ABSTRACT
A prospective study was designed to (a) investigate the rate of daily weight gain among kittens less than 9 weeks old presented to an animal shelter, (b) identify factors (e.g., sex, clinical signs of disease, diet, and medical treatment) that affect daily weight gain, and (c) investigate the mortality of study kittens. The study of 203 kittens was conducted at a managed admission, no-kill animal shelter in upstate New York, USA, from April 2014 through October 2014. Body weight was measured daily from day of intake to adoption or 12 weeks of age. Fecal score, clinical signs of disease, food type, and medical treatments were recorded daily. Lethargy and being female were significantly associated with lower daily weight gain. Despite the challenges of shelter and foster care, the average daily weight gain for study kitten was higher than that reported in other settings such as catteries and laboratories. Five study kittens (2.5%) died or were euthanized. Daily monitoring systems provide opportunities for interventions, increased live outcomes, and improved welfare for kittens in shelter and foster care.
Introduction
Kittens are a substantial source of intake into shelters in the United States. In 2019, data from 2722 shelters reflecting cats up to 5 months of age represented 48% (642,182/1,350,535) of feline intake and 24% (642,182/2,682,373) of all intake into shelters (Shelter Animals Count, The National Database, Citation2021). This number likely under-represents neonatal and pediatric cats, given that many kitten programs operate in shelters, rescues, and foster-based organizations not providing statistics to national databases. Scientific literature on growth, nutrition, and mortality of kittens primarily reflects those raised in catteries and laboratories, very different environments from fostered and shelter-housed kittens. Birth weight, growth rate, disease occurrence, and mortality are greatly impacted by environmental factors. Kittens entering animal shelters may be orphaned, ill, injured, and malnourished, rendering them more vulnerable than kittens housed in other environments. Very few studies have been conducted on growth, disease occurrence, and mortality of shelter-housed kittens, and none has been done on those residing within foster care (Dolan, Doyle, Tran, & Slater, Citation2020; Murray, Skillings, & Gruffydd-Jones, Citation2008; Strong, Gookin, Correa, & Banks, Citation2020).
Seminal studies of kitten growth were performed in purebred cattery and laboratory populations. Published literature suggests that kittens weigh approximately 100 grams at birth, gain 7–15 grams per day, and double their birth weights by day 10 (Fascetti & Delaney, Citation2013; Moik & Kienzle, Citation2011; Sparkes et al., Citation2006; Willoughby, Citation2008). Unlike puppies, which show exponential growth rates regardless of adult size, the growth rate of kittens in these settings has been found to be remarkably linear (Peterson, Citation2011b; Willoughby, Citation2008), with a slight increase postweaning (after 5 weeks of age) in laboratory and cattery kittens (Lawler, Citation2008; Lawler & Evans, Citation1995; Pedersen, Citation1991). Clinical guidelines for aging kittens by weight advise estimating 1 pound (0.45 kg) per month until adult weight is reached around 6 months of age (Root Kustritz, Citation2011). One study demonstrated this to be effective up to 10 weeks of age, although it was much more reliable in specific pathogen free (SPF) neonates (2–8 weeks of age) maintained in a single breeding facility than in pediatric kittens (6–20 weeks) sourced from private homes (DiGangi et al., Citation2020).
Although healthy kitten growth rates are remarkably linear in laboratory and breeding facilities, they have been demonstrated to be impacted by clinical factors (Willoughby, Citation2008). Weight measurements aid in the recognition of illness in pediatric patients (Willoughby, Citation2008). Reasons for poor growth in kittens in breeding colonies include large litter size, poor maternal health, inadequate or poor nutrition, congenital endocrine conditions or cardiac abnormalities, and infectious diseases such as gastrointestinal parasitism and viruses (Deag, Lawrence, & Manning, Citation1987; Peterson, Citation2011b; Willoughby, Citation2008).
Life-stage appropriate nutrition of adequate amounts is essential to kitten growth. Overall, energy requirements per body weight peak at 5 weeks of age and remain high until they plateau at 6 months of age (Case, Danstotle, Hayek, & Raasch, Citation2011). Although peak lactation occurs in week four, the yield and nutrient composition of queen’s milk is no longer sufficient as a sole source of nutrition for this stage of growth (Case et al., Citation2011; Hendriks & Wamberg, Citation2000). Although kittens may continue to take in the same relative volume of queen’s milk, they may show decreased growth (Hendriks & Wamberg, Citation2000). These factors, along with kitten development, drive the weaning process. Deciduous teeth erupt between days 21 and 35, and most kittens are readily consuming semi-solid food by day 35 (Prendergast, Citation2011). When comparing kitten growth rates pre- and postweaning, there is a biological precedent for using day 35 as the cutoff point between these two important life-stages (Case et al., Citation2011; Cave, Thompson, Reid, Hodgson, & Addie, Citation2002; Lawler, Citation2008; Lawler & Evans, Citation1995; Pedersen, Citation1991).
The weaning period is stressful, especially if orphaned (Greco, Citation2014; Peterson, Citation2011a). For bottle-fed kittens or those in highly controlled settings like laboratories, formula or food can be metered to match nutritional needs. However, as sheltered and fostered kittens begin to transition to solid food, it becomes increasingly difficult to track caloric intake in individual kittens. Likewise, various physiologic factors (e.g., illness, stress, and physical activity) and environmental factors (e.g., ambient temperatures) impact daily energy needs (Greco, Citation2014).
Weaning also impacts incidence of disease. Nutritional changes and physiologic stress at weaning alter intestinal pH and flora, as well as impact absorption of nutrients and stool consistency; although microbial colonization of the gut begins with nursing, it continues during the weaning period and transitions to solid food (Greco, Citation2014). Protection offered by the kitten’s initial intake of colostrum starts to wane (Greco, Citation2014; Lawler & Evans, Citation1995). Kittens with adequate intake of colostrum demonstrate a more stable microbial population, which in turn decreases susceptibility to gastrointestinal disease (Greco, Citation2014). Those who received no colostrum are at greater risk in this window as they start to explore the environment (Prendergast, Citation2011).
