (-180 °C, 3 min) and thaw (45 °C, 7 min), which can be used for preparation of unilamellar liposomes with increased drug loading (Zhang et al. Citation2015). In SEC-HPLC, no changes were detected in the retention time nor the peak shape. The trastuzumab concentration remained within 5% variance to the original concentration in both the high and low concentration groups (). A single band without fragmentation was visualised in SDS-PAGE ().
Repeated freeze-and-thaw cycles are commonly used in liposome formulations to develop unilamellar liposomes (Zhang et al. Citation2015). During freezing, a few mechanisms are involved in inducing protein instability (Thorat et al. Citation2020). Firstly, the decreased temperature itself can cause cold denaturation by increasing the solubility of hydrophobic groups within the antibody; secondly, the proteins can adsorb to the newly created ice and liquid interface, which further leads to protein unfolding and aggregation; thirdly, crystallisation of the pH buffer molecules in the formulation can decrease the buffer capacity and induce pH shift. However, trastuzumab solutions subjected to freeze and thaw appeared stable in the current study. Mohamed et al. (Mohamed et al. Citation2018) also confirmed the stability of concentrated trastuzumab solution (22 mg/ml) after freezing at −80 °C for 24 h and thawing at room temperature for 3 cycles. However, Pabari and coworkers showed that the stability of trastuzumab after freeze-and-thaw is cycle dependent (Pabari et al. Citation2011). When frozen at −80 °C for 50 min and thawed at 4 °C, trastuzumab maintained its structural integrity (at 0.4 mg/ml and 4 mg/ml) initially, but had a decrease in its peak area on SEC-HPLC when cycle number increased to six. Therefore, the current results only suggested trastuzumab solutions were stable at the freeze-and-thaw conditions specified in the study, and cannot be extrapolated to other experimental conditions.
3.3.3. Effect of pH
In an acidic environment of pH 4.0, the increased trastuzumab concentrations measured on SEC-HPLC () and a protein band corresponding to 250 kDa on SDS-PAGE suggested a possible aggregation of the antibody. Interestingly, the aggregates showed a similar retention time as trastuzumab (6.3 min) instead of being eluted faster from the column. This indicated that the aggregates were likely formed via non-covalent binding of the intact antibody. A larger number of antibodies was withdrawn during sampling, and dissociated into individual trastuzumab molecules upon dilution, thus had no effect on the retention time. The non-covalent aggregation of trastuzumab at pH 4.0 had been reported in a previous study, in which aggregation was observed on SEC-HPLC and dynamic light scattering, but not on SDS-PAGE due to the dissociation by SDS (Mohamed et al. Citation2018). As the pH further drops to pH 2.0, remarkable decreases of trastuzumab were measured on SEC-HPLC (), and a higher molecular weight band observed on SDS-PAGE (). Trastuzumab is believed to be degraded at pH 2.0 due to the disruption of the tertiary structure from the protonation of the carboxyl group and breakage of the disulphide bonds (Sert et al. Citation2022). The higher molecular weight protein on SDS-PAGE could result from the accumulation of degraded fragments.
At pH 10.0, SEC-HPLC measured decreases in trastuzumab concentrations in both solutions (), indicating losses of the antibody either through chemical degradation or covalent aggregation. SDS-PAGE found an aggregate in high concentration trastuzumab sample, and it is consistent with previous studies in which non-reducible covalent binding of trastuzumab at pH 8.0 were reported (Mohamed et al. Citation2018; Sert et al. Citation2022). Similarly, at pH 12.0, only approximately 20% of the initial trastuzumab were found () and no clear bands were detected on SDS-PAGE (). A significant loss of trastuzumab at this high pH is likely caused by chemical degradation.
