Abstract
Background: During resuscitation in the field, intraosseous (IO) access may be achieved using a variety of available devices, often attempted by inexperienced users. Aim: We sought to examine the success rate and ease-of-use ratings of an IO device, the NIO® (New Intraosseous Persys Medical, Houston, TX, USA) in comparison to the Arrow® EZ-IO® (Teleflex Medical Research Triangle Park, NC, USA) by novice users. Methods: We performed a randomized crossover trial. The study model was a porcine hind leg which was cut distally in order to expose the marrow. The Study population was composed of pre-graduate medical students without prior experience in IO use, all designated future field physicians. The students underwent instruction and practiced the use of both devices. After practice completion, each student attempted a single IO insertion with both devices sequentially in randomized fashion. Success was defined as a flow of fluid through the bone marrow after a single IO attempt. Investigators which determined the success rate were blinded to the used device. Results: 50 users (33 males, 17 females) participated in the trial, mean age of 21.7 years (±1). NIO users were successful in 92% (46/50) attempts while EZ-IO user success rate was 88% (44/50). NIO success rates were comparable to those of EZ-IO (p = NS). Results were similar when examining only the initial device used. Median score of ease of use was 4 (5 point Likert scale) in both devices (p = NS). 54% (27/50) of the participants preferred using the EZ-IO over the NIO (p = NS). Conclusion: Novice users were equally successful in establishing IO access with the NIO® in comparison to the EZ-IO® in a porcine model.
Key words::
Introduction
In patients undergoing resuscitation for whom intravenous (IV) access is not readily available, the American Heart Association (AHA) and the European Resuscitation Council (ERC) recommend the establishment of an intraosseous access (IO).Citation1,2
This procedure is also used widely for casualty treatment in military and other field scenarios. The 2015 update of the U.S. Army Committee on Tactical Combat Casualty Care (CoTCCC) recommends using IO access in any resuscitation scenario in which IV access is not obtainable.Citation3 IO access can be established using manually inserted IO needles or using mechanical semi-automatic devices such as the FAST1® (Pyng Medical Corporation, BC, Canada), the spring loaded device BIG® (Bone Injection Gun, Waismed Ltd., NY, USA) and the battery powered drill such as the Arrow® EZ-IO® (Teleflex Medical Research Triangle Park, NC, USA). A systematic review of available IO devices reported a superiority of the EZ-IO® over manual needles, and other semi-automatic IO devices.Citation4 The EZ-IO® and the FAST-1® are commonly used by emergency service organizations worldwide.Citation5,6 The BIG® had been the standard issued IO device by the Israeli Defense Forces (IDF) for over a decade due to its light weight, portability, storage durability in extreme climates and single packaging. However, unsatisfactory results with the device in field use by IDF Medical Corps (IDF-MC) advanced life support providers (physicians and paramedics) have been reported.Citation7 These providers are often inexperienced in establishing IO access which might have contributed to such a study outcome.
The aim of this study was to examine the success rate of establishing IO access and ease-of-use ratings of an IO device, the NIO® (New Intraosseous Persys Medical, Houston, TX, USA) in comparison to the EZ-IO® by novice users.
Methods
Study Design
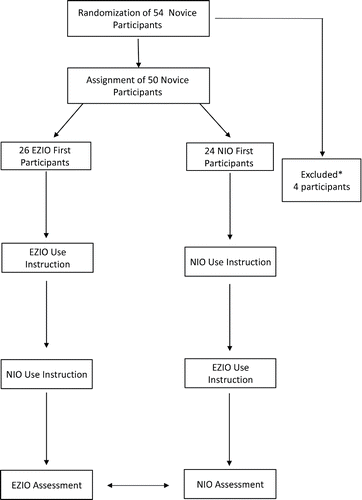
We performed a randomized crossover single blinded simulation study comparing the use of the NIO® and the EZ-IO®, as illustrated in . The study was conducted at the Military Medical School of the IDF-MC. The sequence of device insertion was randomized to either NIO®-first or EZ-IO®-first using a computerized random-number generator.Citation8 The Institutional Review Board of IDF Medical Corps exempted the investigators from attaining written consent from study participants as this was a voluntary model based study.
Figure 1. The study flow diagram: After giving their consent, participants were randomized into the NIO® first and EZ-IO® first groups according to the device they were allocated to begin the study with. They underwent oral and “hands-on” instruction and practice with either device. After practice completion, participants proceeded to the IO procedure according to their group allocation. Upon completion with the initial device participants immediately repeated the procedure with the second IO device. After completing both procedures, participants rated the devices' ease of use and device preference.
