Abstract
Study Objective: National Park Service (NPS) Parkmedics provide medical care in austere environments. The objective of this study was to evaluate the stability of specific medications used by Parkmedics at extremes of temperatures likely to be faced in the field. Methods: This is a bench research study conducted in the laboratory setting over a 4-week period. Parenteral medications were separated into 4 temperature exposure groups: A) 45°C (hot); B) −20°C (cold); C) hot then cold temperatures alternating weekly; and D) cold then hot temperatures alternating weekly. At study start and the end of each week, three aliquots from each group were sampled to determine the remaining drug concentration through liquid chromatography-quadrupole time-of-flight mass spectrometry (Agilent LC 1260- QTOF/MS 6550). Quantitative analysis was done using Agilent MassHunter Quantitative Analysis software. The mean drug concentration from triplicate aliquots was expressed as percentage of its baseline concentration to monitor the drug's stability during storage. Results: Eight medications were analyzed (atropine, diphenhydramine, fentanyl, hydromorphone, midazolam, morphine, naloxone, ondansetron). Hydromorphone, morphine, and ondansetron showed the greatest stability, at above 90% of original concentration in all study arms. Diphenhydramine, fentanyl and midazolam showed heat independent degradation, degrading the same way regardless of heat exposure. By the end of the study period, 51–56% midazolam remained in all groups. Atropine and naloxone showed heat dependent degradation, degrading more when exposed to heat. Atropine had the most degradation, being undetectable after 4 weeks of heat exposure. Conclusions: We recommend that EMS providers replace atropine, naloxone, diphenhydramine, fentanyl, and midazolam frequently if they are practicing in low call volume or high-temperature environments. Further studies will be needed to determine if re-dosing midazolam, naloxone, and atropine is the appropriate clinical strategy in this setting if adequate clinical effect is not reached with a single dose.
Introduction
The National Park Service (NPS) utilizes park rangers with medical training (Parkmedics) to provide medical care in austere environments. A Parkmedic's training is at an advanced level Emergency Medical Technician (AEMT), but with an expanded pharmacological and procedural scope of practice reflecting their wilderness practice environment.Citation1 Parkmedics carry parenteral medications to treat a variety of backcountry injuries and illnesses (). During the course of the year, these medications are subject to extreme temperature variations. At our affiliated national parks, Sequoia and Kings Canyon, the coldest temperatures at middle elevations in January are −4°C on average and −21°C at extremes, while the warmest temperatures in the foothills in July are 36°C on average and 46°C at extremes.Citation2 Parkmedics may carry medications from three months to one year until they are used or expired.
Table 1. Medications in National Park Services Parkmedic Kit
Evidence of the effect of prehospital medication storage on drug stability is mixed. In a study by Johansen et al., atropine, naloxone, lidocaine, and epinephrine did not show significant alteration in chemical structure after exposure to temperatures of −20°C, 70°C, or a combination of these two temperatures for four-hour periods.Citation3 De Winter et al. examined refrigerated medications stored at room temperature or exposed to ambient temperature in EMS vehicles for up to a year, finding significant degradation in lorazepam and succinylcholine as early as 4 weeks.Citation4
Temperature is clearly an important variable in drug stability.Citation4,5 It is unknown to what extent Parkmedic medications are affected by prolonged exposure to extreme temperatures. Significant degradation may impact the clinical effectiveness of the drug, and changes in dosage, storage, or frequency of replacement may be required. The medications tested in this study are carried by many EMS agencies; therefore, our results could apply to any location at the extremes of hot or cold or with wide temperature variability.
The objective of this study was to evaluate the stability of specific medications used by Parkmedics at extremes of temperatures likely to be faced in the field. Our primary outcome measure was the percentage of original medication remaining in each study sample at weekly intervals over a four-week study period.
Methods
Study Design
This is an original bench research study conducted in the laboratory setting. It was approved by our local Institutional Review Board.
Study Protocol
Parkmedic drugs met study inclusion criteria if they were formulated for immediate parenteral use (not requiring reconstitution) and were amenable to analysis by liquid chromatography–quadrupole time-of-flight mass spectrometry (LC-QTOF/MS) [small organic compounds (75–1000 amu) that could be ionized using a soft ionization technique such as electrospray ionization]. Samples of Parkmedic drugs used in the study were provided by Sequoia and Kings Canyon National Parks. Medications were purchased by NPS staff simultaneously from pharmaceutical companies (), were not used in any Parkmedic field packs prior to the study and were not expired.
