Abstract
Objective: Drug dosing errors pose a particular threat to children in prehospital emergency care. With the Pediatric emergency ruler (PaedER), we developed a simple height-based dose recommendation system and evaluated its effectiveness in a pre–post interventional trial as the Ethics Committee disapproved randomization due to the expected positive effect of the PaedER on outcome. Methods: Pre-interventional data were retrospectively retrieved from the electronic records and medical protocols of the Cologne Emergency Medical Service over a two-year period prior to the introduction of the PaedER. Post-interventional data were collected prospectively over a six-year period in a federal state-wide open trial. The administered doses of either intravenous or intraosseous fentanyl, midazolam, ketamine or epinephrine were recorded. Primary outcome measure was the number and severity of drug dose deviation from recommended dose (DRD) based on the patient's weight. Results: Fifty-nine pre-interventional and 91 post-interventional prehospital drug administrations in children were analyzed. The rate of DRD > 300% overall medications were 22.0% in the pre- and 2.2% in the post-interventional group (p < 0.001). All administrations of epinephrine occurred excessive (DRD > 300%) in pre-interventional and none in post-interventional patients (p < 0.001). Conclusions: The use of the PaedER resulted in a 90% reduction of medication errors (95% CI: 57% to 98%; p < 0.001) and prevented all potentially life-threatening errors associated with epinephrine administration. There is an urgent need to increase the safety of emergency drug dosing in children during emergencies. A simple height-based system can support health care providers and helps to avoid life-threatening medication errors.
Introduction
Medication errors are a major source of morbidity and mortality in both adults and children.Citation1,2 Potential adverse drug events (ADEs) are three times more common in children when compared with adults.Citation3 The main reasons are the necessity of individual dose calculationCitation4 and the lack of dose familiarity. An unintentional tenfold higher amount of a drug solution is commonly administered during resuscitationCitation5,6 and in the case of epinephrine is likely fatal.Citation7 It is impossible to provide a specialized pediatric team during prehospital emergency careCitation8 and these scenarios occur in unpredictable circumstances and locations. In addition, the pediatric caseload of prehospital emergency teams is too low in order to generate sufficient experience and expertiseCitation8 and most prehospital health-care providers do not feel comfortable with their abilities to administer a correct dose for small children and infants.Citation9 It is, therefore, not surprising, that the worst medication errors repeatedly occur during the prehospital emergency care of children.Citation10
Several measures are available to reduce medication errors in pediatric emergencies with a reduction of cognitive input requirements to calculate drug doses as the main principle.Citation11 Systems for length-based dosing recommendations have the advantage to combine the most reliable method to estimate a child's unknown weightCitation12 with a reference for drug doses, estimated sizes for airway equipment and normal physiological values. The first device that offered such was the Broselow Pediatric Resuscitation System (known as “Broselow-Tape” (BT), Armstrong Medical Industries Inc., Lincolnshire, IL, USA) and has repeatedly been shown to have a positive impact on medication errors in simulated resuscitations.Citation13 However, the only preclinical study published focused on an improvement of the rates within 20% dose deviations and mentions a not precisely reported reduction of tenfold errors.Citation14 Nevertheless, such errors still occurred and difficulties in using this system have been described.Citation15 Since the BT was never soldCitation16 nor licensed as a medical product in Europe it was additionally unsuitable for its use in Europe. We therefore developed and introduced a certified and licensed length-based dosing recommendation system the “Pediatric emergency ruler” (PaedER; Alpha 1 e.K., Falkenberg, Germany) in 2008. Before the development, we determined the requirement on this device, that all information must be available directly on one spot and for administration of an adequate dose and volume of each drug, no further calculation steps arenecessary.
Therefore, we hypothesized that such a length-based dosing recommendation systems can reduce the rate of life-threatening dosing errors in preclinical pediatric emergencies. A deviation from the recommended dose (DRD) > 300% is considered as a life-threatening overdose, especially for epinephrine and is explicit beyond the recommendations of the actual guidelines.Citation8 We tested this hypothesis in a pre-post interventional observational trial.