Study Participants
Study participants were undergraduate medical students of the Military Track, Hadassah and Hebrew University School of Medicine who completed two years of their bachelor of medical sciences degree without any clinical exposure. All are designated future IDF-MC field physicians. Participants with any previous practical or theoretical knowledge of IO device use were excluded from the study.
Study Instruments
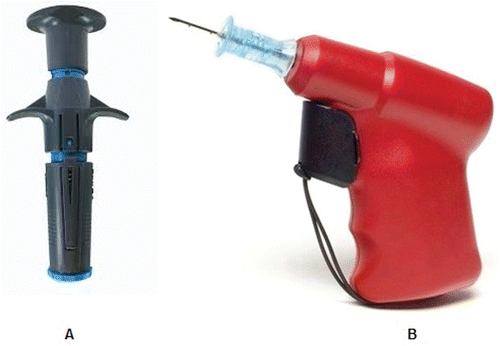
The NIO® (New Intraosseous Persys Medical, Houston, TX, USA) is a spring loaded IO device. It weighs approximately100 grams and is a single use spring-loaded device with a twist-to-unlock handle and a trigger mechanism. This single-package device contains a 15-gauge needle and stylet as well as a needle stabilizer ().
The IO drill Arrow® EZ-IO® (Teleflex Medical Research Triangle Park, NC, USA) is a device composed of a battery-powered vascular access driver with an integrated driller stylet-tipped 15-gauge needle. The operator has an arsenal of needles (provided separately) to use along with the drill in relation to the patient size. This study utilized the 25-mm long needle that is recommended for placement in the proximal tibia of patients weighting 40 kg or higher ().
IO Model
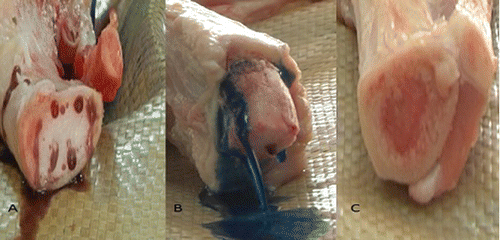
The IDF-MC field care clinical practice guideline (CPG) specifies that the preferable IO placement site is the tibia plateau.Citation9 The chosen IO study model was a fresh adult-male porcine hind leg because of its similarity to the human adult anatomy.Citation10 All porcine models were retrieved from 5 months old mix breed of Landrace and Large White swine of 80 kgs in approximate weight. The designated area of placement was one centimeter medial to the tibia plateau similar to previous studies on porcine models.Citation11 Each leg was inspected in a systematic fashion for the presence of joint integrity and absence of fractures. In order to better visualize the flow of infused fluids inside the marrow cavity the bones were stripped of their skin and overlying meat and the tibias were sawed approximately 6-cm distal to the IO placement site (). Leaving the entire overlying tissue may allow for a more realistic tactile simulation, but we opted to remove it as the primary outcome was the success rates between the two devices on the model and we were concerned that the differences in the overlying tissue thickness between each model might alter the results. In order to ascertain if the suitable needle length and size was chosen for the porcine model, we made sagittal incisions in several porcine models and measured the average cortex width of the tibia at the point of insertion (Figure S1).
Study Procedure
Study participants received a 40-minute general lecture regarding intraosseous infusion in critically injured casualties. After the lecture, participants received a 30-minute instruction session by the study investigators (AvS and ENB) on the use of each device, including a 5-minutes video demonstration of the NIO® and the EZ-IO®. Each participant in turn practiced using both devices on the adult intraosseous bone models as much as they felt was needed. Sessions included hands on practice with the porcine model. When each and every participant felt sufficiently adept and confident in using both devices the sessions ended. Thereafter, participants began the experiment according to the randomization allocation. Each participant was asked by a study investigator to perform a single IO insertion attempt independently, using one of the devices (NIO®-first or EZ-IO®-first) on the porcine model. Participants were asked to infuse two 20 ml syringes filled with normal saline dyed with Methylene Blue to the needle when they believed the insertion was successful. Upon completion of the IO access attempt with the first device, the participant entered a second room where a single IO access attempt was performed using the second IO device. The study investigators (AvS, ENB) did not intervene or provide any consultation or feedback and participants were not allowed to watch others perform the procedure or relay tips to their peers. Each needle was used on one bone only; a new needle was used for each insertion attempt; a new porcine bone model was used for each of the attempts.
