Abstract
Background: The American Heart Association guidelines (AHA) guidelines list tachycardia as a contraindication for the administration of nitroglycerin (NTG), despite limited evidence of adverse events. We sought to determine whether NTG administered for chest pain was a predictor of hypotension (systolic blood pressure <90 mmHg) in patients with tachycardia, compared to patients without tachycardia (50≥ heart rate ≤100). Methods: We performed a retrospective cohort study using patient care reports completed by basic life support (BLS) providers in a large urban Canadian EMS system for the period 2010–2012. We used logistic regression to test the association between post-NTG hypotension and tachycardia, independent of pre-NTG blood pressure, age, sex, and comorbidities. Using identical models, we tested four secondary outcomes (drop in blood pressure, reduced consciousness, bradycardia, and cardiac arrest). Results: The cohort included 10,308 patients who were administered NTG by BLS in the prehospital setting; 2,057 (20%) of patients were tachycardic before NTG administration. Hypotension occurred in 320 of all patients (3.1%): 239 without tachycardia (2.9%) and 81 with tachycardia (3.9%). Compared to non-tachycardic patients, tachycardic patients showed increased adjusted odds of hypotension (AOR: 1.60; 95% CI: 1.23–2.08) or of a drop in blood pressure of 30mm Hg or greater (AOR: 1.11; CI: 1.00–1.24). Tachycardia was associated with decreased odds of bradycardia (OR: 0.33; CI: 0.17–0.64). We did not find a significant association between tachycardia and either post-NTG reduced level of consciousness or cardiac arrest. We did find a strong, significant association between pre-NTG blood pressure and post-NTG hypotension (AOR for units of 10mmHg: 0.64; CI: 0.61–0.69). Conclusion: Hypotension following prehospital administration of NTG was infrequent in patients with chest pain. However, while the absolute risk of NTG-induced hypotension was low, patients with pre-NTG tachycardia had a significant increase in the relative risk of hypotension. In addition, hypotension occurred most frequently in patients presenting with a lower pre-NTG blood pressure, which may prove to be a more discriminating basis for future guidelines. EMS medical directors should review BLS chest pain protocols to weigh the benefits of NTG administration against its risks.
Introduction
Nitrates, such as nitroglycerin (NTG), are commonly used in suspected myocardial ischemia due to the beneficial dilation of the coronary arteries. Specifically, patients with chest pain of suspected cardiac origin may experience symptomatic relief of chest discomfort when NTG is administered by prehospital care providers.Citation1
NTG is described as a relatively safe drugCitation1-3 with adverse effects occurring in 0.7% to 3.6% of administrations in the prehospital setting.Citation1-4 Typically, adverse events associated with sublingual nitroglycerin include headache, nausea, vomiting, hypotension, and tachycardia. Syncope, severe hypotension, bradycardia, and cardiac arrest occur rarely.Citation1,Citation3-6 Given the uncertainty in identifying patients who may experience potentially serious adverse events, some have questioned the safety of administering NTG in the prehospital setting.Citation1,2,5,6
NTG administration in tachycardic patients was given a Class III, LOE C recommendation in the American Heart Association ECC and CPR Guidelines based upon the risk of hypotension.Citation7 Tachycardia can diminish preload since it decreases right ventricular filling time. When combined with the effect of NTG on decreasing venous return, administration in tachycardia could plausibly cause a severe drop in blood pressure.
In the province of Quebec, BLS care providers cannot administer intravenous fluid to control NTG-associated hypotension. BLS care in the study area includes use of semi-automatic defibrillation, a dual lumen supraglottic airway device, a C-PAP mechanical ventilator device, 12-lead ECG acquisition, and the administration of symptom relief medications (salbutamol, epinephrine, acetylsalicylic acid, glucagon and NTG). Chest pain patients rarely receive care by advanced life support providers in this jurisdiction.
To our knowledge, despite the recommendation to withhold nitroglycerin in the presence of tachycardia, no published research has evaluated the risk of NTG administration in the presence of tachycardia.