Method
Development of the Pediatric Emergency Ruler (PaedER)
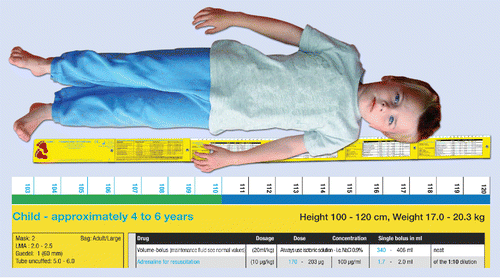
The weight and length distribution of German children was collected in a large national survey (KiGGS-study),Citation17 but the weight-for-length correlation remains to be published. Analysis of the raw data by the principal statistician of this census confirmed that the weight-for-length percentiles of German children were identical to data available in the US (H. Stolzenberg, personal communication). Therefore, data provided by the American Centers for Disease Control, and PreventionCitation18 were used, to determine the best length-weight estimation. Considering the decelerating proportional weight gain related to the absolute length gain, appropriate segments on the PaedER were chosen (). In the ruler segment at the head-end, information about normal values for age and weight, intubation material and sizes and weight-adjusted doses and volumes for the emergency drugs are provided with no further calculations required (). Due to the potential impact of the ruler on medical treatment, the national authority for medical products (BfArM) classified this product as a medical device. Therefore, the PaedER was Europe-wide registered as medical product (class 1m, non-invasive with measurement function) after compliance with legal requirements and certificated by a notified body of the European Union (DQS MED, identification number 0297). The PaedER holds a granted patent by the European Design and Patent Agency (Office for Harmonization in the internal marked – OHIM; No 002909382–0001). An English version of the PaedER is under preparation and currently submitted for licensing for international distribution.
Table 1. Length-segments and associated estimated weights of the PaedER
Figure 1. Illustration for the use of the Pediatric Emergency Ruler (PaedER): The supine child is measured with the unfolded ruler from the heel over the straightened leg to the head, where the height is displayed. Normal values for age, size to tracheal tubes, and weight adjusted doses for the emergency drugs are provided at the head end. The lower part of the figure contains a translated excerpt from the table.
Study Design, Outcome Measures, and Committee Review
We conducted a pre–post interventional study in prehospital pediatric emergencies to assess the impact of the PaedER on the correct administration of drug doses with emphasis on life-threatening medication errors. All doses of the investigated drugs were evaluated as percentage with respect to the recommended dose (defined in ). Primary outcome was the rate of DRD of more than 20% and more than 300% with respect to the medications fentanyl, ketamine (including esketamine), midazolam or epinephrine. Deviation from the recommended dose (DRD) greater than 20% (<80% or >120% of the recommended dose) were classified as errors and larger than 300% (<33% or >300% of the recommended dose) as life-threatening errors.
Table 2. Drug doses as deviations from the recommended dose
Table 3. Demographic data
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional Ethics Committee and with the 1964 Helsinki declaration and its later amendments. The trial was approved by the Ethics Committee of the University of Cologne (file reference 08–161) with parental consent waived due to a lack of feasibility and no expected harm. However, due to an expected positive impact of the PaedER, the Committee disapproved randomization or just recruitment into a cohort without the use of the PaedER. Therefore, the study used a historical control group, collecting all electronic datasets and hand-written medical protocols of all children (<18 years) who were treated by Cologne Emergency Medical Service (EMS) during a 20-month period in 2007 and 2008 immediately prior to the development of the PaedER. Missing patient's weights were retrieved from hospital records or the department of forensic medicine, whenever available. Prospective data collection of the post-interventional part following the introduction of the PaedER into the clinical services were planned with the participation of four different EMS´s (Cologne, Leverkusen, Bonn and Aachen). Although these study centers attend more than 900 pediatric emergencies per annum combined, recruitment numbers were insufficient. The IRB approved a change of the study design to extend to federal state recruitment and permitted a personal remuneration of 25.- Euro for each EMS physician completing a study form.