All solvents including acetonitrile and water were LC-MS grade and obtained from Honeywell Burdick and Jackson (Muskegon, MI, USA). Reference standards and deuterated internal standards used to quantify the drugs in the study were purchased from Sigma (St. Louis, MO, USA) and Cerilliant (Round Rock, TX, USA). Calibration standards for each drug were prepared directly from their respective reference and internal standards in 10% acetonitrile.
Figure 1. Study Design. A) Group A was at a constant temperature of 45 C (hot) and Group B was at a constant temperature of −20 C (cold) over they study period. B) Group C was subjected to hot then cold temperatures alternating weekly. C) Group D was subjected to cold then hot temperatures alternating weekly.
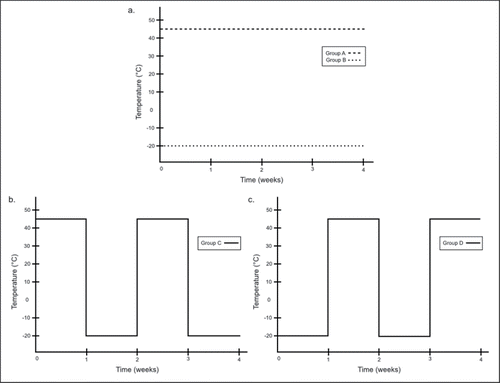
Figure 2. Compounds exhibiting stability. A) Hydromorphone, B) Morphine, and C) Ondansetron concentrations expressed as a percentage of week 0 concentration with standard error bars during each study week.
Each drug was initially divided into four equal 0.5 mL aliquots in 1 mL amber vials. Each aliquot was then incubated in one of four different storage conditions for four weeks: Group A) 45°C (hot); Group B) −20°C (cold); Group C) hot then cold temperatures alternating weekly; and Group D) cold then hot temperatures alternating weekly (). A heat bath was utilized for the hot and −20°C freezer for the cold storage conditions. Medications vials were removed from the heat bath and freezer at the end of each week and placed in the room temperature environment until frozen samples had thawed. At that time, three 10-uL aliquots from each amber vial were sampled to determine the remaining drug concentration through LC-QTOF/MS (Agilent LC 1260- QTOF/MS 6550, Santa Cruz, CA, USA). Sampling was done through the end of the fourth week of storage. A 10-uL aliquot of each drug was also sampled prior to the start of the stability study to determine baseline drug concentration (week 0).
Each 10-uL aliquot sampled during the stability study was dried and reconstituted with enough acetonitrile to make the equivalent of a 1-mg/mL (w/v) solution of the drug. From this stock solution, 100 ug/mL, 10 ug/mL, 1 ug/mL, and 200 ng/mL dilutions were prepared in 10% acetonitrile. The last dilution was used in the LC-QTOF/MS analysis, each of which was run twice.
In each LC-QTOF/MS run, a 2.5-uL diluted sample of each drug was injected in an Agilent Poroshell 120 C-18 column (2.1×100mm, 2.7 um) maintained at 55°C. Chromatographic separation was achieved by gradient elution using LC-MS grade water with 0.05% formic acid and 5 mM ammonium formate as mobile phase A and methanol with 0.05% formic acid as mobile phase B. The elution gradient employed was: 0–0.5 min = 5% B; 1.5 min = 30% B; 4.5 min = 70% B; 7.5 min = 100% B; 7.5–10 min = 100% B; and 10.01–12 min = 5% B.
Eluates from the chromatographic column were ionized in the QTOF/MS using an electrospray ionization source in positive polarity operated in the following conditions: gas temperature at 225°C; sheath gas temperature at 350°C; drying gas flow at 14 L/min; sheath gas flow at 11 L/min; nebulizer pressure at 14 psi; voltage cap at 3000 V; and, nozzle voltage at 500 V. Data acquisition was run at 2 GHz in extended dynamic range mode. Both TOF/MS and MS/MS spectra were collected in automated MS/MS mode using 500 arbitrary units as threshold for inducing MS/MS data collection.