While it seems logical that clinical signs of disease would be associated with lower growth rates or weight loss in shelter cats, there are few studies. In one study of newly admitted adult shelter cats, upper respiratory infection (URI) was not associated with weight loss (Tanaka, Wagner, Kass, & Hurley, Citation2012). Diarrhea is a common concern in kittens, with published incidence as high as 72% in a shelter nursery (Dolan et al., Citation2020). However, diarrhea in kittens has not been studied in relation to growth rates. Diarrhea in kittens is associated with many factors, including viral and bacterial causes, nutritional changes, inappropriately reconstituted milk replacer, and overfeeding (Bucheler, Citation1999; Marks & Willard, Citation2005; Peterson, Citation2011a; Watson et al., Citation2017).
Excluding stillborn births, reported mortality rates for kittens less than 9 weeks of age in laboratory and breeding colonies range from 6% to 20% (Festing & Belby, Citation1970; Fournier et al., Citation2017; Romagnoli, Bensala, Ferre-Dolcet, Sontas, & Stelletta, Citation2019; Root Kustritz, Johnston, & Olson, Citation1995; Rutty & Smith, Citation1967; Sparkes et al., Citation2006; Ström Holst & Frössling, Citation2009; Young, Citation1973). Overall kitten mortality has been reported to be as high as 29.1% in a research setting (Root Kustritz et al., Citation1995). In one shelter-based study, kittens 0–7 weeks of age were four times more likely to die than cats 1–3 years of age (Murray et al., Citation2008). Preweaning mortality rates for purebred cats are most commonly attributed to maternal, congenital, or developmental abnormalities, with a substantial portion due to being still-born (Bucheler, Citation1999; Fournier et al., Citation2017; Lawler, Citation1984; Pedersen, Citation1991; Romagnoli et al., Citation2019; Ström Holst & Frössling, Citation2009; Young, Citation1973). Postweaning deaths are attributed most often to infectious disease and trauma. In a study of kittens housed in a shelter nursery, those diagnosed with URI were four times more likely to die (Dolan et al., Citation2020). Other clinical signs that correlated with increased mortality included weight loss (nine times greater), anorexia (three times greater), and diarrhea (two times greater) (Dolan et al., Citation2020).
Low birth weight has been correlated with increased mortality in kittens (Dolan et al., Citation2020; Lawler, Citation1984, Citation2008; Moik & Kienzle, Citation2011). However, at least one study of purebred cats demonstrated that surviving low-birth weight kittens compensate in the postnatal period to reach normal weights at maturity (Moik & Kienzle, Citation2011). A laboratory study of underweight kittens demonstrated that although preweaning growth rates were lower than average (7.3–9.0 g/day) and dipped at day 28, kittens rebounded after day 35 to gain 9–12 g/day (Lawler, Citation2008). Although low birth weight generally serves as a prognostic indicator, nutritional and environmental measures may improve outcomes for individuals (Bucheler, Citation1999; Lawler, Citation2008).
Tracking weight gain is considered an effective, inexpensive way to quantitatively monitor the health of growing animals. The current study investigated the rate of daily weight gain of kittens less than 9 weeks old presenting to an animal shelter, most of whom were placed in foster care. Additional objectives were to identify associations between daily rate of growth and several factors (sex, presence of clinical signs of disease, treatment, nutrition type, neutering), and to investigate the mortality (died or were euthanized) of study kittens.
Materials and methods
The project was reviewed in the IACUC office and exempted from IACUC review because the procedures performed were clinically required for the animal(s) and their conditions. The project was approved by the Cornell University Veterinary Clinical Studies Committee (CUVCSC) following an ethical and scientific review.
Study population
This study was conducted at a managed admission, no-kill animal shelter in upstate New York from April 14 2014 through October 10 2014. The shelter held the animal control contracts for seven towns in the county and had an annual intake of 1342 cats and 457 dogs in 2014.
Kittens <63 days (<9 weeks) of age presenting to the shelter were initially enrolled in the study. The shelter routinely placed kittens in this age range in foster care homes. Exceptions occurred when insufficient homes were available. Sick kittens were often foster-housed with experienced and trained care providers, but sometimes remained in the shelter short term for treatment and management by the shelter’s medical team.
Medical care for kittens was provided by a licensed veterinary technician (LVT) employed by the shelter under supervision by shelter medicine faculty from a nearby veterinary college. The standard intake protocol for kittens included a physical examination by a trained medical staff member (usually an LVT), followed by administration of a modified live FVRCP vaccination subcutaneously for kittens over 4 weeks of age; oral administration of pyrantel pamoate (0.05 ml/kg) and ponazuril (30 mg/kg); application of a flea and tick product titrated by weight; and recording of weight. If medical conditions were noted, a veterinarian was consulted, evaluated the kitten, and determined diagnosis and treatment.
Kittens with deciduous teeth were offered both canned and dry food and monitored to be sure that they ate prior to being sent to foster care. Kittens without fully erupted deciduous teeth remained with the queen whenever possible. If orphaned, kittens were offered bottles of milk replacer, shallow dishes of milk replacer, canned food, and/or a gruel of canned food and milk replacer. Standard, consistent diets included kitten milk replacer (KMR, Pet Ag) and kitten formula dry kibble provided through a shelter feeding program (Hill’s Science Diet).Footnote1 Canned food included a variety of flavors of kitten pate products available in grocery stores that were purchased or donated (i.e., Purina Fancy Feast Kitten Pate). Kittens with deciduous teeth that would not eat the standard dry kibble were provided with an alternate kibble (e.g., Purina Kitten Chow) as well as canned pate or a gruel of pate with milk replacer.