The primary goal of the study was to determine whether NTG administered for chest pain was associated with an increased risk of hypotension (systolic blood pressure < 90 mmHg) in patients with tachycardia (HR > 100bpm) compared to patients without tachycardia (50bpm ≥ HR ≤ 100bpm). Secondarily, we sought to determine whether tachycardia was associated with other post-NTG outcomes, two of which are AHA contraindications for NTG administration and two of which are undesirable effects. Respectively, these are: a drop in blood pressure, bradycardia, reduced level of consciousness, and cardiac arrest.
Methods
Study Design and Setting
This retrospective cohort was based on chart review of EMS patient care records. Urgences-santé, a non-profit urban emergency medical service, completes patient care reports for all patients. These paper reports are scanned into a computerized database. Data is double checked against the original patient care report by a technician. In 2012, Urgences-santé's territory included approximately 2.3 million people over a area of 744 kmCitation2. Urgences-santé received 373,000 calls that year with approximately 228,000 prehospital patient transports. The vast majority (99%) of prehospital care providers at Urgences-santé are BLS-level. In the case of chest pain of suspected cardiac origin, the province of Quebec BLS protocols stipulate cardiac monitoring, acquisition of a 12-lead ECG, administration of oxygen (adjusted according to blood saturation), acetylsalicylic acid (320 mg PO) and sublingual nitroglycerin spray (Nitrolingual® 0.4mg Sanofi Aventis) repeated every five minutes as indicated. If pain remains unchanged after four doses, NTG is stopped. However, if pain is reduced after administration of NTG, there is no restriction as to the maximum number of doses. IV placement is not performed by BLS providers. The inclusion and exclusion criteria of the nitroglycerin protocolCitation8 applicable to the study period are presented in Table . Blood pressure was measured using either a manual sphygmomanometer or by the integrated non-invasive blood pressure measurement of the Zoll E-series® monitor defibrillator. Typically, the first blood pressure reading was taken using a manual sphygmomanometer. Thereafter, the provider had the option of repeating manual measurements or switching to the monitor. None of chest pain patients included in this study had an IV placed by a prehospital care provider, nor were any of the patients treated by ALS paramedics.
Table 1. Provincial prehospital nitroglycerin protocol: 2010-2012 (Quebec, Canada)9
This study was approved by the Research Ethics Board of Sacré-Coeur Hospital, Montreal.
Data Collection and Processing
We performed a computerized database search for all prehospital charts with NTG administered for chest pain patients for the period of January 1, 2010 to December 31, 2012. Case-patients were defined as those who received at least one dose of NTG from a BLS provider. Variables included: age, sex, comorbidities (diabetes, hypertension, dyslipidemia and cardiac condition), time of vital sign readings (heart rate, systolic blood pressure, level of consciousness), and time of medication administration. Prehospital ECG electronic interpretations were not reviewed in this study. Charts were excluded if other medications, with the exception of oxygen and acetylsalicylic acid, were given simultaneously. We also excluded 1) cases with incomplete information; 2) cases where vital signs were not repeated within 15 minutes of the first dose of NTG; 3) cases non-compliant with BLS protocol (i.e., NTG administered when HR < 50 bpm or BP < 100mmHg); and 4) cases where more than two hours elapsed before the first and last vital sign measurement (to remove outliers and cases of data mis-entry). Patients were divided into two groups. The tachycardic group was defined by a heart rate over 100 bpm on the measurement that preceded NTG administration. The non-tachycardic group was defined by a heart rate between 50 bpm and 100 bpm inclusively. We attributed NTG-induced hypotension to any patient with a blood pressure of less than 90 mmHg measured at any time within 15 minutes of the first dose of NTG. Secondary outcomes were defined as: 1) drop in blood pressure for patients with a 30 mmHg or greater decrease difference in systolic BP between vital signs preceding and following the first dose of NTG; 2) bradycardia for patients with a HR lower than 50 bpm on any vital signs following the first dose of NTG; 3) reduced level of consciousness for patients whose post-NTG score on the Alert-Verbal-Pain-Unresponsive scale (responds to verbal stimuli or pain, or is unresponsive) was lower than their pre-NTG score; and 4) cardiac arrest for patients who coded after the first dose of NTG and before delivery to hospital. We grouped age into quartiles to allow for non-linear effects. We measured pre-NTG BP in 10 mmHg units to improve the interpretability and comparison of our model results. A trained extractor collected the data using a standardized data abstraction form with defined study variables and clear inclusion and exclusion criteria.Citation9,10 Trained reviewers extracted any missing data from the ZOLL GE Medical Systems Marquette ® 12SL™ Analysis Program, version 14, or from the original patient care reports. An investigator reviewed database entries that extended beyond normal range and compared them to the original patient care report. The trained extractor was not blinded to the purpose of the study. A second trained reviewer examined a random sample of 5% of all charts.Citation10 Inter-rater agreement was high (Cohen's kappa: 0.91).