Quantitative analysis of the drugs was run after each sampling time point. Quantification was done by isotope dilution method using an eight-point calibration curve that was run alongside aliquoted samples. A deuterated standard of each drug was used as an internal standard.
Data Analysis
To confirm the presence of each drug, the total ion chromatogram (TIC) obtained from the LC-QTOF/MS run was analyzed using Agilent MassHunter Qualitative Analysis software (Santa Cruz, CA, USA). All sample TICs were analyzed to confirm the presence of each drug using the “Find by Formula” algorithm. A database of 550 drugs including all Parkmedic drugs served as the reference for compound matching using the following criteria: mass error ≤ 10 ppm; retention time ≤ 0.15 min; target score ≤ 70 (indication of isotopic pattern match) for peaks that did not exhibit detector saturation; and, the presence of at least one fragment ion peak in its MS/MS spectra.
Quantitative analysis of each drug was done using Agilent MassHunter Quantitative Analysis software. The peak area ratios between varying amounts of each drug's reference standard to a fixed amount of its internal standard was plotted against known drug concentration to generate each drug's calibration plot. The drug concentration from each sample aliquot was then determined from the linearly fitted calibration plot. The mean drug concentration from triplicate aliquots obtained from a given drug at each storage condition was expressed as percentage of its baseline concentration to monitor the drug's stability during storage and standard errors were calculated.
Results
Of the 32 medications in the Parkmedic medication kit, 11 were parenteral and thus considered for analysis in this study. Of these 11, amiodarone, dexamethasone, and epinephrine were excluded from analysis, leaving 8 in the study group (atropine, diphenhydramine, fentanyl, hydromorphone, midazolam, morphine, naloxone, ondansetron). For the three excluded medications, the inter-batch precision of the calibrants were observed to have >20% CV (coefficient of variation); hence, data obtained from these drugs were deemed unreliable with our techniques.
Overall, hydromorphone, morphine, and ondansetron showed the greatest stability, remaining at greater than 90% of original concentration in all study arms (10% variation is the maximum accepted by the FDACitation17) (). Diphenhydramine, fentanyl, and midazolam showed heat independent degradation, degrading the same way regardless of heat exposure (). Atropine and naloxone showed heat dependent degradation, degrading more when exposed to heat (). Atropine had the most significant degradation; in all three of the study arms incorporated heat exposure.
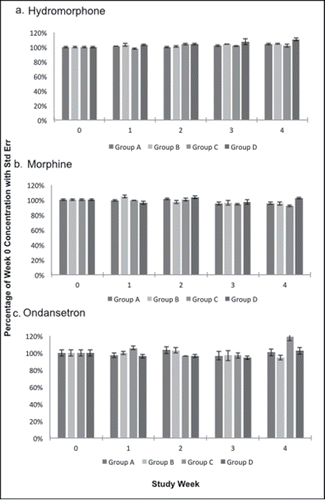
Figure 3. Compounds exhibiting heat independent degradation. A) Diphenhydramine, B) Fentanyl, and C) Midazolam concentrations expressed as a percentage of week 0 concentration with standard error bars during each study week.
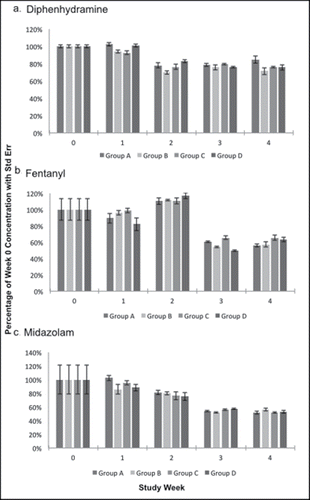
Figure 4. Compounds exhibiting heat dependent degradation. A) Atropine and B) Naloxone concentrations expressed as a percentage of week 0 concentration with standard error bars during each study week.
Three of eight compounds in Group A (heat exposure only), hydromorphone, morphine, and ondansetron, remained stable by the end of the study period (). Most notably, by week 2, group A atropine concentrations were negligible (). Naloxone was stable through week 2, then dropped to 73% of original concentration by week 3, and 39% of original concentration by week 4 (). About half of fentanyl and midazolam concentrations remained at week 4 (56% and 51%, respectively) ().
