ABSTRACT
Pasture access can benefit dairy cows’ behavior, health, and welfare, but herds are increasingly housed indoors full-time. Recent infrared thermal-imaging (thermography) studies suggest that higher eye temperatures may be a physiological indicator of chronic stress. We, therefore, hypothesized that, compared to cows with pasture access, cows housed indoors full-time would have higher eye temperatures. In a two-phase crossover experiment, 29 Holstein-Friesian dairy cows experienced 18 days of overnight pasture access and 18 days of full-time indoor housing. We measured each animal’s eye temperature 16 times (eight/phase). During Phase One, cows with pasture access had higher eye temperatures than cows housed indoors full-time (contrary to our hypothesis). However, during Phase Two, cows with pasture access had lower eye temperatures than cows housed indoors full-time. It is, therefore, unclear whether eye temperature reflected disparities in dairy cow welfare between different housing treatments.
Introduction
Dairy cattle are increasingly housed indoors throughout the year (Knaus, Citation2016; USDA, Citation2016; van den Pol-van Dasselaar, Hennessy, & Isselstein, Citation2020). Full-time indoor housing means that grazing area accessibility no longer constrains herd size, enabling farmers to keep more animals (Arnott, Ferris, & O’connell, Citation2017). Moreover, cattle housed indoors are typically fed energy-dense diets, which meet their high metabolic requirements better than grass (Olmos et al., Citation2009b). Indoor housing also shelters cows against adverse weather conditions (Schütz, Rogers, Poulouin, Cox, & Tucker, Citation2010; Tucker et al., Citation2007) so, in temperate regions, even cows with summer pasture access are usually kept indoors during winter. Most dairy cows, therefore, spend long periods indoors.
Animal welfare scientists (Arnott et al., Citation2017; Charlton & Rutter, Citation2017; Mee & Boyle, Citation2020; Smid, Weary, & von Keyserlingk, Citation2020) and dairy consumers (Ellis, Billington, McNeil, & McKeegan, Citation2009; Hötzel, Cardoso, Roslindo, & von Keyserlingk, Citation2017; Schuppli, Von Keyserlingk, & Weary, Citation2014) are concerned about full-time indoor housing. Given the choice between pasture and free stall housing, cows usually spend longer at pasture, at least at night (Charlton, Rutter, East, & Sinclair, Citation2011; Kismul, Spörndly, Höglind, Næss, & Eriksson, Citation2018; Legrand, Von Keyserlingk, & Weary, Citation2009). Cattle also work as hard to access pasture as they work to access high-energy feed (total mixed ration; Von Keyserlingk, Cestari, Franks, Fregonesi, & Weary, Citation2017). These findings show that cows prefer – and are motivated to access – pasture. Compared to full-time indoor housing, pasture provides a softer lying and walking substrate, resulting in less disrupted lying behavior (associated with good welfare; Crump et al., Citation2019a, O’Connell, Giller, & Meaney, Citation1989; Olmos et al., Citation2009a). Pasture is also linked to lower risks of lameness (Barker, Leach, Whay, Bell, & Main, Citation2010; Haskell, Rennie, Bowell, Bell, & Lawrence, Citation2006; Olmos et al., Citation2009a) and mastitis (Firth et al., Citation2019; Goldberg et al., Citation1992; Washburn, White, Green, & Benson, Citation2002).
Few studies have explored the physiological effects of pasture access (Arnott et al., Citation2017; but see e.g., Comin et al., Citation2011; Grille, Adrien, Olmos, Chilibroste, & Damián, Citation2019). Sharma, Umapathy, Kumar, and Phillips (Citation2019) recorded an inverse correlation between daily access to outdoor yards and hair cortisol levels (a general stress marker), suggesting pasture access may reduce stress. Olmos et al. (Citation2009b), on the other hand, reported that plasma cortisol concentration did not differ between pasture-based and free stall housing systems. However, blood metabolite levels linked pasture access to more negative energy balance – a sign of nutritional stress and poor welfare. Other studies have found positive correlations between inclement weather and cows’ cortisol levels (Tucker et al., Citation2007; Webster, Stewart, Rogers, & Verkerk, Citation2008). These findings suggest that, when nutritional needs are unmet or weather conditions are poor, pasture access can be stressful.