3.3.4. Effect of temperature
After stored at 60 °C up to 5 days, all trastuzumab samples showed aggregation as well as degradation with fragments at ∼110 kDa and 37–50 kDa on SDS-PAGE (). High temperature is well known to result in a heat-induced denaturation of the protein structure, and promote protein unfolding and/or aggregation (Paul et al. Citation2013). On the other hand, SEC-HPLC showed that the amount of antibody was reduced in 0.21 mg/ml solution after 5 days, whereas, significant aggregation was observed at 24 h as the drug concentration increased to 21 mg/ml (). The results indicated that the protein denaturation and unfolding was likely the main degradation pathway in low concentration samples; and the aggregation was most likely to dominant in high concentrations samples because of stronger protein-protein interaction. The combined results highlighted the importance of using different complementary tests to assess the stability and degradation pathways of protein samples. Thermostability and thermal resistance is best described by the melting temperature Tm, defined as the temperature at which half of the antibody is unfolded (Pucci et al. Citation2014; Sert et al. Citation2022). Trastuzumab was reported to have a Tm of 73–76 °C (Pérez et al. Citation2019). However an unfolding of the protein started at a temperature well below Tm, such as 60 °C has also been reported (Paul et al. Citation2013).
As the temperature increased to 75 °C, which was in the range of Tm, no drug peaks were detected on the SEC-HPLC chromatograph at 24 h () and the antibody concentration was below the limit of detection on SDS-PAGE (Data not shown). A recent study also recorded a denaturation of trastuzumab at 75 °C, as evidenced by an unfolding of the antibody and shifting from its regular secondary β-sheet structure to random coil structure (Sert et al. Citation2022).
3.4. Stability of trastuzumab after long term storage at different temperatures
3.4.1. Stability by SEC-HPLC and SDS-PAGE
According to the manufacturer’s recommendation, Herceptin reconstituted with bacteriostatic water for injection has a shelf life of 28 days at 2–8 °C (FDA Citation1998). However, a longer storage life may be required as part of the formulation development. Therefore, the long term stability of trastuzumab stored at −80 °C, 4 °C, 25 °C and 37 °C was investigated. Interestingly, the long term stability of trastuzumab measured appears to be both temperature and concentration dependent.
At low temperatures of 4 °C and −80 °C, trastuzumab at all tested concentrations (0.21- 21 mg/ml) stored in MilliQ water showed good stability over 12 months (). SEC-HPLC results showed no significant changes in the peak area (p > 0.05); and no bands corresponding to aggregation nor fragmentation were detected on SDS-PAGE. Low temperatures are commonly used in the long-term storage of antibodies. While 4 °C is more appropriate for storage up to months, very low temperatures at −20 °C or −80 °C is believed to be beneficial for long term storage up to years (Ma et al. Citation2020). Paul et al. (Paul et al. Citation2013) have previously demonstrated that trastuzumab stored in 0.9% sodium chloride (0.8 mg/ml and 2.4 mg/ml) was physically and chemically stable at 4 °C for up to 6 months, as measured by SEC-HPLC and other complementary analytical methods. In a separate study, SEC-HPLC and SDS-PAGE results showed that high concentration of trastuzumab (22 mg/ml) reconstituted with water for injection was stable at −20° and 2–8 °C for 4 weeks (Mohamed et al. Citation2018).
Figure 4. Stability of trastuzumab when stored at (A) -80 °C, (B) 4 °C, (C) 25 °C and (D) 37 °C for up to 12 months. Left: percentage of trastuzumab detected in 0.21 mg/ml (circle), 2.1 mg/ml (square) and 21 mg/ml (triangle) solutions by SEC-HPLC (n = 3, Mean ± SD); Right: SDS-PAGE at the end of 12 months storage.
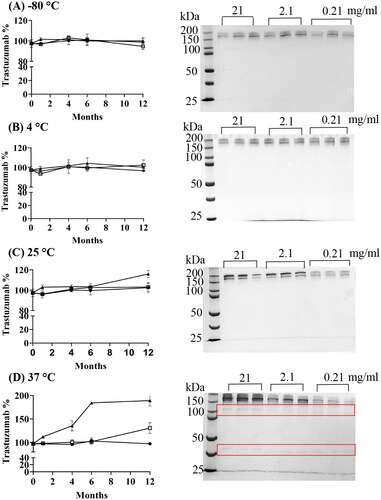
At 25 °C, low (0.21 mg/ml) and medium (2.1 mg/ml) concentrations of trastuzumab samples obtained approximately 100% of the nominal values over 12 months on SEC-HPLC (). SDS-PAGE results also confirmed the good stability of 0.21 mg/ml samples, however, revealed a possible presence of covalent binding in 2.1 mg/ml samples that were not detected on SEC-HPLC. The more concentrated trastuzumab solution (21 mg/ml) was stable for 6 months at this temperature; but showed changes at the end of 12 months in both assays.