At the end of the study period, five of eight Group B compounds (cold exposure only) were stable: atropine, hydromorphone, morphine, naloxone, and ondansetron. Diphenhydramine was stable through week 1, then dropped to 71% of original concentration by week 4 (). Fentanyl and midazolam dropped to 57% and 56% of original concentration by week 4 (). In Group B, all compounds remained at greater than 55% of original concentration by the end of the study period.
When subjected to weekly intermittent hot and cold temperatures (Group C), hydromorphone, morphine, and ondansetron remained stable by the end of the study period (). Atropine showed the most degradation, with only 26% of the original concentration remaining by week 4 (). Diphenhydramine and naloxone remained stable through week 2, with week 4 concentrations at 76% and 55%, respectively (). Midazolam was also a poor performer, with significant degradation after week 1 and about half of the original concentration (52%) remaining at week 4 (). Fentanyl remained stable through week 3, with a final concentration of 65% ().
Hydromorphone, morphine, and ondansetron were the only stable Group D compounds by the end of the study period (). Atropine showed the most degradation, with only 28% of the original drug concentration remaining by week 2, and none remaining by week 4 (). Diphenhydramine, fentanyl, and naloxone remained stable until week 2, after which they had moderate degradation at week 4 to 76%, 63%, and 65% of original concentrations, respectively (). About half (53%) of the original midazolam concentration remained by the end of the study period ().
Discussion
Extreme temperatures cause degradation of medications via chemical reactions such as oxidation, hydrolysis, dehydration, and decarboxylation, which may decrease drug efficacy.Citation5,18,19 For most drugs, manufacturers guarantee a potency of 90–110% for a specified period, but only if it is stored as recommended.Citation4 While factors like light exposure, moisture, and oxygen exposure contribute to drug degradation, temperature variability is extremely important and nearly impossible for an NPS EMS system to control.Citation5,18 Insulated bags are of little value since internal temperatures equilibrate with the ambient environment in a matter of hours, and carrying medications in inner clothing to keep warm is impractical for long rescue operations.Citation5 In one study, plastic storage boxes were shown to cause minor temperature attenuation of 1–4°C with unknown effect on drug concentration.Citation20 A mathematical model of daily stock rotation, moving medications into a temperature controlled environment at 20°C every other day, showed reduction in exposure to excessive heat.Citation21 In the NPS backcountry setting, daily stock rotation is not as feasible as in the urban EMS setting. In the present study, we sought to identify medications that may become unstable with temperature variation, in an effort to maintain the quality and efficacy of these medications. Due to previous research on the recognition of drug instability with temperature variations, improvements have been made in medication storage in EMS vehicles.Citation22 In the same vein we hoped to identify targets for improvements in medication storage, if necessary, for NPS Parkmedics.
Of the medications tested in this study, some maintained a high concentration of active compound despite exposure to extreme temperatures, while some rapidly decreased in concentration. This decrease in concentration translates to decreased clinical efficacy based on a generally accepted definition of loss of potency at a concentration less than 90% of the original product.
Hydromorphone, morphine and ondansetron () maintained concentrations near 100% for the duration of the study period, which makes these medications ideal for use in a temperature variable prehospital setting. In EMS systems like the NPS with long transport times, commonly exceeding two hours, long acting temperature-stable opioids such as hydromorphone and morphine are preferable to shorter acting unstable opioids, like fentanyl. Although parenteral fentanyl has an elimination half-life of about 3.7 hours, its duration of action is fairly short, at 30–60 minutes.Citation23 This may be preferable in an injured trauma patient, where an unwanted opioid-induced drop in blood pressure will improve quickly as the fentanyl effect dissipates. However, our findings show that only 50–65% of the drug remains after 3 weeks regardless of heat or cold exposure. In a busy urban EMS system where Fentanyl is used daily, this is likely not a problem. In the NPS or smaller EMS systems that do not run large call volumes, providers will likely carry fentanyl vials that are well over one month old, which may contain about half of the desired dose. For this reason we recommend low-volume EMS providers to consider carrying morphine or hydromorphone instead of fentanyl. The NPS protocol pain medication was recently changed to hydromorphone due to its fast time of onset, titratability, long duration of action, and stability with temperature variation.