Infrared thermography has been proposed as a noninvasive physiological indicator of animal welfare (Clay-Warner & Robinson, Citation2015; Godyń, Herbut, & Angrecka, Citation2019; McManus et al., Citation2016; for criticism of this approach, see Ede, Lecorps, von Keyserlingk, & Weary, Citation2019). As part of the acute stress response, blood-flow increases to core organs, such as the heart and lungs, preparing the individual for activity (“stress-induced hyperthermia”; Zethof, Van Der Heyden, Tolboom, & Olivier, Citation1994). Some authors suggest that infrared thermal images can record corresponding vasoconstriction in peripheral regions, such as the eyes and ears, with lower surface temperatures indicating higher stress (Travain & Valsecchi, Citation2021). For example, dairy cattle’s eye temperature can drop immediately after exposure to acute stressors (e.g., startle and restraint; Stewart et al., Citation2008a) and painful procedures (e.g., hot-iron disbudding without anaesthetic; Stewart, Stafford, Dowling, Schaefer, & Webster, Citation2008b), which the authors attributed to vasoconstriction of peripheral blood vessels. Infrared thermography has also recorded peripheral temperature drops following aversive stimulus exposure in macaques (Nakayama, Goto, Kuraoka, & Nakamura, Citation2005) and rats (Vianna & Carrive, Citation2005), although other dairy cow studies have linked acute stress to higher peripheral temperatures (e.g., cathaterization: Stewart et al., Citation2007; claw-trimming: Gómez et al., Citation2018).
Over longer time scales, some experimental evidence suggests that chronic stress increases eye temperature. For example, Franchi, Jensen, Herskin, McNeill, and Phillips (Citation2021) found that dairy cow eye temperature was higher 2 days after abrupt dry-off and regrouping (a stressful event; Zobel, Weary, Leslie, & Von Keyserlingk, Citation2015), compared to the day before dry-off. Uddin, Phillips, Goma, and McNeill (Citation2019) also found that right lateralized dairy cows had higher eye temperatures than left lateralized cows. Because the right hemisphere primarily controls the fight-or-flight response, passing on the right enables cows to easily view potential threats with their contralateral left eye (Robins & Phillips, Citation2010). Right lateralization, therefore, indicates an anxious temperament in dairy cows (Goma et al., Citation2018; Phillips, Oevermans, Syrett, Jespersen, & Pearce, Citation2015), so Uddin et al. linked higher eye temperatures to anxiety. Several studies have reported similar findings (Goma et al., Citation2018; Lee et al., Citation2018; Phillips et al., Citation2015), although Uddin, Phillips, Auboeuf, and McNeill (Citation2021) failed to replicate the association between eye temperature and right lateralization. Moreover, stress-induced spikes in eye temperature were higher in fearful than non-fearful calves (Lecorps, Kappel, Weary, & von Keyserlingk, Citation2018) and horses (Dai et al., Citation2015).
In past papers, we compared dairy cow welfare at pasture and indoors using behavioral and cognitive indicators (Crump et al., Citation2019a, Citation2019b, Citation2021). Cows at pasture had longer overnight lying durations than those indoors, as well as longer lying bouts and more synchronous herd lying behavior (Crump et al., Citation2019a). These findings suggest that cows at pasture were more comfortable, less restless, and encountered less aggression (but see Tucker, Jensen, de Passillé, Hänninen, & Rushen, Citation2021). Cows also had higher daily step counts at pasture. In humans, exercise improves psychological wellbeing (Bailey, Hetrick, Rosenbaum, Purcell, & Parker, Citation2018; Ernst, Olson, Pinel, Lam, & Christie, Citation2006). As well as measuring behavior, we individually trained the cows on a spatial judgment bias task – a measure of emotional state (Burman, Parker, Paul, & Mendl, Citation2008; Harding, Paul, & Mendl, Citation2004; Neave, Daros, Costa, von Keyserlingk, & Weary, Citation2013). Buckets on one side of an alley were rewarded with 130 g of grain-based concentrate feed, whereas buckets on the other side of an alley were unreinforced. Housing treatment did not affect animals’ responses on test trials to ambiguous buckets, intermediate between the rewarded and unreinforced locations (Crump et al., Citation2019b, Citation2021). However, PAS cows were slower to the rewarded bucket location than PEN cows. Reduced reward anticipation in the PAS treatment suggests that cattle with pasture access had more rewarding lives (see Spruijt, Van den Bos, & Pijlman, Citation2001; Watters & Krebs, Citation2019).
In this study, we took infrared thermal images of each cows’ right eye after the aforementioned judgment bias testing (see Crump et al., Citation2019b, Citation2021). We hypothesized that dairy cows would have higher eye temperatures when they were housed indoors full-time compared to when they had overnight pasture access. This effect would indicate chronic stress during full-time indoor housing.
Materials and methods
All experimental procedures were approved by the Animal Research Ethics Committee, School of Biological Sciences, Queen’s University Belfast (approval number: QUB-BS-AREC-18-005).