Healthy kittens were transferred to foster care within 24 to 48 hours with trained foster caretakers. Caretakers presented kittens for recheck appointments with the medical staff every 2 weeks until 8 weeks of age, at which time kittens returned to the shelter for neutering and adoption. Caretakers were further instructed to contact the shelter immediately in the event of injury or illness for consultation, which included a veterinarian on-call after business hours.
All kittens in this study were routinely dewormed with oral administration of pyrantel pamoate (0.05 ml/kg) and ponazuril (30 mg/kg) at two-week intervals starting at 14 days of age. Kittens with diarrhea >2 days commonly received fenbendazole (50 mg/kg PO SID for 5 days), ponazuril (30 mg/kg PO SID for 5 days), and a probiotic added to their food daily (Fortiflora, Purina, St Louis, MO, USA). Additionally, at the veterinarian’s discretion and based on clinical signs, fecal samples of kittens with vomiting and/or diarrhea might also have been tested using the Canine Parvovirus Antigen Test (Idexx SNAP Parvo, Westbrook, ME, USA) for feline panleukopenia.
Data collection
When kittens entered the shelter, their ages were collected from owners when known or estimated by trained medical personnel using two standard methods: dental eruption guidelines and the 1 pound (0.45 kg) per month estimate. Emphasis was placed on dentition if discrepancies occurred between methods.
During the shelter’s 2014 kitten foster care orientation, investigators explained the study protocols. Shelter personnel were trained to communicate salient aspects of the study and answer questions for care providers. The voluntary and confidential nature of the study was explained during the orientation and written in the study packets.
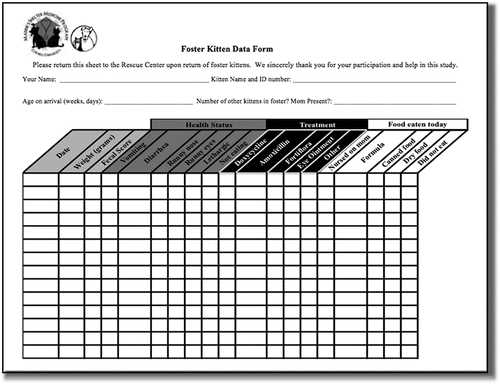
When care providers picked up their foster kitten(s), they received a study packet: a written introduction to the study, a data collection form for each kitten (), a digital kitchen scale (Etekcity EK3551, Anaheim, CA, USA) with removable bowl, a 7-point fecal scoring chart (Purina Veterinary Diets, Citationn.d.), and explicit instructions to complete the data forms daily, including use of the scale and fecal scoring chart. Foster care providers were also given supplies, including life-stage appropriate food, litter, heating discs, crates or kitten boxes, toys, and other implements.
Care providers were instructed to weigh each kitten daily at approximately the same time each day and to record information on the data collection form. Data collected included fecal quality score, clinical signs (nasal and ocular discharge, diarrhea, vomiting, inappetence, and lethargy), medical treatments (systemic antibiotics, probiotics, ophthalmic ointments, de-wormers, or other), and the primary type of food eaten (queen’s milk, commercial milk replacer, canned kitten food, and dry kibble). Dates of neutering were collected from the shelter’s medical records.
Foster care providers were contacted by e-mail approximately 1 day after receiving their kittens to verify all information and answer questions. Those who failed to respond or lacked e-mail accounts were contacted by telephone. All participants were contacted once weekly to encourage participation and address any questions. Medical questions were referred to medical personnel.
The study manager visited the shelter daily to identify kittens housed in the shelter in the event of a delay in moving to foster care due to illness or management issues (e.g., short-term shortage of a foster care provider). She collected study data for those kittens during their residence in the shelter.
Three of the five kittens (meeting study inclusion criteria) that died or were euthanized during the study period were submitted for necropsy by a board-certified pathologist.
Inclusion criteria
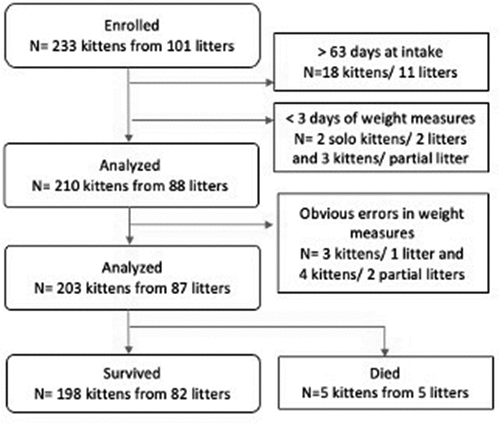
Only kittens 60 days of age or younger at the time of entry into the shelter and with at least 3 days of weight measurements were included. Of 233 kittens from 101 litters initially enrolled in the study, 203 kittens and 87 litters completed the study. The number and reasons that litters and kittens were excluded from study are presented in .
Data management and analysis
Data were entered into Microsoft Excel (Microsoft Corporation, Redmon, CA, USA) and then analyzed in Statistix (v.9, Tallahassee, FL, USA) and JMP (v.13, SAS Institute Inc, Cary, NC, USA). Since the daily rate of weight gain has been reported to increase after 5 weeks of age in laboratory and cattery kittens (Cave et al., Citation2002; Lawler, Citation2008; Lawler & Evans, Citation1995; Pedersen, Citation1991), growth rates were compared between those <35 and ≥35 days of age. In order to calculate daily weight gain for each kitten <35 days old, the daily weights of each kitten were regressed on the kitten’s age (up to 35 days) and the slope of the linear regression was used to estimate the daily rate of growth. This process was repeated for kittens 35 through 90 days of age. Average growth rates in each age group were calculated and compared using the Student's t-test with the Satterthwaite correction for unequal variances (Statistix, Citation2008)
Since kittens ≥35 days old grew at a significantly higher daily rate than those <35 days, two new files (one for each age group) were then created. They contained each kitten’s growth rate and data regarding potential risk factors during the respective age interval, litter identification number, and days observed. Each kitten was required to have at least 3 daily weight observations to be included in either age group to improve the precision of measurement. Factors potentially affecting the rate of growth of kittens in both groups were defined as follows: upper respiratory tract signs treated with an antibiotic (yes or no), diarrhea (fecal score >5/7) for >2 days (yes or no); fecal score (Purina fecal score of 6 or 7 for at least one day – yes or no), lethargy of at least one day’s duration (yes or no), sex (male or female), neutering (yes or no), and age of neutering. For un-weaned kittens <35 days of age, a variable representing primary source of food (nursing, formula fed, or eating soft food) was also evaluated.