The procedure was recorded by video cameras (Panasonic AG-AC160A AVCCAM HD Handheld Camcorder, Japan) that were fixed to a tripod located 50-cm from the bone. Once the participant indicated readiness, a video recording was started. Only the bone and the fluid line tubing connected to it were visible for the blinded assessment of success, and recording discontinued when the infusion of dyed water ended.
Outcome Measures
Primary Outcome Measure
The primary outcome measure was the establishment of successful IO access. A successful attempt was defined by visualization (in the recorded attempt) of fluid emerging from the IO cavity without extravasation around the drilled hole (). If fluid did not emerge from the bone marrow or extravasation was witnessed around the hole, the attempt was defined as an unsuccessful (). If fluid emerged from the cortex itself, the attempt was defined as inconclusive as the overlying skin and meat were removed from the porcine model and potential microscopic damage may have been caused to the cortex. To ensure investigator blinding, video films were edited by an independent editor before being assessed. The IO needle in each frame was blackened and the sound muted, making it unrecognizable on video (editing software Sony VEGAS PRO version 11.0). The study investigators (AvS and ENB, AmS), blinded to the group allocation, reviewed the video films together, and rated each procedure as successful, unsuccessful or inconclusive. When there was a disagreement amongst the three investigators or the attempt was rated as inconclusive, the edited video in question was reviewed by a fourth blinded investigator (IS) unaware of the previous scoring. Failed attempts were reviewed once more with unedited video footage in order to analyze and record causes for technical device failure.
Secondary Outcome Measures
Following the study procedure, participants were asked to complete a two-item questionnaire. In the first item, participants were asked to rate the ease-of-use of the NIO® and the EZ-IO® by a 5-point Likert Scale (“The device is easy to use”; 1 strongly disagree, 2 disagree, 3 neither agree nor disagree, 4 agree, 5 strongly agree). In the second item, they were asked to record their “device of choice” (NIO® or EZ-IO®). Data was collected anonymously.
Data Analysis
Sample Size Calculation
In a previous crossover study comparing the BIG® versus the EZ-IO®, the difference in success rate between the devices was 31% (65.5% vs 96.5%, respectively).Citation12 We estimated that a minimum of 47 participants would be required to detect a 20% difference in success rate between the devices with a power of 90% and α of.05.
Statistical Analysis
As this was a crossover trial, pairing was taken into account in the statistical analysis. McNemar's test was used for comparing the success rate of the NIO and the EZ-IO. A two-sided Wilcoxon signed rank test was used for comparing the scoring of the “ease-of-use.” All statistical analysis was performed using SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0, Armonk, NY: IBM Corp).
Results
A total of 54 medical students were randomized into two groups and 4 declined to participate, thus 50 medical students were included in the study. The NIO®-first group consisted of 24 participants with a mean participant age of 21.7 years (±1) and 8:16 female: male ratio. The EZ-IO®-first group had 26 participants with a mean participant age of 21.7 years and a 9:17 female: male ratio.
Successful IO Insertion Attempts
Participants had a similar single-attempt success rates with the NIO® compared to the EZ-IO®, 92% versus 88% respectively (p = NS) ().
Table 1. Results of primary and secondary outcome measures.
Unsuccessful Attempts
Four participants used the NIO® unsuccessfully: two participants were unable to properly fixate the NIO® to the bone, thus the device safety mechanism prohibited the spring release; one participant pulled out the IO needle while trying to remove the stylet; the fourth participant was unable to attach the syringe to the IO needle. 6 participants were unsuccessful with the EZ-IO®: three participants dislodged the IO needle while trying to unplug the driller stylet; two participants drilled in a non-perpendicular angle thus placed the needle tangentially to the marrow; and one participant was unable to unplug the driller stylet. We noted no technical malfunction in either device.
Assessment of ”Ease-of-Use” and Device Preference
A Similar “Ease-of-use” rating was given for both the NIO® and the EZ-IO® ().
A total of 46% of the participants preferred the NIO®, while 54% preferred the EZ-IO®. The difference between the groups was not statistically significant ().
Discussion
In this randomized crossover study with blinded success assessment, we found comparable IO access success rates between the EZ-IO® and a new spring loaded device, the NIO® in a novice population (88% vs. 92%, respectively, p = NS). These results persisted when only the success rate of the first device used was calculated. We also did not find a difference in ease of use ratings or device preference by our study participants. These results suggest that even in the hands of a novice population, both devices may be equally effective in achieving intraosseous access.