Animals and housing
This experiment was carried out at the Agri-food and Biosciences Institute (AFBI), Hillsborough, County Down, Northern Ireland (54°5’ north; 6°1’ west). We tested 29 lactating Holstein-Friesian dairy cows, who were 2.7–8.7 years old (mean: 4.3 years) and 209–273 days since calving (mean: 241 days) at recruitment (May 2, 2018). Three cows not tested in the experiment were used to increase the total group size to 32. All animals had experience of pasture, although they were housed indoors for eight weeks before testing. Indoor housing comprised two interconnected pens 13.3 × 8.5 m. Each pen had 16 free stalls fitted with rubber mats, and concrete walking areas that were scraped clean six times/day. Grass silage, provided at 0900 h, was available through an open feed barrier ad libitum. Water was also offered ad libitum. At 0630 h and 1500 h, animals were milked in a rotary parlor. The indoor housing was naturally ventilated, with no additional ventilation.
Experimental procedure and treatments
Before the experiment, we installed plywood barriers to separate the pens, and divided the herd into two groups of 16. A treatment-blind veterinary graduate pseudorandomly assigned animals to each group, based on mobility score (following the AHDB, Citation2019 scoring system). We then used a repeated-measures crossover design, with both phases lasting 18 days (Phase One: June 25-July 13, 2018; Phase Two: July 16-August 3, 2018). The two treatments were overnight pasture access (PAS) and full-time indoor housing (PEN). Animals in the PAS treatment were managed on a rotational grazing system for 18 h from 1600 h to 1000 h (area grazed: 1,370–3,950 m2; distance to parlor: 190–295 m), and then kept in their indoor pen during the day from 1000 h to1600 h. Herbage was higher quality in Phase One than Phase Two, measured three times per phase as mean oven dry matter (DM) content (mean ± SD; Phase One: 239 ± 8.6 g/kg; Phase Two: 215 ± 8.6 g/kg), mean crude protein content (Phase One: 226 ± 11.5 g/kg DM; Phase Two: 207 ± 11.5 g/kg DM), and mean metabolizable energy content (Phase One: 12.0 MJ/kg DM; Phase Two: 10.9 MJ/kg). Animals in the PEN treatment were kept in their indoor pen 24 h/day, except during milking.
Eye temperature was recorded immediately after judgment bias sessions (between 1000–1600 h), with subject order (and thus time) newly randomized each day. We took one good-quality infrared thermal image of each cows’ right eye (eight images/cow/treatment; 16 images/cow total), because the right eye gives highly repeatable thermal images (Scoley, Gordon, & Morrison, Citation2019). We used a FLIR E8 Infrared Camera (FLIR Systems, Kent, UK) at a 90° angle and 1.5 m distance. According to the manufacturer, this model has a temperature measurement accuracy of ±2%, thermal sensitivity of < 0.06°C, spectral range of 7.5–13 μm, and resolution of 320 × 240 pixels. We used an emissivity setting of 0.95 (as in Church et al., Citation2014; Gloster, Ebert, Gubbins, Bashiruddin, & Paton, Citation2011). Images were captured during cows’ daytime indoor housing to reduce the effects of sunlight, rain, and wind (Church et al., Citation2014). The same experimenter took every image. This process always took < 2 min after the last judgment bias trial. We did not restrain cows for thermography so, throughout the study, we returned animals to their home pen without capturing an image in 20 trials (when the animal was agitated). Due to equipment malfunction, we also did not collect thermographic data on the seventh testing day of Phase Two (July 302,018).
After downloading each thermal image onto a computer, we extracted data using FLIR Tools software (FLIR Systems, Kent, UK). We recorded temperature in the lacrimal caruncle region of the eye, due to the consistency of this region’s temperature (Stewart et al., Citation2008b). Data were extracted treatment blind. To explore the effects of weather conditions, we used recordings from a UK Met Office weather station 17 km from AFBI Hillsborough (Katesbridge). We also entered contemporary mean temperature and humidity into the FLIR Tools software during image processing, and set object distance to 1.5 m.
Statistical analysis
Data were analyzed using a general linear mixed-effects model in the statistics program R (R Core Team, Cran-r-project, Vienna, Austria; version 3.6.2; package “lme4”; Bates, Mächler, Bolker, & Walker, Citation2014). To improve model fit, we log-transformed the response variable: eye temperature. The fixed effects were treatment, treatment order, latency to rewarded bucket locations during the preceding judgment bias testing session (accounting for general activity; see Nakayama et al., Citation2005), as well as three weather variables (daily mean environmental temperature, daily mean windspeed, and h/day with humidity > 90%). Cow ID and day number were included as random effects. We included the treatment × treatment order interaction and all interactions contained within treatment × bucket latency × temperature × humidity × windspeed. We dropped interactions if this lowered the Akaike Information Criterion value by > 5 (Burnham & Anderson, Citation2002). Throughout this manuscript, we regard P-values < 0.05 as statistically significant.