Risk factors were evaluated for their relationship with daily rate of weight gain (as the outcome variable) adding litter as a random effect in two multiple regression mixed-effects models – one for each age group. Litter was included because of potential correlation of data among litter members with regard to their management, disease risk, and growth rates. The length of observation (days) was also evaluated for its potentially confounding or modifying effect on other variables. The multivariable models were built using forward stepwise regression.
To evaluate the source of food for kittens and its association with growth rate, the source was first coded into three categories: “queen” if the kittens nursed and were supplemented with canned or dry food, “formula” if the kittens were bottle-fed formula and supplemented with canned or dry food, or “soft food only” if they were fed only canned or dry food. Then, the mean growth rate for each litter was calculated. Finally, the mean growth rate for all litters receiving each source of food was calculated and these three means were compared using a one-way ANOVA. Source of food was not evaluated in the multivariable models because kittens in the same litter had the same food source (resulting in singularity). P values < 0.05 for all analyses were considered statistically significant.
Results
Study population
Of 88 litters that met the inclusion criteria, 87 (99%) litters had at least one kitten in the litter participating in the study. Of the 210 eligible kittens, 203 (96%) had usable data. Daily weight data and risk factors for growth rates from these litters and kittens were analyzed. Thirty-seven foster care providers participated in the study. Kittens were followed for a median of 24 days with quartiles of 15 and 33 days and a range of 3 to 90 days. Follow-up was truncated at 12 weeks (90 days) of age for kittens still belonging to the shelter.
Population characteristics
Each kitten was considered a part of a litter. The median litter size was two kittens (with a range of 1 to 8 kittens per litter). Thirty-eight litters (43.7%) had 1 kitten (singletons), and the rest had two or more. Thirty-seven percent of kittens were 34 days of age or younger at entry to the study. Of these with feeding data, 41.8% of kittens were bottle-fed with some canned/dry supplementation, 30.9% nursed on their queen with some canned/dry supplementation, and 27.3% ate only canned food as their primary food source. The youngest and oldest kittens were 8 and 60 days of age, respectively, when enrolled. The sex distribution slightly favored females ().
Table 1. Characteristics of litters and kittens included in the study.
Frequency of health issues
Clinical signs of illness were reported by trained caretakers based on scoring systems and treatment. Diarrhea defined as a fecal score >5 on at least one day was the most frequently reported sign (66.3%). The second most reported condition was upper respiratory tract disease requiring treatment (44.8%). These were followed by diarrhea of >2 days (31.5%) and lethargy of at least one day’s duration (7.9%) ().
Table 2. Frequency of health issues in kittens <9 weeks of age.
When the kittens and litters were divided by age at entry into the study, 60/75 (80%) of kittens had three or more weight measurements in the interval 8–34 days of age; 195/198 (98.5%) had three or more weight measurement in the interval 35–90 days of age. Among the kittens with at least 3 weight measurements in each age grouping (i.e., those included in the multivariable models), the frequency of clinical signs of illness did not differ significantly between kittens <35 and ≥35 days of age ().
Factors associated with daily weight change in surviving kittens
Litters with kittens <35 days of age had an average daily weight gain of 13.2 g/day (95% CI:8.9–17.5). Litters with kittens ≥35 days of age grew at an average daily rate of 19.2 g/day (95% CI: 17.6–20.8). Since litters with kittens ≥35 days old grew at a significantly (p < 0.05) higher than average daily rate, risk factors were evaluated for each age group separately.
Risk factors studied included clinical signs of disease (diarrhea, upper respiratory signs, and lethargy), sex, neuter surgery, and, for kittens <35 days of age, primary type of food consumed (queen’s milk, formula, and canned food). The length of observation was initially included in both age group models. Since it did not change the models appreciably and was not significant, it was removed from the final models.
Adjusting for litter, in the younger kittens, only the presence of lethargy was significantly associated with the rate of weight gain. In the older kittens, both sex (p = 0.01) and lethargy (p = 0.04) were significantly associated with daily rate of weight gain in kittens (). Using the regression equation, kittens <35 days without lethargy had a growth rate of 13.6 g/day, and those with lethargy, only 6.6 g/day. For kittens ≥35 days old, females and males with lethargy grew an average of 15.4 and 16.6 g/day, respectively. Among kittens ≥35 days of age with no lethargy, males had a remarkably high growth rate of 19.5 g/day, and females 18.3 g/day.
Table 3. Multiple regression mixed-effects model* of factors associated with daily weight gain in sheltered and fostered kittens 8–62 days of age followed to 90 days of age.
Neutering and age of neutering were not significantly associated with rate of weight gain in kittens ≥35 days of age. Neutering was not examined for kittens <35 days of age since kittens were not neutered until 8 weeks of age.
Sources of nutrition did not have a significant effect on weight gain. No significant differences were observed in the growth rates of litters <35 days of age based on their source of nutrition. Kittens <35 days of age grew at an average rate of 13.5 g/day (95% CI: 2.5–24.5), 12.1 g/day (95% CI: 4.3–19.9), and 14.3 g/day (95% CI: 5.3–23.3) if nursed with supplementation, were bottle fed with supplementation, or ate soft food only, respectively.