For the prospective post-interventional part, every pediatric patient (<18 years) with at least one of the above medications using the PaedER was eligible for inclusion. Initial data were entered into a secure database with an individual access code. Further entries were possible using a secured web gateway on a non-public server (ANIMANIACS GmbH, Cologne, Germany). In addition, all included patients had their original medical protocol uploaded to verify the authenticity of the data. As requested by the Ethics Committee, all person identifiable data were deleted once reviewed and confirmation of each full data set was completed. The trial was registered at the International Clinical Trials Registry platform (DRKS00000502, date of registration 2010/07, recruitment from 2010/08 until 2015/08).
Statistical Planning and Analysis
The sample size was calculated based on the assumption that the use of PaedER would half the number of drug errors (DRD >20%). Therefore, a prospective group of 200 (50 for each studied drug) was planned (Chi-square-test; power 0.97; alpha 0.05). Qualitative data were summarized by count and percentage, quantitative data by mean (standard deviation) or median (minimum to maximum), contingent on apparent skewness. Differences concerning demographic data were evaluated by two-sample t-test. Group differences between rates of drug deviations were evaluated by two-tailed Fisher´s exact test. If repeated administrations of the same drug were documented in the same patient, only the initial dose was included. When a patient received different drugs, each of the drugs was analyzed separately. Analyses were performed using SPSS 21 (IBM Corp., Armonk, NY, USA) and Stata/SE 12.1 (StataCorp LP, College Station, TX, USA).
Results
In the historical group 437 children were identified that received intravenous or intraosseous medications during preclinical emergency care. Only 2 children (0.5%) had a weight documented on their prehospital medical record. It was possible to identify a further 37 patients with a measured weight identifiable from the treating hospital or department of forensic medicine, receiving 59 administrations of a distinct dose of the observed medications. The prospective observational part included 60 patients with 91 distinct drug administrations (). Children treated in the prospective group were significant smaller and lighter ().
Figure 2. Study flow chart (PaedER - Pediatric emergency ruler; i.v. – intravenous; i.o. intraosseous).
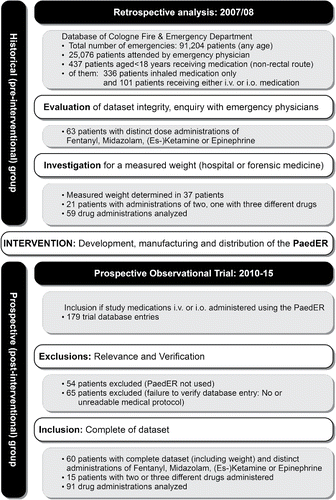
The use of the PaedER resulted in a reduction of life-threatening dosing errors (DRD > 300%) when compared with the retrospective group (). The PaedER prevented nine out of ten of these errors (2.2% vs 22.2% of all drug administrations; RRR 90%, 95% CI: 57% to 98%; p < 0.001). Whereas all administrations of epinephrine in the historical group were with life-threatening dosing errors, no such episodes were registered in the post-interventional study part. No difference was observed for administrations with a DRD > 20 %.