Results
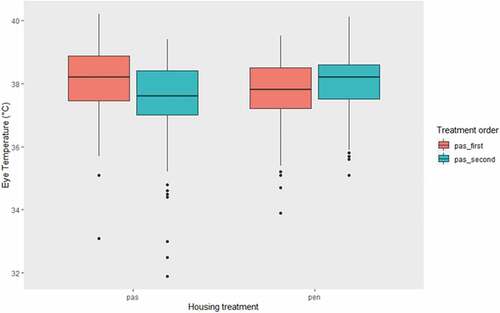
In the 415 thermal images captured, eye temperature ranged from 31.9 to 40.2°C (mean: 37.8°C). There was a significant treatment × treatment order interaction effect on eye temperature (χ21 = 7.70, P = 0.005; ). In the group that underwent the PAS treatment during Phase One, eye temperature was higher in the PAS treatment than the PEN treatment. In the group that underwent the PAS treatment during Phase Two, eye temperature was lower in the PAS treatment than the PEN treatment. We found no significant relationship between eye temperature and latency to the rewarded bucket location (χ22 = 3.20, P = 0.20), and the treatment × bucket latency interaction was not significant (χ21 = 0.00, P = 0.96).
Figure 1. Effect of housing treatment (PAS/PEN) and treatment order (PAS-first/PAS second) on eye temperature during our two-phase crossover experiment. There was no significant association between either treatment or treatment order and eye temperature, but the treatment × treatment order interaction was significant. PAS: 18-day treatment where cows had overnight pasture access; PEN: 18-day treatment where cows were housed indoors full-time; PAS-first: subjects that underwent the PAS treatment during the first experimental phase; PAS-second: subjects that underwent the PAS treatment during the second experimental phase.
Throughout our study, daily environmental temperature ranged between 11.2 and 20.7°C (mean: 15.7°C), daily windspeed was 2–8 kn (mean: 4.4 kn), and h/day with humidity > 90% was 0–15 h (mean: 6.9 h). We found two significant three-way interactions involving weather variables. First, a treatment × temperature × windspeed interaction (χ21 = 5.66, P = 0.02). At high windspeeds, there was a negative correlation between environmental temperature and eye temperature in the PAS treatment, but a positive correlation in the PEN treatment. This effect was smaller at lower windspeeds. Second, a treatment × windspeed × humidity interaction (χ21 = 6.97, P = 0.01): at high windspeeds, there was a positive association between humidity and eye temperature in the PAS treatment, but a negative association in the PEN treatment. At low windspeeds, there was a negative association between humidity and eye temperature in both treatments, but the effect was greater for the PAS treatment. No other treatment × weather variable interactions were significant.
Discussion
Our findings indicate that housing treatment influenced dairy cows’ eye temperature – a result that carried over into daytime housing and was independent of current weather conditions. However, treatment order changed the effect’s direction. Cows that went outside in Phase One (PAS-first) had higher eye temperatures following overnight pasture access than during full-time indoor housing, whereas cows that went outside in Phase Two (PAS-second) had lower eye temperatures following pasture access than during indoor housing.
These results partially support our hypothesis. If higher eye temperatures indicate chronic stress (Uddin et al., Citation2019), then our findings suggest that pasture access increased stress in PAS-first cows. On the other hand, pasture access reduced eye temperature in PAS-second cows, suggesting lower stress at pasture. This discrepancy may reflect the greater maximum ambient temperature during Phase One (30.0°C) than Phase Two (25.8°C). Higher ambient temperatures heat surfaces (including cows’ eyes) – a major confound of infrared thermography (Church et al., Citation2014). Phase One ambient temperatures also exceeded dairy cows’ thermal tolerance, likely leading to heat stress (Polsky & von Keyserlingk, Citation2017). The shaded PEN treatment may have alleviated this effect more than the unshaded PAS treatment, so eye temperature could have accurately reflected lower stress indoors during Phase One. Phase One’s higher-quality herbage is another possible explanation, perhaps reducing nutritional stress and, thus, increasing metabolic heat production in PAS-first cows (Savory, Kostal, & Nevison, Citation2006). Nonetheless, selective grazing may have negated the small between-phase difference in herbage quality. Future infrared thermography studies could record forage intake and milk yield as possible indicators of nutritional stress. We also did not measure animals’ core temperatures directly, so can only speculate about the relationship between eye temperature, core temperature, and chronic stress.