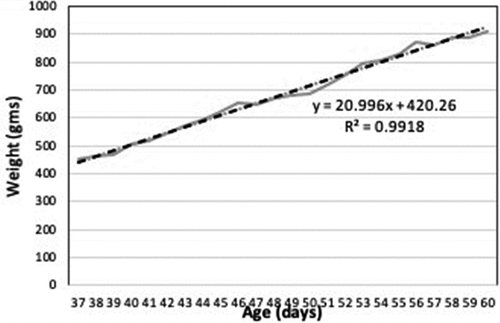
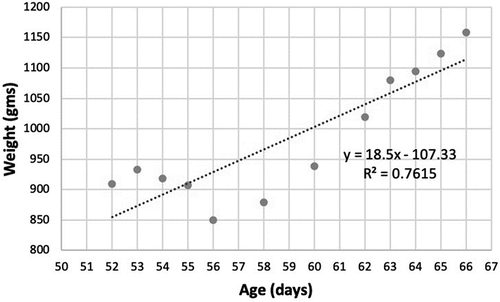
The weight gain for many kittens was remarkably linear with little variability (R2 values of >0.97) around the predicted lines over time as demonstrated in the kitten in . Other kittens, while they ultimately experienced steady weight gain, often had several days – usually corresponding to when they were displaying signs of illness – during which gains in weight were not observed (either they plateaued or lost weight) as shown in the kitten in . Surviving kittens resumed their weight gain following recovery to rebound to steady weight gains consistent with their healthy counterparts. Protocols in this shelter generally required a veterinary visit and treatment interventions for kittens displaying clinical signs of illness.
Mortality
The mortality in study kittens was 2.5% (95% CI: 0.8–5.7%). In 2014, the postbirth mortality rate of all kittens <9 weeks of age entering the shelter was 6.5% (95% CI: 4.4–9.2%), significantly higher than the mortality rate of the study kittens (p = 0.03). Since only five study kittens died, the study lacked statistical power to identify risk factors associated with weight changes in these kittens.
All kittens that died were ≥35 days of age at intake, and all were members of different litters. Necropsy was performed on three of the five. With the exception of a single kitten that died of postoperative respiratory arrest, all the kittens that died had less than average weight gain when compared to surviving kittens ≥35 days of age; three actually experienced weight loss. Characteristics and the cause of death or euthanasia are described in .
Table 4. Characteristics of kittens that died.
Discussion
Weight gain in study kittens
Shelters and foster homes are less controlled settings for the recruitment of kittens and the provision of care than catteries or laboratories. Kitten foster programs generally incorporate varied sources of kittens, foster homes, types of kitten food, queen health status, and seasonal changes. Nonetheless, in this study population, litters with kittens <35 days of age grew at an average daily rate at the high end of the range reported in the literature; litters with kittens ≥35 days of age grew at an average daily rate well over previously reported ranges from other settings (Fascetti & Delaney, Citation2013; Moik & Kienzle, Citation2011; Peterson, Citation2011b; Sparkes et al., Citation2006; Willoughby, Citation2008).
The high rate of daily weight gain in study kittens may be attributed to the robust training and monitoring in this shelter’s foster program. Foster care providers were provided in-person training, mentorship, and instructions to regularly weigh their kittens. During the study, recording methods were more formalized and standardized than in previous years. Recognition of a failure to gain weight and implementation of medical and nutritional interventions may have occurred more readily during the time of the study. Free-feeding of kittens postweaning, which is standard practice in foster programs, likely resulted in overfeeding and contributed to the high rate of growth in this population.
Nutritional stage and weaning
Overall, the daily weight gain for kittens was remarkably linear both pre- and postweaning. This is consistent with previously published literature from laboratory and breeding facilities (Peterson, Citation2011b; Willoughby, Citation2008). Primary source of nutrition did not have a significant effect on average daily growth rate of litters <35 days of age whether they nursed, were bottle fed, or ate soft food. Average daily growth rates for this group regardless of food type were higher than rates reported in the literature for preweaned kittens in other settings (Festing & Belby, Citation1970; Hendriks & Wamberg, Citation2000; Prendergast, Citation2011). Litters ≥35 days of age were primarily fed kitten soft food and kibble, and their average daily weight gain was higher than both the younger study group and the 7–15 g/day reported in the literature for breeding and laboratory kittens (Fascetti & Delaney, Citation2013; Moik & Kienzle, Citation2011; Peterson, Citation2011b; Sparkes et al., Citation2006; Willoughby, Citation2008).
In this study, data on food source in weaning and weaned kittens were the most difficult to track. Bottle-feeding kittens are typically fed according to caloric needs. Energy requirements for neonatal kittens equal to 20–26 kcal/100 g body weight daily divided over multiple feedings, with the maximum stomach capacity being 4 ml/100 g body weight (Lawler, Citation2008). It is common practice to provide bottle-feeding kitten caretakers with a feeding chart to ensure caloric intake is adequate for orphaned nursing kittens but not excessive. These materials are readily available in online training forums for kitten foster care providers (Maddie’s Fund, Citationn.d.). For weaning and weaned kittens, care providers generally provide varying forms and combinations of food to ensure that kittens eat. In this study, data on food provision were often missing from daily logs and likely subject to daily alterations based on perceived kitten needs. Consistent diets were provided by the shelter, but foster providers might have purchased diets on their own despite shelter instructions.
Most prevalent diseases in this population
Not surprisingly, diarrhea was the most common disease reported in the study population. Common causes of diarrhea in kittens include infectious (e.g., parasitic, bacterial, and viral) and nutritional causes. None of the study kittens were diagnosed with panleukopenia, and all were routinely dewormed prophylactically. Noninfectious causes of diarrhea in kittens are associated with the transition to solid food, diet changes, bloating, and alterations in the gut microbiome, especially if food offered is not consistent and/or high quality (Greco, Citation2014; Lawler, Citation2008). It is also associated with overfeeding (Greco, Citation2014). Although diets provided to study kittens were fairly consistent and of high quality, over-feeding may be very relevant to this study population given the general free-feeding of solid food and higher than average weight gain in these kittens postweaning.