Analysis
Descriptive statistics were used to compare the tachycardic and non-tachycardic groups. We used multivariate binary logistic regression to calculate adjusted odds ratio with 95% confidence intervals (CI) for the primary outcome and the four secondary outcomes. All five regression models controlled for pre-NTG blood pressure, age group, sex and four comorbidities (diabetes, cardiac condition, dyslipidemia and hypertension). Statistical analysis was completed using the R language (version 3.1.0).
Results
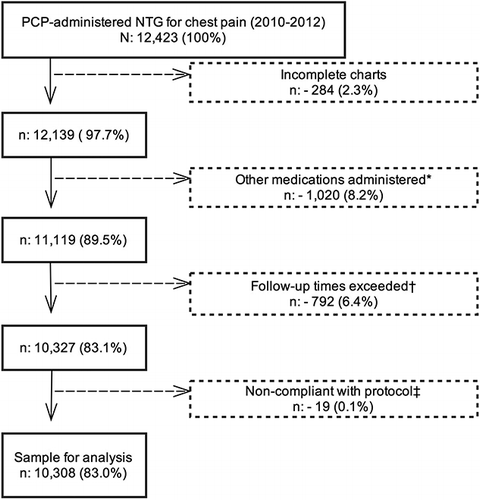
BLS providers administered NTG to 12,423 patients presenting with ACS symptoms during the study period. We excluded 2,115 cases as described in Figure . Among the remaining 10,308 subjects, 8,251 (80.0%) were non-tachycardic and 2,057 (20.0%) were tachycardic before NTG administration.
Figure 1. Inclusion/exclusion diagram. *Epinephrine and salbutamol cases were excluded for potential confounding effects on heart rate. ASA and oxygen cases were not excluded. † No vital signs taken within 15 minutes after the first dose of NTG, or calls lasting more than 2 hours ‡ Patient presenting with pre-NTG BP under 100 mmHg
Bivariate comparison between non-tachycardic patients and tachycardic patients are shown in Table . Hypotension occurred in 320 of all cases (3.1%). Non-tachycardic patients became hypotensive in 239 cases out of 8,251 (2.9%) and tachycardic patients 81 cases out of 2,057 (3.9%); the difference was statistically significant (p = 0.02). There were significant differences in baseline characteristics between the two groups. The non-tachycardic group had higher rates of CAD, dyslipidemia and hypertension, and the mean age was higher (68.2 versus 65.9). Moreover, systolic blood pressure pre-NTG was 145 mmHg in the non-tachycardic group versus 150 mmHg in the tachycardic group.