Long and winding roads, pain, immobilization, and Trendelenburg positioning lead to frequent nausea in patients transported in the National Parks. Ondansetron, whose stability in variable temperatures has not been studied before, is a good choice for prehospital nausea and vomiting due to its stability over this four-week study. Although the role of antiemetics in the prehospital setting has been debated, airway compromise, risk of changing mental status, and pleasantness of the transport for both the patient and provider have convinced most EMS medical directors to allow some use of these agents.Citation24
In addition to fentanyl, diphenhydramine, and midazolam also showed temperature independent degradation over time. Diphenhydramine is a key medication in EMS and NPS allergic reaction and anaphylaxis protocols and has not been examined in prior drug stability studies. It maintained a stable concentration until week 2 where it declined to about three-quarters of original concentration for the duration of the study. Midazolam is used in our local EMS system and the NPS for seizures, agitation, and sedation during cardioversion or transport. With about half gone by week three regardless of temperature exposure, this key medication may need to be utilized quickly and replaced often for the drug to have maximum effect. Two studies examining midazolam stability in the prehospital setting found no degradation over 60 and 120 days.Citation25,26 However, the mean kinetic temperatures recorded in these studies were 8–30°C and 23–40°C.Citation25,26 Perhaps our study showed degradation due to the extreme hot and cold environments which were sustained for a week at a time.
Although prior studies have examined drug stability in a variety of temperatures, the study is the first to describe temperature sensitive degradation of atropine and naloxone, two key resuscitation medications. Atropine is an ester, and placing it in an acidic solution or exposing it to heat will facilitate hydrolysis, greatly shortening its half-life.Citation27–29 It reverses bradycardia by antagonizing parasympathetic input at the sinoatrial node and is the primary medication for symptomatic bradycardia in ACLS and NPS Parkmedic protocols. The results of this study lead us to believe, however, that the atropine that has been carried by our Parkmedics would be, to a large degree, ineffective. It would be nearly impossible to determine whether a lack of response to atropine was due to ineffective medication dose or severity of illness.
Johansen et al. showed that the stability of atropine was not significantly changed after exposure to extreme temperatures, however the study length was 2 days.Citation3 A study by Schier et al. found that a significant amount of atropine remains in samples after their expiration dates. There was minimal degradation seen even in a sample from World War II. The storage of the samples is not described, and so it is unknown if they were exposed to significant heat.Citation27 In the event of a terrorist attack with an organophosphate agent, the best option may be to rapidly prepare solutions from atropine powder, suggested in a study by Dix et al., as atropine was stable for at least 72 hours at various temperatures when protected from light.Citation30 In this study, our findings are different from these prior atropine studies. When exposed to even one week of high temperature, atropine concentrations were significantly decreased and almost completely gone by week 3. For that reason we recommend that atropine be stored in a cold environment as much as possible and replaced frequently in the NPS or low volume EMS setting.
Naloxone is an opiate antagonist that can reverse opioid effects through intravenous, intramuscular, intranasal, and subcutaneous routes of administration. From 1991 to 2010, the annual number of opioid prescriptions in the U.S. almost tripled, from 76 to 210 million, reflecting the current opioid epidemic.Citation31 Oral opioid abuse has led to a resurgence of heroin abuse due to heroin's cheaper price.Citation32 In 2012, about 669,000 Americans reported using heroin, a number that's been on the rise since 2007.Citation33 This has led to national debate about first responders carrying naloxone, since they frequently arrive on the scene before ALS personnel. As of July 2015, forty-one states have legalized first responders to carry naloxone. The heat intolerance of this drug raises concerns about frequency and cost-effectiveness of restocking first responders' supplies.
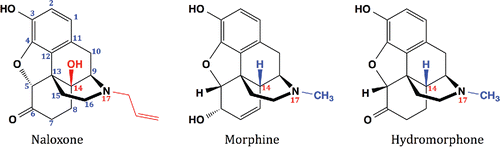
Naloxone shares a common parent structure with hydromorphone and morphine, which remained stable in our study. Two features in naloxone's molecular structure can explain this stark contrast in stability profile: (1) the presence of a tertiary alcohol group instead of H at C-14 and (2) the presence of an allyl group instead of a methyl group at N-17 (). Tertiary alcohol groups are prone to acid-catalyzed dehydration (via E1 elimination) that is facilitated by high temperature. The injectable naloxone used by Parkmedics is available as an HCl salt in acidic saline solution (pH 3–4.5). At ambient conditions, this dehydration reaction is most likely minimal, but an increase in temperature serves as a catalyst for this reaction. In both morphine and hydromorphone, this hydroxyl group is replaced by hydrogen, which makes both compounds unreactive to dehydration. The presence of an allyl group at N-17 also makes naloxone vulnerable to acid catalyzed hydrolysis through its alkene moiety. This feature is also lacking in both morphine and hydromorphone.