Our findings point to an improvement of the NIO® over its predecessor, the BIG® with success rates of 65.5%Citation12 in comparison to 92% in the current study. In that previous study by Shavit et al., 6 out of 10 BIG® failures were due to a “stuck” stylet, where participants were unable to remove the stylet from within the IO needle.Citation12 In our study there was only one occurrence of such a “stuck” stylet out of 4 NIO® failures. This adjustment in device mechanism might account for the improved results of the NIO®. Three EZ-IO® failures were attributed to dislodgement of the needle when attempting to remove the stylet. Recently, Pasley et al. also reported placement issues in 3 out of 16 EZ-IO® attempts in a cadaveric modelCitation13 suggesting that thickness of soft tissues overlying the tibia insertion site may play a role in the EZ-IO® dislodgement especially in the hands of novice users.
The results of equal device effectiveness are similar to a study conducted on a manikin simulator which found identical success rates between the NIO® and EZ-IO®.Citation14 The relatively high success rate using both devices in our study is encouraging as we previously demonstrated relatively poor in-field success rates of 50% with the BIG®.Citation7 Recently, a large retrospective series of over 1000 IO attempts in military casualtiesCitation15 displayed very high success rates of IO access. However, it should be noted that approximately 75% of the IO attempts in this series were undertaken by a helicopter evacuation team or at field hospitals, probably where a skilled and experienced user was available,Citation15 unlike our study population of inexperienced future point of injury care givers. Macnab et al.Citation16 displayed similar overall success rates by all users and 74% novice success rates with the sternal FAST1® (Pyng Medical Corporation, BC, Canada) IO device.
The study population consisted of medical students without any previous experience with establishing IO access. The technique of achieving IO access was easily acquired by them as the high success rates with both devices show. Previous studies have also demonstrated similar success rates between paramedics and less skilled advanced emergency medical technicians in the field.Citation17 It is noteworthy that all participants are designated future advanced life support providers of the IDF-MC. We believe that this chosen novice study population well represents the IDF-MC field physicians and paramedics, which often lack any experience in IO placement when attempting to achieve vascular access in a casualty at the point of injury. Conducting our study on this population enabled us to devoid a previous-experience bias, which could have significantly affect the results of such a study comparing a new device (NIO®) to a more commonly used one (EZ-IO®). Moreover, users were instructed by study investigators while adhering to the NIO® and EZ-IO® official instruction and practice guidelines. Thus, the skill acquisition process performed in our study represented the actual process which novice IO users undergo, allowing for both the reliability of our design and reproducibility of results.
Our study has a few limitations. A non-human bone model stripped of the overlying skin and meat was used in the assessment the IO devices. However, as all participants practiced on the porcine model beforehand and the randomized trial design, we believe that the use of this model did not alter the primary outcome measure of comparing between the two devices. Moreover, the chosen porcine IO model is commonly accepted for IO access evaluationCitation18–20 and allowed for a high quality success assessment () while providing the participants with a more realistic simulation with tactile feel. Practice sessions ended when each of the participants felt confident enough to perform the procedure and this may be a potential limitation as they may have practiced in different amounts. However, the randomization process addresses potential competency differences between the study groups and participants. The tibia IO model is also compatible with the current IDF-MC clinical practice guidelines of IO insertion with place the tibia as the initial site of insertion.Citation9 Additionally, our study was designed to compare success rates only in a single IO insertion attempted. The EZ-IO®, in contrast to the NIO®, allows for multiple attempts while using the same IO needle. We designed the study in this way as we expected a high IO access success rate in a single attempt with the EZ-IO® as previously demonstrated.Citation6,12,21 Our results may have been different if participants attempted insertion multiple times. Finally, the use of the IO devices was conducted in a “sterile,” safe laboratory environment, which does not represent the stressful environment of real life prehospital resuscitation.
The strengths of the study include a blinded assessment of success and its randomized crossover design which eliminated any potential investigator bias. Previous studies were not blindedCitation21,22 and some used manikin simulators as the chosen IO model.Citation14 We also used a new needle and device and IO model with each attempt in order to avoid any technical device malfunction and stylet blunting, better depicting real life resuscitation. This was not adhered to in previous study designs.Citation22 In conclusion, we found comparable IO access success rates between the EZ-IO® and the NIO® novice users in a porcine model. These results warrant future point of injury studies with the NIO® in human casualties.
ORCID
Erez Nissim Baruch http://orcid.org/0000-0001-6001-6598
References
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: Executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. Nov 3 2015;132(18 Suppl 2):S315–67.
- Khalifa GEA, Alfonzo A, Arntz H-R, et al. European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015(95):1–80.