Discussion
The use of the PaedER resulted in a tenfold reduction of life threatening medication errors and prevented all life-threatening errors associated with epinephrine administration. Although a DRD of 20% is most commonly used as a definition of drug dose errorCitation10,14,19,20 it is hardly conceivable, that such an deviation could really cause harm. Whereas the 10-fold amount of epinephrine (DRD of 1,000%) is known to threaten the survival,Citation7,21 the threshold of an overdose to cause harm or missing effect due to a too low dose is unknown. Nevertheless, following clinical experience, one third of or the three times the recommended dose of any drug is likely to cause a clinical meaningful adverse effect. With regards to epinephrine, such an amount is clearly beyond the recommendations for patients of any age, and all international guidelines explicitly caution against such doses.Citation8,22 Therefore, we selected this threshold (DRD > 300%) as a dose error to be potentially life-threatening. It would be highly desirable to define an universally acceptable clinically relevant DRD for each drug in future studies. The absence of such definitions is the main reason why meta-analyses for medication errors are currently limited or not possible.Citation11,23
The beneficial impact of simple measures on life-threatening errors was repeatedly shown in simulated scenarios. These include simple weight based tables that provide dose recommendations. This prevented 90% of tenfold errors when compared to not using this tabular aid.Citation24 Nevertheless, length-based devices are superior to other dose recommendation sources due to their greater abilities. First of all, they offer a better weight estimation method than age-related estimationCitation12 and provide the average weight for a certain height, which is correlating closely to the lean body weightCitation25 and the extracellular fluid compartment for the volume of distribution for hydrophilic drugs.Citation26 The reliability and feasibility of this weight-estimation method was proven for the preclinical setting.Citation27 The knowledge of age-related physiological values is also essential to guide resuscitationCitation28 and was included in the PaedER. It was grouped together with all other length-based recommendations negating the need to look them up in emergency situations.
This current evaluation of the PaedER is a real-life clinical trial for a device designed to improve the prehospital pediatric emergency care explicitly addressing potentially life-threatening drug dosing errors. Another trial preclinical evaluated a height-based drug dose recommendation system, but focused on DRD > 20%,Citation14 failing to provide detailed information on errors with a potentially significant clinical impact. The use of the PaedER avoided 9 out of 10 life threatening DRD (> 300%) when compared with pre use data in the same clinical setting. This observation was not solely attributable to the total avoidance of such DRD with epinephrine, since a summary analysis of all other drug excluding epinephrine was also highly significant (). The lack of significance during analysis of each drug separately was due to the number being too small for subgroup analysis.
One patient in the pre-interventional group (8 months old weighing 8 kg, without any underlying disease) received an initial dose of intra-osseous epinephrine with a DRD of 2,500% (250 µg/kg). Despite successful tracheal intubation and ventilation, the child died. This epinephrine DRD was likely to have contributed to this outcome. Avoidance of all life-threatening DRD with the use of the PaedER may contribute to saving children's lives.
The reported DRD > 20% in this study was high but is consistent with other results.Citation10,14 The primary reason is the individual response of patients to analgesic or sedative medications. The initial recommended dose of the PaedER is based on safety avoiding potentially compromising the patient and should be titrated until an adequate effect is achieved. Since several study participants report the total dose of analgesics and sedatives and not the initial intended dose, the high rate of DRD > 20% is likely due to individual titration and, therefore, clinically insignificant. The administration of epinephrine during resuscitation, however, does not follow this titration principle but requires a specified amount. This underlines that epinephrine DRD > 300% are clinically meaningful for patient's safety. Regardless of the drugs investigated, a threefold amount must be accepted as potentially harmful.