Signs of upper respiratory infection requiring treatment were the second most common medical concern. Many of these kittens presented with signs at the time of intake and were started on treatment in the shelter. Although URI in cats is primarily viral in nature, antibiotics are often used in shelters for secondary bacterial issues and, especially in kittens and other immune-deficient shelter populations, can help prevent life-threatening sequela such as pneumonia and sepsis. In this shelter, while mild URI signs (including serous discharge) might just be monitored, more serious signs including conjunctivitis, mucopurulent discharge, or paroxysmal sneezing result in antibiotic administration. Of the five kittens that died, two had URI signs present on intake examination.
Risk factors for reduced weight gain
Kittens reported to be lethargic for at least one day were more likely to demonstrate lower than average weight gain; however, reports of diarrhea or URI did not significantly impact overall daily weight gain. Kittens <35 days reported to have lethargy and had an average daily weight gain lower than the lowest average rate reported in the literature from laboratory and breeding facilities and significantly lower than the study kittens in the same group without lethargy. For study kittens ≥35 days old, females and males with lethargy grew at an average rate just slightly higher than that reported from laboratory and breeding settings. In kittens ≥35 days without lethargy, males and females had remarkably high growth rates, with males being slightly higher. Overall, sheltered and fostered kittens fared as well as or better than kittens in laboratory and breeding facilities in regard to average daily weight gain and live outcomes.
While healthy kittens often had strikingly linear growth, it was common in study kittens experiencing illness to experience a dip in their rate of weight gain, followed by a rebound (see ). This rebound was often associated with treatment interventions enacted by the medical staff.
In this study, female kittens had a significantly lower rate of the growth than male kittens in the group ≥35 days of age. This is consistent with studies of laboratory and breeding kittens (DiGangi et al., Citation2020; Festing & Belby, Citation1970).
Neutering and the age at neutering did not impact the rate of weight gain in this study. Literature that reported neutering resulted in increased weight gain postsurgically was performed in a population of kittens altered at 19 weeks of age and followed for a year postsurgically (Alexander, Salt, Thomas, & Butterwick, Citation2011). Long-term weight gain is thought to result from the reduced metabolism energy requirement in altered pets (Fascetti & Delaney, Citation2013). The findings in our study population are not surprising as any impact on metabolism due to neutering would not be expected to be seen in the immediate postoperative period, and the kittens recovered quickly following surgery.
Mortality and cause of death
Of the 203 kittens enrolled in the study, only 5 kittens (2.5%) died or were euthanized during the study period. Three of five had experienced weight loss, and the fourth had minimal gain. Notably, the fifth kitten had an overall weight gain much higher than average (24 g/day), but arrested postoperatively after an eye enucleation surgical procedure; although he had a history of URI that had been treated, at the time of his death, he was evaluated as stable for surgery. All five of the kittens had abnormalities noted on examination at the shelter, and four of the five were started on treatment for these conditions (). Pneumonia was the cause of death or euthanasia for four of the five kittens that died, including the postsurgical arrest: three were confirmed on necropsy, and one was diagnosed based on clinical signs.
A mild URI can act as a portal of entry to result in pneumonia and/or sepsis. Pneumonia is more subtle than diarrhea in terms of recognition. Cats may not even demonstrate a fever in the face of pneumonia, and sepsis can progress rapidly in the kitten. Kittens who fail to get colostrum in the first 24 hours of life experience failure of passive transfer of protective maternal antibodies; this renders them subject to life-threatening infectious diseases, particularly as they begin to transition to solid foods and become more active in their environment (Bucheler, Citation1999; Greco, Citation2014). Even in kittens with adequate intake of colostrum, maternal antibodies wane at the same time as weaning begins (Bucheler, Citation1999). Kittens entering a shelter have unknown status in terms of colostrum intake, and maternal health and immunity varies widely. Due to a fragile immune status, nutritional transitions, an evolving gastrointestinal microbiome, and the physiologic challenges of entering a shelter environment, kittens are particularly subject to infectious agents in the window of time they are most likely to enter the shelter or foster care.
At 6.5%, the mortality rate for all kittens entering the shelter at <9 weeks of age in 2014 was at the low end of the previously published mortality rates reported for kittens born alive in laboratories and breeding facilities (Festing & Belby, Citation1970; Fournier et al., Citation2017; Romagnoli et al., Citation2019; Root Kustritz et al., Citation1995; Rutty & Smith, Citation1967; Sparkes et al., Citation2006; Ström Holst & Frössling, Citation2009; Young, Citation1973) and significantly higher than the mortality rate of the study kittens. These findings are likely due to increased observations and early interventions as required by the study and this shelter’s foster program, providing opportunities to improve kitten outcomes. Additionally, kittens not included in the study included those too ill to be supported in foster care or in the shelter. These kittens would not have had 3 days of weight data recorded, and those entering the shelter at greater than 60 days of age were not included.
This study has several limitations. The study was performed in a single organization, and kittens were cared for in multiple environments. Foster care volunteers and shelter staff were provided an orientation reviewing elements of neonatal and kitten care, as well as disease recognition. Reporting was standardized as much as possible: written protocols, a fecal scoring chart, a standard digital kitchen scale, and data collection forms were used. Nonetheless, subjectivity in data collection undoubtedly occurred. Foster homes varied in terms of length of care, time of weighing, quality of data, and consistency of reporting. A minimum of three daily weight measurements for every kitten in an age group (<35 days, ≥35 days) was required to increase the precision of weight gain estimates, but some forms had missing data for particular days; this most likely impacted disease reporting and may have affected estimates of daily weight gain. Consistent with the limitations of applied clinical research, kitten care pathways were not altered for study purposes and time of follow-up varied based on the point of entry and time to reach adoption status.