Figure 5. Molecular structure comparison among opioid receptor agonists and antagonist used in the study. Naloxone's difference in stability profiles from morphine and hydromorphone may be explained by the presence of a tertiary alcohol group instead of H at C-14 and the presence of an allyl group instead of a methyl group at N-17.
The results of our study suggest that if first responders (Police, Fire, EMTs) are going to carry this drug in warm climates, they may need to replace the drug every 3 weeks or keep it in a cool environment for this initiative to be cost effective. We recommend first responders to place a drug “born on” date on the packaging upon receipt. This would enable first responders to estimate that the drug is more than half gone once it is one month old if they live in a hot environment, which are most places in the United States during the summer months. Another option is to have first responders carry multiple doses, knowing that after 4 weeks an overdose patient may need twice the normal dose, since over 50% of the active ingredient is gone.
Limitations
There are significant limitations to this study, which may limit its applicability to clinical use. First, all arms of the study involved exposure to either extreme of hot and cold temperatures. We did not keep any controls at the manufacturer's recommended temperatures. However, because these are FDA-approved medications with FDA-approved packaging, we trusted that the package insert recommendations were tested for stability by the pharmaceutical companies. Our laboratory procedures may have theoretically contributed to some measured degradation of study medications. However, in the setting of a group of medications (hydromorphone, morphine, ondansetron) that remained completely stable throughout the course of the study, this is highly unlikely. This was a relatively short study, conducted over a four-week period. The results may not be applicable to medications kept at certain temperatures for longer periods of time. For example, in the summer medications may be kept in a warm environment for months, and the converse applies in the winter. Further studies may explore a more longitudinal model that mimics the seasons of the year. Our methodology prevented us from testing the Parkmedic medications amiodarone, epinephrine and dexamethasone. Two of those medications are key factors in cardiac arrest and resuscitation and it would be useful to include these medications in a future study. We also assumed that the initial drug compound itself was the only physiologically active entity in each sample, not taking into account the effect of active degradation products. Therefore, in vitro experiments would be necessary to truly establish efficacy and dosing changes.
Conclusions
Hydromorphone, morphine, and ondansetron remained stable in extremes of hot and cold over this four-week study. Diphenhydramine, fentanyl, and midazolam showed heat independent degradation, with midazolam concentrations at half of the original by the end of the study period. Atropine and naloxone were stable in cold temperatures but showed extensive degradation in heat, with atropine all but absent at week 4. In this era of opioid abuse of epidemic proportions, the finding that naloxone is unstable in heat is concerning and warrants further research. We recommend that EMS providers consider replacing atropine, naloxone, diphenhydramine, fentanyl, and midazolam frequently if they are practicing in either low call volume or high-temperature environments. Further studies are needed to determine if re-dosing midazolam, naloxone, and atropine is indicated in this setting if adequate clinical effect is not reached with a single dose.
References
- Kaufman TI, Knopp R, Webster T. The parkmedic program: prehospital care in the national parks. Ann Emerg Med. 1981;10(3):156–60.
- National Park Service. Weather-sequoia and kings canyon. Available at: www.nps.gov/seki/planyourvisit/weather.htm. Updated 2016. Accessed October 9, 2016.
- Johansen RB, Schafer NC, Brown PI. Effect of extreme temperatures on drugs for prehospital ACLS. Am J Emerg Med. 1993;11(5):450–2.
- De Winter S, Vanbrabant P, Vi NT, et al. Impact of temperature exposure on stability of drugs in a real-world out-of-hospital setting. Ann Emerg Med. 2013;62(4):380–7.e1.
- Kupper TE, Schraut B, Rieke B, Hemmerling AV, Schoffl V, Steffgen J. Drugs and drug administration in extreme environments. J Travel Med. 2006;13(1):35–47.