At 37 °C, only 0.21 mg/ml samples demonstrated good stability over 12 months with a constant peak area measured (). However, trastuzumab solutions at high concentrations both showed aggregation, with peaks significantly increased at the end of 12 months. Degradation in all samples were also detected by SDS-PAGE with fragmentation bands at 110 kDa and 37 kDa. High temperature induced protein denaturation can result in protein unfolding and aggregation at the same time (Paul et al. Citation2013), but further study is still required to clarify whether the degradation products could form aggregates which co-eluted with trastuzumab peak in the SEC-HPLC and contribute to a false higher measurement.
In summary, samples stored at high concentrations and high temperature were less stable during long-term storage. Increased protein concentrations have enhanced protein-protein interactions within the samples, thus increased propensity to aggregate (Le Basle et al. Citation2020). High temperatures cause protein aggregation through the increased frequency of molecular collisions, as well as other effects, such as reduction of activation energy and enhancing hydrophobic interactions (Wang et al. Citation2010).
3.4.2. Anti-proliferation activity of trastuzumab using cell viability assay
Trastuzumab solutions stored at 4 °C showed the best long term stability profiles in the current study, and is the storage condition recommended by the manufacturer (FDA Citation1998). Therefore, biological stability of trastuzumab solutions (0.21 mg/ml and 21 mg/ml) stored at 4 °C was assessed.
The anti-proliferation activity of trastuzumab was measured on HER2 positive BT-474 cells with freshly reconstituted trastuzumab formulation as a control. A decrease in cell viability to approximately 50% was observed up to a concentration of 5 nM, after which increasing the trastuzumab concentration could not reduce the cell viability any further (). This lack of a measurable effect at high concentrations could be explained by trastuzumab-mediated antibody-dependent cell-mediated cytotoxicity (ADCC), in which the Fc region on the trastuzumab binds to the Fc receptors (FcRs) on Natural Killer (NK) cells, and activates NK cells to provoke cell death (Mandó et al. Citation2021). In the absence of NK cells, the ADCC effect could not be accurately measured by the in vitro MTT assay.
Figure 5. Anti-proliferation activity of trastuzumab solutions of (A) 0.21 mg/ml and (B) 21 mg/ml stored at 4 °C after 6 and 12 months. Cell viability of HER2+ breast cancer cell BT-474 was measured by MTT assay after 5-day treatment (Mean ± SD, each concentration was measured in 4 wells, n = 3).
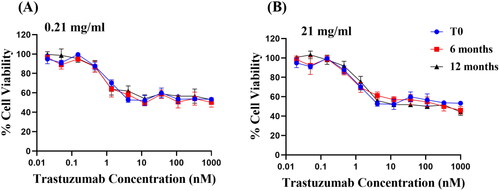
The trastuzumab solution tested in long term storage demonstrated no statistical significance in inhibiting the cell proliferation as compared to the control after 6 months (p = 0.1324 and p > 0.9999 for 0.21 mg/ml and 21 mg/ml respectively), and 12 months (p > 0.9999 for both 0.21 mg/ml and 21 mg/ml). The biological stability alone is not sufficient to predict the overall stability, since it only implies a conservation of the binding site and has no indication on the structural integrity of the whole antibody (Le Basle et al. Citation2020). In particular, the function of Fc region and trastuzumab-mediated ADCC effect were not measurable in the current study. Nevertheless, since physicochemical stability tests in the present study did not detect any aggregation nor fragmentation of trastuzumab at 4 °C, it was reasonable to predict that the function of Fc region and thus the ADCC effect in vivo would also be preserved; and the biological test acted as a complementary test to suggest that the trastuzumab is stable at 4 °C for 12 months.