Amounts of food consumed daily were not recorded in this study. For nursing kittens without a queen, kittens were fed milk replacer according to guidelines based on the body weight (Maddie’s Fund, Citationn.d.). Those weaning to solid food were offered gruel of pate mixed with milk replacer, pate, and free-feed dry food progressively, and based on what they consumed. The type of food eaten each day was recorded, but kcal intake was neither calculated nor tracked. While standard life-stage diets were used, several types of food might be mixed to encourage a kitten to eat. Overfeeding likely impacted both high growth rates and incidence of diarrhea in study kittens. More reliable metering of food (commonly practiced in bottle-feeders on milk replacer since it is easily tracked) may result in lower occurrence of diarrhea in shelter and foster housed kittens.
Finally, this shelter’s kitten program may not be representative of kitten programs in other organizations. This shelter has relatively intensive veterinary oversight due to its collaboration with an academic shelter medicine training program and a history of participating in clinically applied research. Additionally, the community provides a robust and motivated volunteer force that has been fostering kittens for decades. The significantly higher rate of weight gain and low mortality in this study may in part be attributed to elements of this organization not found in other shelters.
Additional studies investigating mortality of sheltered and fostered kittens are needed. Pneumonia was the most prevalent cause of death in study kittens. Clinical signs of pneumonia are often subtle in cats and kittens and could be easily missed, especially in litters containing many kittens. Diarrhea and URI, more easily tracked, were surprisingly not significantly associated with below average daily weight gain. Additional studies of kitten mortality in shelters including postmortem necropsy with histopathology would better elucidate the causes of death in sheltered and fostered kittens. Additional studies are also needed to investigate the role overfeeding may play in the high incidence of diarrhea in sheltered and foster-housed kittens.
Conclusion
Compared to those in research and breeding facilities, study kittens in shelter and foster care demonstrated average daily weight gain preweaning and higher than average daily weight gain postweaning. This study suggests that lethargy, while often a subtle sign, is a significant risk factor for a decreased rate of weight gain in kittens both pre- and postweaning. Study kittens had lower mortality rates than those reported in the literature for kittens in laboratory and breeding facilities; this mortality rate was also significantly lower than that of the overall population of kittens entering the shelter the year the study was conducted. Training and mentoring staff and foster care providers to use monitoring and data collection systems and provide early medical interventions, especially for subtle signs, such as lethargy and weight plateau or loss, are likely to result in better outcomes and welfare for kittens.
Acknowledgments
The authors thank Maddie’s Fund for their generous donation of 20 digital kitchen scales that were essential in this study as well as their ongoing support of the Maddie’s Shelter Medicine Program at Cornell. They also thank Anne Marie McPartlin, Suzanne Nelson, Karen Nieves, and the rest of the staff of the SPCA of Tompkins County, the foster care providers who participated in this study, and Cornell University College of Veterinary Medicine.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author, subject to consent from the organization in which the study was conducted.
Additional information
Funding
Notes
1. Commercial materials are identified in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement.
References
- Statistix manual 9. (2008). Tallahassee, FL: Analytical software.
- Alexander, L. G., Salt, C., Thomas, G., & Butterwick, R. (2011). Effects of neutering on food intake, body weight and body composition in growing female kittens. British Journal of Nutrition, 106(S1), S19–S23.doi:10.1017/S0007114511001851
- Bucheler, J. (1999). Fading kitten syndrome and neonatal isoerythrolysis. The Veterinary Clinics of North America. Small Animal Practice, 29(4), 853–870.doi:10.1016/S0195-5616(99)50077-6
- Case, L., Danstotle, L., Hayek, M. G., & Raasch, M. F. (2011). Nutritional care of neonatal puppies and kittens. In Canine and feline nutrition (pp. 209–219). Maryland Heights, MO: Mosby.
- Cave, T. A., Thompson, H., Reid, S. W., Hodgson, D. R., & Addie, D. D. (2002). Kitten mortality in the United Kingdom: A retrospective analysis of 274 histopathological examinations (1986 to 2000). Veterinary Record, 151(17), 497–501.doi:10.1136/vr.151.17.497
- Deag, J. M., Lawrence, C. E., & Manning, A. (1987). The consequences of differences in litter size for the nursing cat and her kittens. Journal of Zoology, 213(1), 153–179.doi:10.1111/j.1469-7998.1987.tb03687.x
- DiGangi, B. A., Graves, J., Budke, C. M., Levy, J. K., Tucker, S., & Isaza, N. (2020). Assessment of body weight for age determination in kittens. Journal of Feline Medicine and Surgery, 22(4), 322–328.doi:10.1177/1098612X19844846
- Dolan, E. D., Doyle, E., Tran, H. R., & Slater, M. R. (2020). Pre-mortem risk factors for mortality in kittens less than 8 weeks old at a dedicated kitten nursery. Journal of Feline Medicine and Surgery, 1098612X2097496. doi:10.1177/1098612X20974960
- Fascetti, A. J., & Delaney, S. J. (2013). Feeding the healthy dog and cat. Applied Veterinary Clinical Nutrition, NRC, 2006, 75–94.doi:10.1002/9781118785669.ch7
- Festing, M. F. W., & Belby, J. (1970). Breeding performance and growth of SPF cats. Journal of Small Animal Practice, 11, 533–543.doi:10.1111/j.1748-5827.1970.tb05610.x
- Fournier, A., Masson, M., Corbière, F., Mila, H., Mariani, C., Grellet, A., & Chastant-Maillard, S. (2017). Epidemiological analysis of reproductive performances and kitten mortality rates in 5,303 purebred queens of 45 different breeds and 28,065 kittens in France. Reproduction in Domestic Animals, 52, 153–157.doi:10.1111/rda.12844
- Greco, D. S. (2014). Pediatric nutrition. Veterinary Clinics of North America: Small Animal Practice, 44(2), 265–273.doi:10.1016/j.cvsm.2013.11.001
- Hendriks, W. H., & Wamberg, S. (2000). Milk intake of suckling kittens remains relatively constant from one to four weeks of age. Journal of Nutrition, 130(1), 77–82.doi:10.1093/jn/130.1.77
- Lawler, D., & Evans, R. (1995). Nutritional and environmental considerations in neonatal medicine. In Bonagura, J. D. (Ed.), Kirks current vet therapy XII (pp. 37–40). Philadelphia, PA: W.B. Saunders Company.