Although not a primary outcome measure, the lack of pre-interventional weight documentation of only 0.5% is alarming. The standard medical record for preclinical emergencies used by the German interdisciplinary federation for intensive care and emergency medicine (DIVI) does not contain weight entry and requires further clarification and amendment.Citation29
This trial has several limitations. First, the Ethics Committee disapproved randomization within a clinical trial. The Committee assumed a benefit of the PaedER a priori and hence unethical to withhold this in children in preclinical emergencies. The gap between the pre-intervention group of 2007/08 and the start of the prospective observation (2010–15) was attributable to the extensive requests from national authorities and the need to certify and distribution of the device. Nevertheless, no other measures concerning preclinical medication safety were implemented between 2007 and 2015. The pre-interventional data were collected from only one single Emergency Medical Service; the prospective data were collected from one federal state. Nevertheless, German federal state regulates standardized qualifications for emergency physicians supervised by the medical council and uniform structural specifications of EMS are governed by federal law. Therefore, the different areas are comparable. Of all 101 patients that received intravenous or intraosseous medications in the historical control group, 63 received a distinct drug dose of one of the study medications. Of them, a measured weight could just be determined in 37 patients (59%, ), making a sampling bias for this selection possible. Despite a wide distribution of the PaedER (> 15,000 copies in Germany), the recruitment and collection of data was unexpectedly difficult. With no study coordinators at each EMS, emergency physicians were reluctant to enter data into a secure database. Therefore, a reporting bias can be assumed with only enthusiastic emergency physicians with a special interest in drug safety in pediatric emergencies participating. Also the payment of 25 Euros for completing study forms in the prospective part of the trial is a potential source of bias. Both influences could have resulted in an overestimation of the impact of the PaedER. There are two other possibilities for bias, which may have resulted in an underestimation of the effect of the ruler: The children in the interventional group were significantly younger and smaller and also required more resuscitation interventions (). The ambitious sample size calculation was attributable to the lack of comparable data.
There are inherent difficulties in conducting prospective trials in real-life prehospital pediatric emergencies and these are the main reasons as to why similar trials are rare. The main problem is the relatively small proportion of pediatric emergencies requiring emergency drug administration in comparison to the total case load of EMS´s. Although simulation studies do not have such problems, they do not reproduce the real life scenarios.
We were unable to assess the impact of the observed dose errors on the outcome (like harm or survival) due to the study design. Further clinical trials would be highly desirable to investigate a correlation between drug errors and such outcomes, which simulation studies are on principle unable to report. Nevertheless, safe pediatric emergency care surely requires more than a single but multiple measures to enhance the quality, including thorough training programs.Citation30 Currently, only a German version of the PaedER is available, referring to the drug preparations available in Germany and fulfilling the requests from national authorities. For using the PaedER in other countries, an adaption to the local conditions would be necessary. Additionally, other legislation may exist in other countries and need to be followed accordingly.
In summary, despite of the study limitations the presented data provide evidence for the use of preventive measures for the avoidance of medication errors in real-life pediatric emergencies. This study will, therefore, contribute to increased vigilance of the emergency health care providers and provide the basis for further investigations.
Conclusion
This study demonstrated that there is still an urgent need to increase the awareness of emergency drug dosing errors in children. A simple height based tool was suitable to prevent most life-threatening preclinical pediatric emergency care medication errors and was a simple device to estimate children´s weight if a documented weight is not available. Further studies, also addressing the impact on harm or survival as outcome measures, are urgently needed.
Additional information
Funding
References
- Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. NE J Med. 1991;324(6):377–84.
- Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington, DC: National Academy Press, 1999.
- Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–20.
- Davis T. Paediatric prescribing errors. Arch Dis Child. 2011;96(5):489–91.
- Kozer E, Scolnik D, Jarvis AD, Koren G. The effect of detection approaches on the reported incidence of tenfold errors. Drug Saf. 2006;29(2):169–74.
- Kozer E, Seto W, Verjee Z, et al. Prospective observational study on the incidence of medication errors during simulated resuscitation in a paediatric emergency department. BMJ (Clinical Research ed.). 2004;329(7478):1321.
- Perondi MB, Reis AG, Paiva EF, Nadkarni VM, Berg RA. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. The New England journal of medicine. 2004;350(17):1722–30.
- Maconochie IK, Bingham R, Eich C, et al. European Resuscitation Council Guidelines for Resuscitation 2015. Section 6. Paediatric life support. Resuscitation. 2015;95:223–48.
- Hoyle JD, Sleight D, Henry R, Chassee T, Fales B, Mavis B. Pediatric prehospital medication dosing errors: a mixed-methods study. Prehosp Emerg Care. 2016;20(1):117–24.
- Hoyle JD, Davis AT, Putman KK, Trytko JA, Fales WD. Medication dosing errors in pediatric patients treated by emergency medical services. Prehosp Emerg Care. 2012;16(1):59–66.