Our inconsistent results reflect previous conflicting findings on the relationship between stress and peripheral temperatures in dairy cattle. While some studies have linked acute stress to lower peripheral temperatures (Stewart et al., Citation2008a, b), others linked acute stress to higher peripheral temperatures (Gómez et al., Citation2018; Stewart et al., Citation2007). For chronic stress, Uddin et al. (Citation2019) associated right lateralization (an indicator of chronic) with higher eye temperatures, but Uddin et al. (Citation2021) could not replicate this result. A further source of confusion is that positive experiences also affect dairy cows’ peripheral temperature. Gómez et al. (Citation2018) found that both positive (feeding) and negative (restraint for claw-trimming) interventions increased eye temperature, whereas Proctor and Carder (Citation2016) reported the opposite: both positive (unexpectedly high-value rewards) and negative interventions (unexpectedly inedible feed) decreasing nose temperature. Such findings suggest that peripheral temperature changes are a non-directional indicator of physiological arousal (Ede et al., Citation2019), casting doubt on infrared thermography as a welfare indicator for dairy cows.
Finally, this study had various limitations. To facilitate measuring other welfare indicators (Crump et al., Citation2019a, Citation2019b, Citation2021), we sometimes collected infrared thermography data hours after cows returned from pasture. Indoor housing conditions could, therefore, have influenced results (Gómez et al., Citation2018). We do not have data on conditions in the indoor housing, so unfortunately cannot explore this potential source of variation. However, cows in both treatments were tested indoors in a random order, ensuring that any such variability affected both treatments equally and did not introduce systematic bias. In addition, we only captured one thermal image of each cow per testing day. Some studies have found that extracting average eye temperatures from multiple replicate images can improve reliability (e.g., Scoley et al., Citation2019), although Uddin, McNeill, Lisle, and Phillips (Citation2020) reported little improvement above two images.
In summary, this study only partially supported the hypothesis that pasture access would reduce eye temperature – suggesting lower stress – in dairy cows. Our previous work on behavior and cognition indicated that welfare was better when cattle had pasture access than during full-time indoor housing. Nonetheless, different eye temperatures were not consistently associated with different housing treatments. It is, therefore, unclear whether eye temperatures indicated dairy cow welfare.
Data availability
The datasets generated and analysed in this study are available from the corresponding author on reasonable request.
Acknowledgments
A.C. was funded by a postgraduate studentship from Northern Ireland’s Department for the Economy. We thank Mike Davies, Helen Kabboush, Deborah McConnell, Gillian Scoley, and Jennifer Weller. AFBI Hillsborough hosted this study, and the Met Office Library and Archive provided meteorological data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- AHDB (2019). Agriculture and horticulture development board. Available online: https://dairy.ahdb.org.uk/resourceslibrary/technical-information/health-welfare/mobility-score-instructions/#.W2w3w9JkjIU (accessed: 15th July 2021).
- Arnott, G., Ferris, C. P., & O’connell, N. E. (2017). Welfare of dairy cows in continuously housed and pasture-based production systems. Animal, 11(2), 261–273.
- Bailey, A. P., Hetrick, S. E., Rosenbaum, S., Purcell, R., & Parker, A. G. (2018). Treating depression with physical activity in adolescents and young adults: A systematic review and meta-analysis of randomised controlled trials. Psychological Medicine, 48(7), 1068–1083.
- Barker, Z. E., Leach, K. A., Whay, H. R., Bell, N. J., & Main, D. C. J. (2010). Assessment of lameness prevalence and associated risk factors in dairy herds in England and Wales. Journal of Dairy Science, 93(3), 932–941.
- Bates, D., Mächler, M., Bolker, B., & Walker, S. (2014). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48.
- Burman, O. H., Parker, R., Paul, E. S., & Mendl, M. (2008). A spatial judgement task to determine background emotional state in laboratory rats, Rattus norvegicus. Animal Behaviour, 76(3), 801–809.
- Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information-theoretic approach (2nd edn.). New York: Springer.
- Charlton, G. L., & Rutter, S. M. (2017). The behaviour of housed dairy cattle with and without pasture access: A review. Applied Animal Behaviour Science, 192, 2–9.
- Charlton, G. L., Rutter, S. M., East, M., & Sinclair, L. A. (2011). Effects of providing total mixed rations indoors and on pasture on the behavior of lactating dairy cattle and their preference to be indoors or on pasture. Journal of Dairy Science, 94(8), 3875–3884.
- Church, J. S., Hegadoren, P. R., Paetkau, M. J., Miller, C. C., Regev-Shoshani, G., Schaefer, A. L., & Schwartzkopf-Genswein, K. S. (2014). Influence of environmental factors on infrared eye temperature measurements in cattle. Research in Veterinary Science, 96(1), 220–226.
- Clay-Warner, J., & Robinson, D. T. (2015). Infrared thermography as a measure of emotion response. Emotion Review, 7(2), 157–162.
- Comin, A., Prandi, A., Peric, T., Corazzin, M., Dovier, S., & Bovolenta, S. (2011). Hair cortisol levels in dairy cows from winter housing to summer highland grazing. Livestock Science, 138(1–3), 69–73.