- Lawler, D. (1984). Morbidity and mortality in neonatal kittens. American Journal of Veterinary Research, 45(7), 1455–1459.
- Lawler, D. (2008). Neonatal and pediatric care of the puppy and kitten. Theriogenology, 70(3), 384–392.
- Maddie’s Fund. (n.d.). Kitten bottle feeding and stomach capacity chart. Retrieved from https://www.maddiesfund.org/assets/documents/Institute/Kitten%20Bottle%20Feeding%20and%20Stomach%20Capacity%20Chart.pdf
- Marks, S. L., & Willard, M. D. (2005). Diarrhea in kittens. In Consultations in feline medicine (pp. 133–143). Elsevier. doi:10.1016/B0-72-160423-4/50018-4
- Moik, K., & Kienzle, E. (2011). Birth weight and postnatal growth of pure-bred kittens. The British Journal of Nutrition, 106(S1), S32–4.
- Murray, J. K., Skillings, E., & Gruffydd-Jones, T. J. (2008). A study of risk factors for cat mortality in adoption centres of a UK cat charity. Journal of Feline Medicine and Surgery, 10(4), 338–345.
- Pedersen, N. C. (1991). Common infectious diseases of multiple cat environments Pedersen, N. C ed . In Feline husbandry: Diseases and management in the multiple cat environment (pp. 163–288). Goleta, CA: American Veterinary Publications.
- Peterson, M. E. (2011a). Care of the orphaned puppy and kitten. In M. E. Peterson, and M. A. Kutzler (Eds.), Small animal pediatrics (pp. 67–72). St. Louis, MO: Saunders.
- Peterson, M. E. (2011b). Growth. In M. E. Peterson, and M. A. Kutzler (Eds.), Small animal pediatrics (pp. 34–43). St. Louis, MO: Saunders. doi:10.1016/B978-1-4160-4889-3.00005-X
- Prendergast, H. (2011). Nutritional requirements and feeding of growing puppies and kittens. In M. Peterson, and M. A. Kutzler (Eds.), Small animal pediatrics (pp. 58–67). St. Louis, MO: Saunders.
- Purina Veterinary Diets. (n.d.). Purina fecal scoring chart. Retrieved from https://www.proplanveterinarydiets.ca/sites/g/files/auxxlc696/files/2021-02/180107_PPPVD-Fecal-Scoring-Chart-UPDATE-EN-FINAL.pdf
- Romagnoli, S., Bensala, C., Ferre-Dolcet, L., Sontas, H. B., & Stelletta, C. (2019). Fertility parameters and reproductive management of Norwegian Forest Cats, Maine Coon, Persian and Bengal cats raised in Italy: A questionnaire-based study. Journal of Feline Medicine and Surgery, 21(12), 1188–1197.
- Root Kustritz, M. V., Johnston, S. D., & Olson, P. N. (1995). Estrous length, pregnancy rate, gestation and parturition lengths, litter size, and juvenile mortality in the domestic cat. Journal of the American Animal Hospital Association, 31(5), 429–433.
- Root Kustritz, M. V. (2011). History and physical examination of the weanling and adolescent. In M. E. Peterson & M. A. Kutzler (Eds.), Small animal pediatrics (pp. 28–33). Elsevier. doi:10.1016/B978-1-4160-4889-3.00004-8
- Rutty, D. A., & Smith, G. K. (1967). The control of disease in a cat breeding colony. Laboratory Animals, 1(2), 111–115.
- Shelter Animals Count, The national database. (2021). Retrieved from https://www.shelteranimalscount.org/data-dashboards
- Sparkes, A. H., Rogers, K., Henley, W. E., Gunn-Moore, D. A., May, J. M., Gruffydd-Jones, T. J., & Bessant, C. (2006). A questionnaire-based study of gestation, parturition and neonatal mortality in pedigree breeding cats in the UK. Journal of Feline Medicine and Surgery, 8(3), 145–157.
- Ström Holst, B., & Frössling, J. (2009). The Swedish breeding cat: Population description, infectious diseases and reproductive performance evaluated by a questionnaire. Journal of Feline Medicine and Surgery, 11(10), 793–802.
- Strong, S. J., Gookin, J. L., Correa, M. T., & Banks, R. E. (2020). Interventions and observations associated with survival of orphaned shelter kittens undergoing treatment for diarrhea. Journal of Feline Medicine and Surgery, 22(4), 292–298.
- Tanaka, A., Wagner, D. C., Kass, P. H., & Hurley, K. F. (2012). Associations among weight loss, stress, and upper respiratory tract infection in shelter cats. Journal of the American Veterinary Medical Association, 240(5), 570–576.
- Watson, V. E., Jacob, M. E., Flowers, J. R., Strong, S. J., Debroy, C., & Gookin, J. L. (2017). Association of atypical enteropathogenic Escherichia coli with diarrhea and related mortality in kittens. Journal of Clinical Microbiology, 55(9), 2719–2735.
- Willoughby, K. (2008). Paediatrics and inherited diseases. In Feline medicine and therapeutics (3rd ed.). doi:10.1002/9780470690727.ch14
- Young, C. (1973). Preweaning mortality in specific pathogen free kittens. Journal of Small Animal Practice, 14(7), 391–398.