The long term stability study demonstrated that evaluation of an antibody stability requires a range of different analytical techniques to assess the physiochemical properties, as well as complementary tests to measure the biological stability. Trastuzumab at 0.21 mg/ml and 21 mg/ml both demonstrated satisfactory physiochemical stability at 4 °C and −80 °C, and biological stability at 4 °C up to 12 months without consideration of microbiological contamination.
Overall, the current stability data covered a wide range of conditions that may be used in trastuzumab-involved formulation development. It is worth noting that all the samples used were prepared by dissolving a lyophilised trastuzumab injection in MilliQ water without removing the excipients. The presence of lipids, polymers or surfactants during the development of nanoformulation may affect the stability of the antibody, and was not covered in the study.
4. Conclusion
In the present study, an SEC-HPLC method to quantify anti-HER2 antibody trastuzumab was developed. The validated method was accurate and precise, and was more sensitive than previous method reported. As water constituted with the presence of the formulation excipients (Herceptin), trastuzumab was able to withstand mechanical stress commonly applied during nanoparticle formulation, including sonication, vortexing, ultracentrifugation and repeated freeze-and-thaw, although the stability of freeze-and-thaw depends on the number of cycles. Trastuzumab was more vulnerable to both low and high pH conditions and elevated temperatures above 60 °C, especially when it is stored at high concentrations. The data suggested that the stability of trastuzumab is both temperature- and concentration-dependent; concentrated trastuzumab stored at high temperature is more likely to form aggregates over time. Diluted trastuzumab samples in the presence of excipients (0.21 mg/ml) stored at −80 °C or 4 °C appeared to be chemically stable for at least 12 months. Overall, this study provided valuable stability information for the development of trastuzumab based nano-formulation as well as in clinical settings. This study also demonstrated that SEC-HPLC, SDS-PAGE along with biological tests were sufficient to determine the antibody stability.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arakawa T, Ejima D, Li T, Philo JS. 2010. The critical role of mobile phase composition in size exclusion chromatography of protein pharmaceuticals. J Pharm Sci. 99(4):1674–1692.
- Bana AA, Mehta P, Ramnani KA. 2022. Physical instabilities of therapeutic monoclonal antibodies: a critical review. Curr Drug Discov Technol. 19(6):e240622206367.
- Le Basle Y, Chennell P, Tokhadze N, Astier A, Sautou V. 2020. Physicochemical stability of monoclonal antibodies: a review. J Pharm Sci. 109(1):169–190.
- Bekard IB, Asimakis P, Bertolini J, Dunstan DE. 2011. The effects of shear flow on protein structure and function. Biopolymers. 95(11):733–745.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G, Untch M, Smith I, Gianni L, Herceptin Adjuvant (HERA) Trial Study Team, et al. 2017. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 389(10075):1195–1205., al et.
- Chan C, Seki JT, Kwong R, Reilly RM. 2020. The pharmaceutical stability of trastuzumab after short-term storage at room temperature assessed by analytical techniques and tumour imaging by microSPECT/CT. Int J Pharm. 588:229786.
- Den Engelsman J, Garidel P, Smulders R, Koll H, Smith B, Bassarab S, Seidl A, Hainzl O, Jiskoot W. 2011. Strategies for the assessment of protein aggregates in pharmaceutical biotech product development. Pharm Res. 28(4):920–933.
- Ferraro E, Drago JZ, Modi S. 2021. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions. Breast Cancer Res. 23(1):1–11.
- Gao Y, Tang M, Leung E, Svirskis D, Shelling A, Wu Z. 2020. Dual or multiple drug loaded nanoparticles to target breast cancer stem cells. RSC Adv. 10(32):19089–19105.