- Crump, A., Jenkins, K., Bethell, E. J., Ferris, C. P., & Arnott, G. (2019a). Pasture access affects behavioral indicators of wellbeing in dairy cows. Animals, 9(11), 902.
- Crump, A., Jenkins, K., Bethell, E. J., Ferris, C. P., Kabboush, H., O’Connell, N. E., & Weller, J. (2019b). Is the grass half-full? Investigating optimism as a welfare indicator for dairy cows with and without pasture access. Pharmacological Reports, 71(6), 1308.
- Crump, A., Jenkins, K., Bethell, E. J., Ferris, C. P., Kabboush, H., Weller, J., & Arnott, G. (2021). Optimism and pasture access in dairy cows. Scientific Reports, 11(1), 1–11.
- Dai, F., Cogi, N. H., Heinzl, E. U. L., Dalla Costa, E., Canali, E., & Minero, M. (2015). Validation of a fear test in sport horses using infrared thermography. Journal of Veterinary Behavior, 10(2), 128–136.
- Ede, T., Lecorps, B., von Keyserlingk, M. A., & Weary, D. M. (2019). Symposium review: Scientific assessment of affective states in dairy cattle. Journal of Dairy Science, 102(11), 10677–10694.
- Ellis, K. A., Billington, K., McNeil, B., & McKeegan, D. E. F. (2009). Public opinion on UK milk marketing and dairy cow welfare. Animal Welfare, 18(3), 267–282.
- Ernst, C., Olson, A. K., Pinel, J. P., Lam, R. W., & Christie, B. R. (2006). Antidepressant effects of exercise: Evidence for an adult-neurogenesis hypothesis? Journal of Psychiatry & Neuroscience, 31, 84–92.
- Firth, C. L., Laubichler, C., Schleicher, C., Fuchs, K., Käsbohrer, A., Egger-Danner, C., & Obritzhauser, W. (2019). Relationship between the probability of veterinary-diagnosed bovine mastitis occurring and farm management risk factors on small dairy farms in Austria. Journal of Dairy Science, 102(5), 4452–4463.
- Franchi, G. A., Jensen, M. B., Herskin, M. S., McNeill, D. M., & Phillips, C. J. (2021). Assessing response to dry-off in dairy cows kept outdoors using spontaneous behaviours and infrared thermography—a pilot study. Tropical Animal Health and Production, 53(1), 1–4.
- Gloster, J., Ebert, K., Gubbins, S., Bashiruddin, J., & Paton, D. J. (2011). Normal variation in thermal radiated temperature in cattle: Implications for foot-and-mouth disease detection. BMC Veterinary Research, 7(1), 1–10.
- Godyń, D., Herbut, P., & Angrecka, S. (2019). Measurements of peripheral and deep body temperature in cattle–A review. Journal of Thermal Biology, 79, 42–49.
- Goldberg, J. J., Wildman, E. E., Pankey, J. W., Kunkel, J. R., Howard, D. B., & Murphy, B. M. (1992). The influence of intensively managed rotational grazing, traditional continuous grazing, and confinement housing on bulk tank milk quality and udder health. Journal of Dairy Science, 75(1), 96–104.
- Goma, A. A., Pearce, G. P., Uddin, J., Rimon, E., Davies, H., & Phillips, C. J. C. (2018). A forced lateralisation test for dairy cows and its relation to their behaviour. Applied Animal Behaviour Science, 207, 8–19.
- Gómez, Y., Bieler, R., Hankele, A. K., Zähner, M., Savary, P., & Hillmann, E. (2018). Evaluation of visible eye white and maximum eye temperature as non-invasive indicators of stress in dairy cows. Applied Animal Behaviour Science, 198, 1–8.
- Grille, L., Adrien, M. L., Olmos, M., Chilibroste, P., & Damián, J. P. (2019). Diet change from a system combining total mixed ration and pasture to confinement system (total mixed ration) on milk production and composition, blood biochemistry and behavior of dairy cows. Animal Science Journal, 90(11), 1484–1494.
- Harding, E. J., Paul, E. S., & Mendl, M. (2004). Cognitive bias and affective state. Nature, 427(6972), 312–315.
- Haskell, M. J., Rennie, L. J., Bowell, V. A., Bell, M. J., & Lawrence, A. B. (2006). Housing system, milk production, and zero-grazing effects on lameness and leg injury in dairy cows. Journal of Dairy Science, 89(11), 4259–4266.
- Hötzel, M. J., Cardoso, C. S., Roslindo, A., & von Keyserlingk, M. A. (2017). Citizens’ views on the practices of zero-grazing and cow-calf separation in the dairy industry: Does providing information increase acceptability? Journal of Dairy Science, 100(5), 4150–4160.