- Kaufmann J, Laschat M, Wappler F. Medication errors in pediatric emergencies: a systematic analysis. Deutsches Arzteblatt international. 2012;109(38):609–16.
- Krieser D, Nguyen K, Kerr D, Jolley D, Clooney M, Kelly AM. Parental weight estimation of their child's weight is more accurate than other weight estimation methods for determining children's weight in an emergency department? Emerg Med J. 2007;24(11):756–9.
- Shah AN, Frush K, Luo X, Wears RL. Effect of an intervention standardization system on pediatric dosing and equipment size determination: a crossover trial involving simulated resuscitation events. Arch Pediatr Adolesc Med. 2003;157(3):229–36.
- Kaji AH, Gausche-Hill M, Conrad H, et al. Emergency medical services system changes reduce pediatric epinephrine dosing errors in the prehospital setting. Pediatrics. 2006;118(4):1493–500.
- Rappaport LD, Brou L, Givens T, et al. Comparison of errors using two length-based tape systems for prehospital care in children. Prehosp Emerg Care. 2016:1–10.
- Armstrong_Medical_Industries_Inc. “US shipping only”. Available at: https://www.armstrongmedical.com/index.cfm/go/product.detail/sec/3/ssec/14/fam/150. Accessed September 7, 2016.
- Stolzenberg H, Kahl H, Bergmann KE. [Body measurements of children and adolescents in Germany. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)]. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2007;50(5–6):659–69.
- WHO growth standards (growth for infants and children ages 0 to 2 years of age in the U.S); CDC growth charts (for children age 2 years and older in the U.S). Available at: http://www.cdc.gov/growthcharts/. Accessed September 7, 2016.
- Vila-de-Muga M, Colom-Ferrer L, Gonzalez-Herrero M, Luaces-Cubells C. Factors associated with medication errors in the pediatric emergency department. Ped Emerg Care. 2011;27(4):290–4.
- Rinke ML, Bundy DG, Velasquez CA, et al. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014;134(2):338–60.
- Sharman M, Meert KL. What is the right dose of epinephrine? Pediatr Crit Care Med. 2005;6(5):592–4.
- Wyllie J, Bruinenberg J, Roehr CC, Rüdiger M, Trevisanuto D, Urlesberger B. European Resuscitation Council Guidelines for Resuscitation 2015. Section 7. Resuscitation and support of transition of babies at birth. Resuscitation. 2015;95:249–63.
- Maaskant JM, Vermeulen H, Apampa B, et al. Interventions for reducing medication errors in children in hospital. Cochrane database of systematic reviews (Online). 2015;3:Cd006208.
- Bernius M, Thibodeau B, Jones A, Clothier B, Witting M. Prevention of pediatric drug calculation errors by prehospital care providers. Prehosp Emerg Care. 2008;12(4):486–94.
- Young KD, Korotzer NC. Weight estimation methods in children: a systematic review. Ann Emerg Med. 2016;68(4):441–51.
- Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokin. 2005;44(10):1051–65.
- Heyming T, Bosson N, Kurobe A, Kaji AH, Gausche-Hill M. Accuracy of paramedic Broselow tape use in the prehospital setting. Prehosp Emerg Care. 2012;16(3):374–80.
- Chambers IR, Jones PA, Lo TY, et al. Critical thresholds of intracranial pressure and cerebral perfusion pressure related to age in paediatric head injury. J Neurol Neurosurg, Psych. 2006;77(2):234–40.
- DIVI. Medical record for the documetation of preclinical emergencies - Version 5.0. [ Available at: http://www.divi.de/empfehlungen/notarztprotokoll-mind.html. Accessed Sep-tember 7, 2016.
- Shah MI, Carey JM, Rapp SE, et al. Impact of high-fidelity pediatric simulation on paramedic seizure management. Prehosp Emerg Care. 2016:1–9.