- Goyon A, Fekete S, Beck A, Veuthey JL, Guillarme D. 2018. Unraveling the mysteries of modern size exclusion chromatography - the way to achieve confident characterization of therapeutic proteins. J Chromatogr B Analyt Technol Biomed Life Sci. 1092:368–378.
- FDA. 1998. Herceptin (Trastuzumab) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf.
- Hong P, Koza S, Bouvier ESP. 2012. Size-exclusion chromatography for the analysis of protein biotherapeutics and their aggregates. J Liq Chromatogr Relat Technol. 35(20):2923–2950.
- Huang X, Caddell R, Yu B, Xu S, Theobald B, Lee J, Lee RJ. 2010. Ultrasound-enhanced Microfluidic Synthesis of Liposomes. Anticancer Res. 30(2):463–466.
- Hudis CA. 2007. Trastuzumab – Mechanism of action and use in clinical practice. N Engl J Med. 357(1):39–51.
- Kaiser J, Kramer I. 2011. Physiochemical Stability of Diluted Trastuzumab Infusion Solutions in Polypropylene Infusion Bags. Int J Pharm Compd. 15(6):515–520.
- Kirley TL, Norman AB. 2018. Unfolding of IgG domains detected by non-reducing SDS-PAGE. Biochem Biophys Res Commun. 503(2):944–949.
- Kulhari H, Pooja D, Rompicharla SVK, Sistla R, Adams DJ. 2015. Biomedical applications of trastuzumab: as a therapeutic agent and a targeting ligand. Med Res Rev. 35(4):849–876.
- Liu H, Gaza-Bulseco G, Chumsae C, Newby-Kew A. 2007. Characterization of lower molecular weight artifact bands of recombinant monoclonal IgG1 antibodies on non-reducing SDS-PAGE. Biotechnol Lett. 29(11):1611–1622.
- Ma H, Ó'Fágáin C, O'Kennedy R. 2020. Antibody stability: a key to preformance – Analysis, influences and improvement. Biochimie. 177:213–225.
- Maadi H, Nami B, Tong J, Li G, Wang Z. 2018. The effects of trastuzumab on HER2-mediated cell signaling in CHO cells expressing human HER2. BMC Cancer. 18(1):238.
- Mahler HC, Friess W, Grauschopf U, Kiese S. 2009. Protein aggregation: pathways, induction factors and analysis. J Pharm Sci. 98(9):2909–2934.
- Mandó P, Rivero SG, Rizzo MM, Pinkasz M, Levy EM. 2021. Targeting ADCC: a different approach to HER2 breast cancer in the immunotherapy era. Breast. 60:15–25.
- Manjappa AS, Chaudhari KR, Venkataraju MP, Dantuluri P, Nanda B, Sidda C, Sawant KK, Ramachandra Murthy RS. 2011. Antibody derivatization and conjugation strategies: application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J Control Release. 150(1):2–22.
- Meric-Bernstam F, Johnson AM, Dumbrava EEI, Raghav K, Balaji K, Bhatt M, Murthy RK, Rodon J, Piha-Paul SA. 2019. Advances in HER2-Targeted Therapy: novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin Cancer Res. 25(7):2033–2041.
- Meza-Junco J, Au HJ, Sawyer MB. 2009. Trastuzumab for gastric cancer. Expert Opin Biol Ther. 9(12):1543–1551.
- Mohamed HE, Mohamed AA, Al-Ghobashy MA, Fathalla FA, Abbas SS. 2018. Stability assessment of antibody-drug conjugate Trastuzumab emtansine in comparison to parent monoclonal antibody using orthogonal testing protocol. J Pharm Biomed Anal. 150:268–277.
- Nalenz H, Kopf E, Dietel E. 2018. Prolonged In-use Stability of Reconstituted Herceptin in Commercial Intravenous Bags. Int J Pharm Compd. 22(5):417–423.
- Nieto C, Vega MA, Del Valle EMM. 2020. Trastuzumab: more than a guide in her2-positive cancer nanomedicine. Nanomaterials. 10(9):1–20.