Table 2. Univariate comparison between non-tachycardic patients and tachycardic patients
The results of the multivariable logistic regression models are summarized in Tables and . We found that tachycardic patients were at higher odds of experiencing post-NTG hypotension (AOR: 1.60; CI: 1.23–2.08) or post-NTG drop in blood pressure (AOR: 1.11; CI: 1.00–1.24). There were decreased odds of the same group experiencing bradycardia (AOR: 0.33; CI: 0.17–0.64). We did not find a significant association between tachycardia and either post-NTG reduced level of consciousness or cardiac arrest, probably due to a lack of statistical power. After NTG administration, 30 patients (0.003%) had a cardiac arrest; three cases did not have return spontaneous circulation before arrival to hospital. Hypotension was not associated with age, sex, or comorbidities. However, for each incremental increase of 10 mmHg of pre-NTG blood pressure we found that the odds of post-NTG hypotension decreased by 36% (AOR: 0.64; CI: 0.61–0.69). We found similarly significant associations between pre-NTG BP and three of the four secondary outcomes (bradycardia, drop of blood pressure, reduced level of consciousness).
Table 3. Adjusted odds ratios for the primary outcome model [CI: 95%]
Table 4. Adjusted odds ratios for the secondary outcome model [CI: 95%]
Discussion
More than 130 years after the first study published on NTG,Citation11 we still cannot predict reliably which patients will develop adverse effects. In the literature, a minority of adverse effects in prehospital patients were serious. Expert opinion has been that NTG administration in tachycardia should be used with caution as it puts a patient at risk for hypotension. To our knowledge, this is the first study to examine the outcomes of NTG administration in tachycardic chest pain patients.
Our findings suggest that there is an association between tachycardia and hypotension in patients administered NTG for chest pain, with tachycardic patients showing rates of hypotension 33% higher than non-tachycardic patients. However, the results showed an absolute difference in risk of only 1%, which is commensurate with the range of estimates found in the literature. Engelberg et al.Citation1 observed an hypotension rate of 0.003% in patients post-NTG; Wuerz et al. observed a rate of 1.3%;Citation2 Clemency et al. observed a rate of 3.2%.Citation12 The definitions of hypotension, as well as the patient characteristics, varied between the studies. Unfortunately, cardiac arrest occurred in both tachycardic and non-tachycardic groups in our study; the number of arrests was small, which limited our ability to draw inference from these events. We observed no deaths associated with prehospital NTG.
Neither the severity of NTG-induced hypotension nor its effects on patient outcomes are well understood. Consequently, in the absence of clinical characterization of the impact of hypotension in these situations, it is difficult to know how to interpret either a heart rate or blood pressure as a marker of actual patient-oriented harm. Unfortunately, we were unable to collect data on these outcomes, and further study is required to assess the health of hypotensive patients. Some indications can be drawn from the literature. Previous studies were based on advanced life support providers. Consequently, when hypotension was observed, providers were able to intervene with fluids or other treatment. In the Wuerz et al.Citation2 and Engelberg et al.Citation1 studies, all patients had an IV access or medication to reverse adverse effects, as appropriate. By contrast, the hypotensive congestive heart failure patients in the Clemency et al.Citation12 study recovered (BP ≥ 100 mmHg) without treatment. Engelberg et al.Citation1 reported 12 (0.7%) adverse events following a single dose of NTG. Clemency et al.Citation12 had studied multiple simultaneous NTG tabs (0.8 mg if BP > 160 mmHg or 1.2 mg if BP > 200 mmHg) for respiratory distress due to congestive heart failure and reported only 3 (3.2%) hypotension (less than 100 mmHg) cases.
Although the average patients in our study received multiple doses of NTG (mean: 2.5), we chose to calculate the outcome within 15 minutes after the first dose. We wanted to be as sensitive as possible to the class-effect of NTG-induced hypotension while ignoring the dose-based effect. Also, we wanted to identify which patient would be at risk if they received even one dose. Moreover, reported cases of adverse effects were observed within a 15-minute window following NTG administration and adverse effects did not seem to be dose related.