- APP Pharmaceuticals LLC. Package insert, amiodarone hydrochloride injectable, 2008.
- APP Pharmaceuticals LLC. Package insert, dexamethasone sodium phosphate injectable, 2008.
- International Medication Systems Ltd. Package insert, atropine sulfate injectable, 2013.
- International Medication Systems Ltd. Package insert, naloxone hydrochloride injectable, 2012.
- West-Ward Pharmaceuticals. Package insert, fentanyl citrate injectable, 2011.
- Hospira. Product insert, diphenhydramine hydrochloride injectable, 2008.
- Hospira. Package insert, epinephrine injectable, 2004.
- Hospira. Package insert, hydromorphone hydrochloride injectable, 2011.
- Hospira. Package insert, midazolam injectable, 2011.
- Hospira. Package insert, morphine sulfate injectable, 2007.
- Hospira. Package insert, ondansetron injectable, 2013.
- Jenke DR. Chromatographic method validation: a review of common practices and procedures II. J Liq Chromatogr. 1996;19:737–57.
- Brown LH, Campagna JD. Medication storage in the EMS environment: Understanding the science and meeting the standards. Emerg Med Serv. 2005;34(3):71, 73–7, 90.
- Brown LH, Krumperman K, Fullagar CJ. Out-of-hospital medication storage temperatures: a review of the literature and directions for the future. Prehosp Emerg Care. 2004;8(2):200–6.
- Valenzuela TD, Criss EA, Hammargren WM, et al. Thermal stability of prehospital medications. Ann Emerg Med. 1989;18(2):173–6.
- Brown LH, Wojcik SM, Bailey LC, Tran CD. Can stock rotation effectively mitigate EMS medication exposure to excessive heat and cold? Am J Emerg Med. 2006;24:14–8.
- Mehta SH, Doran JV, Lavery RF, Allegra JR. Improvements in prehospital medication storage practices in response to research. Prehosp Emerg Care. 2002;6(3):319–21.
- Product Information: fentanyl citrate intravenous, intramuscular solution, fentanyl citrate intravenous, intramuscular solution. Deerfield, IL: Baxter Healthcare Corporation.
- Salvucci AA, Squire B, Burdick M, Luoto M, Brazzel D, Vaezazizi R. Ondansetron is safe and effective for prehospital treatment of nausea and vomiting by paramedics. Prehosp Emerg Care. 2011;15(1):34–8.
- McMullan JT, Pinnawin A, Jones E, et al., on behalf of the Neurological Emergencies Treatment Trials investigators. The 60-day temperature-dependent degradation of midazolam and lorazepam in the prehospital environment. Prehosp Emerg Care. 2013;17(1):1–7.
- McMullan JT, Jones E, Barnhart B, et al., on behalf of the Neurological Emergencies Treatment Trials investigators. Degradation of benzodiazepines after 120 days of EMS deployment. Prehosp Emerg Care. 2014;18(3):368–74.
- Schier JG, Ravikumar PR, Nelson LS, Heller MB, Howland MA, Hoffman RS. Preparing for chemical terrorism: stability of injectable atropine sulfate. Acad Emerg Med. 2004;11(4):329–34.
- Lund U, Hansen SH. Simultaneous determination of atropine and its acidic and basic degradation products by mixed-column high-performance liquid chromatography. J Chromatogr. 1978;161:371–8.
- Lund W, Waaler T. The quantitative determination of atropine in presence of its hydrolysis and dehydration products. Pharm Acta Helv. 1970;45:701–7.
- Dix J, Weber RJ, Frye RF, Nolin TD, Mrvos R, Krenzelok E. Stability of atropine sulfate prepared for mass chemical terrorism. J Toxicol Clin Toxicol. 2003;41(6):771–5.
- Brady JE, Wunsch H, DiMaggio C, et al. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep. 2014;129(2):139–47.
- Goodloe, et al. Should Naloxone be available to all first responders? J Emerg Med Serv. 2014;39(8):1–6.
- National Institute on Drug Abuse. What is the scope of heroin use in the United States? February 2014. Available at: www.drugabuse.gov/publications/research-reports/heroin/scope-heroin-use-in-united-states. Accessed May 28, 2014.