- Kismul, H., Spörndly, E., Höglind, M., Næss, G., & Eriksson, T. (2018). Morning and evening pasture access–Comparing the effect of production pasture and exercise pasture on milk production and cow behaviour in an automatic milking system. Livestock Science, 217, 44–54.
- Knaus, W. (2016). Perspectives on pasture versus indoor feeding of dairy cows. Journal of the Science of Food and Agriculture, 96(1), 9–17.
- Lecorps, B., Kappel, S., Weary, D. M., & von Keyserlingk, M. A. (2018). Dairy calves’ personality traits predict social proximity and response to an emotional challenge. Scientific Reports, 8(1), 1–9.
- Lee, C., Cafe, L. M., Robinson, S. L., Doyle, R. E., Lea, J. M., Small, A. H., & Colditz, I. G. (2018). Anxiety influences attention bias but not flight speed and crush score in beef cattle. Applied Animal Behaviour Science, 205, 210–215.
- Legrand, A. L., Von Keyserlingk, M. A. G., & Weary, D. M. (2009). Preference and usage of pasture versus free-stall housing by lactating dairy cattle. Journal of Dairy Science, 92(8), 3651–3658.
- McManus, C., Tanure, C. B., Peripolli, V., Seixas, L., Fischer, V., Gabbi, A. M., & Costa, J. B. G., Jr. (2016). Infrared thermography in animal production: An overview. Computers and Electronics in Agriculture, 123, 10–16.
- Mee, J. F., & Boyle, L. A. (2020). Assessing whether dairy cow welfare is “better” in pasture-based than in confinement-based management systems. New Zealand Veterinary Journal, 68(3), 168–177.
- Nakayama, K., Goto, S., Kuraoka, K., & Nakamura, K. (2005). Decrease in nasal temperature of rhesus monkeys (Macaca mulatta) in negative emotional state. Physiology & Behavior, 84(5), 783–790.
- Neave, H. W., Daros, R. R., Costa, J. H., von Keyserlingk, M. A., & Weary, D. M. (2013). Pain and pessimism: Dairy calves exhibit negative judgement bias following hot-iron disbudding. PLOS ONE, 8(12), e80556.
- O’Connell, J., Giller, P. S., & Meaney, W. (1989). A comparison of dairy cattle behavioural patterns at pasture and during confinement. Irish Journal of Agricultural Research, 28, 65–72.
- Olmos, G., Boyle, L., Hanlon, A., Patton, J., Murphy, J. J., & Mee, J. F. (2009a). Hoof disorders, locomotion ability and lying times of cubicle-housed compared to pasture-based dairy cows. Livestock Science, 125(2–3), 199–207.
- Olmos, G., Mee, J. F., Hanlon, A., Patton, J., Murphy, J. J., & Boyle, L. (2009b). Peripartum health and welfare of Holstein-Friesian cows in a confinement-TMR system compared to a pasture-based system. Animal Welfare, 18(4), 467–476.
- Phillips, C. J. C., Oevermans, H., Syrett, K. L., Jespersen, A. Y., & Pearce, G. P. (2015). Lateralization of behavior in dairy cows in response to conspecifics and novel persons. Journal of Dairy Science, 98(4), 2389–2400.
- Polsky, L., & von Keyserlingk, M. A. (2017). Invited review: Effects of heat stress on dairy cattle welfare. Journal of Dairy Science, 100(11), 8645–8657.
- Proctor, H., & Carder, G. (2016). Can changes in nasal temperature be used as an indicator of emotional state in cows? Applied Animal Behaviour Science, 184, 1–6.
- Robins, A., & Phillips, C. (2010). Lateralised visual processing in domestic cattle herds responding to novel and familiar stimuli. Laterality, 15(5), 514–534.
- Savory, C. J., Kostal, L., & Nevison, I. M. (2006). Circadian variation in heart rate, blood pressure, body temperature and EEG of immature broiler breeder chickens in restricted-fed and ad libitum-fed states. British Poultry Science, 47(5), 599–606.
- Schuppli, C. A., Von Keyserlingk, M. A. G., & Weary, D. M. (2014). Access to pasture for dairy cows: Responses from an online engagement. Journal of Animal Science, 92(11), 5185–5192.
- Schütz, K. E., Rogers, A. R., Poulouin, Y. A., Cox, N. R., & Tucker, C. B. (2010). The amount of shade influences the behavior and physiology of dairy cattle. Journal of Dairy Science, 93(1), 125–133.
- Scoley, G. E., Gordon, A. W., & Morrison, S. J. (2019). Use of thermal imaging in dairy calves: Exploring the repeatability and accuracy of measures taken from different anatomical regions. Translational Animal Science, 3(1), 564–576.