Based on our results, we estimate that the AHA tachycardia exclusion prevents one hypotension case while withholding NTG from 99 chest pain patients, which is the traditional definition of medical futility.Citation13 This finding raises the concern that, though the implementation of the AHA guidelinesCitation7 does seem to prevent hypotension, it does so at the expense of a large number of patients not at risk for hypotension who are deprived of the non-trivial pain relief potentially offered by NTG. Moreover, the focus on heart rate may hinder the development of more discriminating exclusion criteria. We found that pre-NTG blood pressure—measured continuously, not as the binary cut-off currently specified by the AHA—to be a better predictor of hypotension than was tachycardia. Further study into the utility of pre-NTG BP-based indicators seems like a promising way to improve future versions of the AHA Guidelines.Citation7
Limitations and Future Research
The BLS protocol restrictions could create selection bias. As per the provincial protocol, NTG was not administered if chest pain patients presented with SBP < 100 mmHg or HR ≥ 150 bpm. It is possible that patients with a pre-NTG HR of > 150 may have an even higher rate of hypotension. Minor differences in the accuracy of blood pressure measures between manual and automatic measurement may have resulted in misclassification in our outcome variable. We found no evidence of lack of robustness when performing sensitivity analyses (not reported; available from the corresponding author upon request).
External validity may be limited because prehospital data were exclusively from a single urban setting. Moreover, despite the fact that patients younger than 35 years-old with chest pain of suspected cardiac origin and known for coronary artery disease were included in the study to comply with the provincial protocols, the sample does not contain any of these patients. Medication, such as beta blockers, may have had a confounding effect. Our dataset did not include medications taken by the patients. Most importantly, hypotension severity and its sequelae were not studied. We did not examine the consequences of hypotension in patients at the emergency department. If it could be shown that there are no significant consequences among patients who develop hypotension following administration of NTG, this would further characterize the risks of prehospital NTG in patients with tachycardia. Since we have demonstrated that NTG increases the risk to develop hypotension in tachycardic patients, yet we are not certain of the clinical impact, further investigations are required to determine short-term outcomes of hypotensive tachycardic patients.
Conclusion
The results of this study indicate that there was a statistically significant increase in the relative risk of hypotension with NTG administration in tachycardic patients. However, the absolute risk of NTG-induced hypotension was low in these patients. Pre-NTG blood pressure seems to be a promising alternative basis for identifying patients at risk of adverse outcomes with NTG. EMS medical directors reviewing PCP chest pain protocols should weigh the potential benefits of NTG administration against its known risks.
References
- Engelberg S, Singer AJ, Moldashel J, Sciammarella J, Thode HC, Henry M. Effects of prehospital nitroglycerin on hemodynamics and chest pain intensity. Prehosp Emerg Care 2000;4:290–3.
- Wuerz R, Swope G, Meador S, Holliman CJ, Roth GS. Safety of prehospital nitroglycerin. Ann Emerg Med 1994;23:31–6.
- Herman LL, Koenigsberg M, Ward S, Sloan EP. The prehospital use of nitroglycerin according to standing medical orders in an urban EMS system. Prehosp Disaster Med 1993;8:29–33.
- Brice JH A-SH, Delbridge TR. Safety of nitroglycerin in an urban EMS system (abstract). Prehosp Emerg Care;2:254.
- Boyle MJ. A dramatic drop in blood pressure following prehospital GTN administration. Emerg Med J 2007;24:225–6.
- Funk D, McGuire TJ. Nitroglycerin in the out-of-hospital treatment of chest pain. Prehosp Emerg Care 1999;3:266–8.
- O'Connor RE, Abdulaziz SAL, Brady WJ, et al. Part 9: Acute coronary syndromes: 2015 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S483–500.
- MSSS. Protocoles d'intervention clinique à l'usage des techniciens ambulanciers-paramédics. 4e ed; 2007, Mise à jour 2010.
- Badcock D, Kelly AM, Kerr D, Reade T. The quality of medical record review studies in the international emergency medicine literature. Ann Emerg Med 2005;45:444–7.
- Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med 1996;27:305–8.
- W M. Nitro-glycerine as a remedy for angina pectoris. Lancet 1879;1:113–5.
- Clemency BM, Thompson JJ, Tundo GN, Lindstrom HA. Prehospital high-dose sublingual nitroglycerin rarely causes hypotension. Prehospital and disaster medicine 2013;28:477–81.
- Schneiderman LJ. Defining medical futility and improving medical care. J Bioethic Inq 2011:2:123–31.