- TCCC Co. Tactical Combat Casualty Care Guidelines for Medical Personnel 2015. Available at: https://www.jsomonline.org/TCCC.html. Accessed April 6, 2016.
- Weiser G, Hoffmann Y, Galbraith R, Shavit I. Current advances in intraosseous infusion–a systematic review. Resuscitation. 2012;83(1):20–26.
- Santos D, Carron PN, Yersin B, Pasquier M. EZ-IO((R)) intraosseous device implementation in a pre-hospital emergency service: a prospective study and review of the literature. Resuscitation. Apr 2013;84(4):440–5.
- Helm M, Haunstein B, Schlechtriemen T, Ruppert M, Lampl L, Gassler M. EZ-IO((R)) intraosseous device implementation in German Helicopter Emergency Medical Service. Resuscitation. Mar 2015;88:43–7.
- Nadler R, Gendler S, Chen J, Lending G, Abramovitch A, Glassberg E. The Israeli Defense Force experience with intraosseous access. Milit Med. 2014;179(11):1254–7.
- Research Randomizer (Version 4.0) [Computer software]. [computer program]. Available at: http://www.randomizer.org/2013. Accessed August 22, 2015.
- Israel Defense forces Medical Corps Trauma and combat medicine branch, The IDF Medical Corps, Surgeon General Headquarters. Intraosseous device use guidelines- Mabat Trauma edition 106 In: Corps TIDFM, 2016.
- Schlimp CJ, Solomon C, Keibl C, et al. Recovery of fibrinogen concentrate after intraosseous application is equivalent to the intravenous route in a porcine model of hemodilution. J Trauma Acute Care Surg. May 2014;76(5):1235–42.
- Uwaydah NI, Hoskins SL, Bruttig SP, et al. Intramuscular versus intraosseous delivery of nerve agent antidote pralidoxime chloride in swine. Prehosp Emerg Care. Jul-Aug 2016;20(4):485–92.
- Shavit I, Hoffmann Y, Galbraith R, Waisman Y. Comparison of two mechanical intraosseous infusion devices: a pilot, randomized crossover trial. Resuscitation. 2009;80(9):1029–33.
- Pasley J, Miller CH, DuBose JJ, et al. Intraosseous infusion rates under high pressure: a cadaveric comparison of anatomic sites. J Trauma Acute Care Surg. 2015;78(2):295–9.
- Szarpak L, Czyzewski L, Woloszczuk-Gebicka B, Krajewski P, Fudalej M, Truszewski Z. Comparison of NIO and EZ-IO intraosseous access devices in adult patients under resuscitation performed by paramedics: a randomized crossover manikin trial. Am J Emerg Med. 2016;34(6):1166–7.
- Lewis P, Wright C. Saving the critically injured trauma patient: a retrospective analysis of 1000 uses of intraosseous access. Emerg Med J. Jun 2015;32(6):463–7.
- Macnab A, Christenson J, Findlay J, et al. A new system for sternal intraosseous infusion in adults. Prehosp Emerg Care. Apr-Jun 2000;4(2):173–7.
- Wolfson DL, Tandoh MA, Jindal M, Forgione PM, Harder VS. Adult intraosseous access by advanced EMTs: A statewide non-inferiority study. Prehosp Emerg Care. Aug 5 2016:1–7.
- Strandberg G, Eriksson M, Gustafsson MG, Lipcsey M, Larsson A. Analysis of intraosseous samples using point of care technology–an experimental study in the anaesthetised pig. Resuscitation. Nov 2012;83(11):1381–5.
- Larabee TM, Campbell JA, Severyn FA, Little CM. Intraosseous infusion of ice cold saline is less efficacious than intravenous infusion for induction of mild therapeutic hypothermia in a swine model of cardiac arrest. Resuscitation. May 2011;82(5):603–6.
- Wong MR, Reggio MJ, Morocho FR, et al. Effects of intraosseous epinephrine in a cardiac arrest swine model. J Surg Res. Apr 2016;201(2):327–33.
- Hammer N, Mobius R, Gries A, Hossfeld B, Bechmann I, Bernhard M. Comparison of the fluid resuscitation rate with and without external pressure using two intraosseous infusion systems for adult emergencies, the CITRIN (Comparison of InTRaosseous infusion systems in emergency medicINe)-Study. PloS one. 2015;10(12):e0143726.
- Levitan RM, Bortle CD, Snyder TA, Nitsch DA, Pisaturo JT, Butler KH. Use of a battery-operated needle driver for intraosseous access by novice users: skill acquisition with cadavers. Ann Emerg Med. Nov 2009;54(5):692–4.