- Sharma, A., Umapathy, G., Kumar, V., & Phillips, C. J. (2019). Hair cortisol in sheltered cows and its association with other welfare indicators. Animals, 9(5), 248.
- Smid, A. M. C., Weary, D. M., & von Keyserlingk, M. A. (2020). The influence of different types of outdoor access on dairy cattle behavior. Frontiers in Veterinary Science, 7, 257.
- Spruijt, B. M., Van den Bos, R., & Pijlman, F. T. (2001). A concept of welfare based on reward evaluating mechanisms in the brain: Anticipatory behaviour as an indicator for the state of reward systems. Applied Animal Behaviour Science, 72(2), 145–171.
- Stewart, M., Schaefer, A. L., Haley, D. B., Colyn, J., Cook, N. J., Stafford, K. J., & Webster, J. R. (2008a). Infrared thermography as a non-invasive method for detecting fear-related responses of cattle to handling procedures. Animal Welfare, 17(4), 387–393.
- Stewart, M., Stafford, K. J., Dowling, S. K., Schaefer, A. L., & Webster, J. R. (2008b). Eye temperature and heart rate variability of calves disbudded with or without local anaesthetic. Physiology & Behavior, 93(4–5), 789–797.
- Stewart, M., Webster, J. R., Verkerk, G. A., Schaefer, A. L., Colyn, J. J., & Stafford, K. J. (2007). Non-invasive measurement of stress in dairy cows using infrared thermography. Physiology & Behavior, 92(3), 520–525.
- Travain, T., & Valsecchi, P. (2021). Infrared thermography in the study of animals’ emotional responses: A critical review. Animals, 11(9), 2510.
- Tucker, C. B., Jensen, M. B., de Passillé, A. M., Hänninen, L., & Rushen, J. (2021). Invited review: Lying time and the welfare of dairy cows. Journal of Dairy Science, 104(1), 20–46.
- Tucker, C. B., Rogers, A. R., Verkerk, G. A., Kendall, P. E., Webster, J. R., & Matthews, L. R. (2007). Effects of shelter and body condition on the behaviour and physiology of dairy cattle in winter. Applied Animal Behaviour Science, 105(1–3), 1–13.
- Uddin, J., McNeill, D. M., Lisle, A. T., & Phillips, C. J. (2020). A sampling strategy for the determination of infrared temperature of relevant external body surfaces of dairy cows. International Journal of Biometeorology, 64(9), 1583–1592.
- Uddin, J., Phillips, C. J., Auboeuf, M., & McNeill, D. M. (2021). Relationships between body temperatures and behaviours in lactating dairy cows. Applied Animal Behaviour Science, 241, 105359.
- Uddin, J., Phillips, C. J., Goma, A. A., & McNeill, D. M. (2019). Relationships between infrared temperature and laterality. Applied Animal Behaviour Science, 220, 104855.
- USDA (2016). United states department of agriculture. Available online: www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_PartI.pdf (accessed: 15th July 2021).
- van den Pol-van Dasselaar, A., Hennessy, D., & Isselstein, J. (2020). Grazing of dairy cows in Europe—An in-depth analysis based on the perception of grassland experts. Sustainability, 12(3), 1098.
- Vianna, D. M., & Carrive, P. (2005). Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. European Journal of Neuroscience, 21(9), 2505–2512.
- Von Keyserlingk, M. A., Cestari, A. A., Franks, B., Fregonesi, J. A., & Weary, D. M. (2017). Dairy cows value access to pasture as highly as fresh feed. Scientific Reports, 7(1), 1–4.
- Washburn, S. P., White, S. L., Green, J. T., Jr, & Benson, G. A. (2002). Reproduction, mastitis, and body condition of seasonally calved holstein and Jersey cows in confinement or pasture systems. Journal of Dairy Science, 85(1), 105–111.
- Watters, J. V., & Krebs, B. L. (2019). Assessing and enhancing the welfare of animals with equivocal and reliable cues. Animals, 9(9), 680.
- Webster, J. R., Stewart, M., Rogers, A. R., & Verkerk, G. A. (2008). Assessment of welfare from physiological and behavioural responses of New Zealand dairy cows exposed to cold and wet conditions. Animal Welfare, 17(1), 19–26.
- Zethof, T. J., Van Der Heyden, J. A., Tolboom, J. T., & Olivier, B. (1994). Stress-induced hyperthermia in mice: A methodological study. Physiology & Behavior, 55(1), 109–115.
- Zobel, G., Weary, D. M., Leslie, K. E., & Von Keyserlingk, M. A. G. (2015). Invited review: Cessation of lactation: Effects on animal welfare. Journal of Dairy Science, 98(12), 8263–8277.