- Niza E, Noblejas-López MDM, Bravo I, Nieto-Jiménez C, Castro-Osma JA, Canales-Vázquez J, Lara-Sanchez A, Moya EMG, Burgos M, Ocaña A, et al. 2019. Trastuzumab-targeted biodegradable nanoparticles for enhanced delivery of dasatinib in HER2+ metastasic breast cancer. Nanomater 9(12):1793.
- Pabari RM, Ryan B, Mccarthy C, Ramtoola Z. 2011. Effect of Microencapsulation Shear Stress on the Structural Integrity and Biological Activity of a Model Monoclonal Antibody, Trastuzumab. Pharmaceutics. 3(3):510–524.
- Patapoff TW, Esue O. 2009. Polysorbate 20 prevents the precipitation of a monoclonal antibody during shear. Pharm Dev Technol. 14(6):659–664.
- Paul M, Vieillard V, Da Silva Lemos R, Escalup L, Astier A. 2013. Long-term physico-chemical stability of diluted trastuzumab. Int J Pharm. 448(1):101–104.
- Pérez LM, Rodríguez Taño AdlC, Martín Márquez LR, Gómez Pérez JA, Garay AV, Santana RB. 2019. Conformational characterization of a novel anti-HER2 candidate antibody. PLoS One. 14(5):e0215442.
- Porterfield JZ, Alotnick A. 2010. A simple and general method for determing the protein and nucleic acid content of viruses by UV absorbance. Virology. 407(2):281–288.
- Pucci F, Dhanani M, Dehouck Y, Rooman M. 2014. Protein Thermostability Prediction within Homologous Families Using Temperature-Dependent Statistical Potentials. PLoS One. 9(3):e91659.
- Rodallec A, Brunel J-M, Giacometti S, Maccario H, Correard F, Mas E, Orneto C, Savina A, Bouquet F, Lacarelle B, et al. 2018. Docetaxel-trastuzumab stealth immunoliposome: development and in vitro proof of concept studies in breast cancer. Int J Nanomedicine. 13:3451–3465.
- Schlam I, Swain SM. 2021. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. Breast Cancer. 7(1):56.
- Sert F, Hız D, Gülmez M, Ezgi Cankurtaran S, Irmak Kayalan C, Kurt H, Yüce M. 2022. Temperature and pH-dependent behaviors of mAb drugs: a case study for trastuzumab. Sci Pharm. 90(:21.
- Singh S, Bandi S, Jones D, Mallela K. 2017. Effect of polysorbate 20 and polysorbate 80 on the higher-order structure of a monoclonal antibody and its fab and Fc fragments probed using 2D nuclear magnetic resonance spectroscopy. J Pharm Sci. 106(12):3486–3498.
- Sundaram Palaniswamy M. 2016. Quantitation of mAb and ADC aggregation using SEC and an aqueous mobile phase.
- Thomas CR, Geer D. 2011. Effects of shear on proteins in solution. Biotechnol Lett. 33(3):443–456.
- Thorat AA, Munjal B, Geders TW, Suryanarayanan R. 2020. Freezing-induced protein aggregation - Role of pH shift and potential mitigation strategies. J Control Release. 323:591–599.
- EMA 2005. Validation of analytical procedures: text and methodology Q2(R1) In: International conference on harmonisation of technical requirements for registration of pharmaceuticals for human USE. https://database.ich.org/sites/default/files/Q2%28R1%29 Guideline.pdf
- Wang W, Nema S, Teagarden D. 2010. Protein aggregation—Pathways and influencing factors. Int J Pharm. 390(2):89–99.
- Wu Z, Medlicott NJ, Razzak M, Tucker IG. 2005. Development and optimization of a rapid HPLC method for analysis of ricobendazole and albendazole sulfone in sheep plasma. J Pharm Biomed Anal. 39(1-2):225–232.
- Zhang W, Wang G, Falconer JR, Baguley BC, Shaw JP, Liu J, Xu H, See E, Sun J, Aa J, et al. 2015. Strategies to maximize liposomal drug loading for a poorly water-soluble anticancer drug. Pharm Res. 32(4):1451–1461.
