Abstract
Objectives: The objectives of this study were to assess comparative effectiveness and harms of opioid and nonopioid analgesics for the treatment of moderate to severe acute pain in the prehospital setting. Methods: We searched MEDLINE®, Embase®, and Cochrane Central from the earliest date through May 9, 2019. Two investigators screened abstracts, reviewed full-text files, abstracted data, and assessed study level risk of bias. We performed meta-analyses when appropriate. Conclusions were made with consideration of established clinically important differences and we graded each conclusion’s strength of evidence (SOE). Results: We included 52 randomized controlled trials and 13 observational studies. Due to the absence or insufficiency of prehospital evidence we based conclusions for initial analgesia on indirect evidence from the emergency department setting. As initial analgesics, there is no evidence of a clinically important difference in the change of pain scores with opioids vs. ketamine administered primarily intravenously (IV) (low SOE), IV acetaminophen (APAP) (low SOE), or nonsteroidal anti-inflammatory drugs (NSAIDs) administered primarily IV (moderate SOE). The combined use of an opioid and ketamine, administered primarily IV, may reduce pain more than an opioid alone at 15 and 30 minutes (low SOE). Opioids may cause fewer adverse events than ketamine (low SOE) when primarily administered intranasally. Opioids cause less dizziness than ketamine (low SOE) but may increase the risk of respiratory depression compared with ketamine (low SOE), primarily administered IV. Opioids cause more dizziness (moderate SOE) and may cause more adverse events than APAP (low SOE), both administered IV, but there is no evidence of a clinically important difference in hypotension (low SOE). Opioids may cause more adverse events and more drowsiness than NSAIDs (low SOE), both administered primarily IV. Conclusions: As initial analgesia, opioids are no different than ketamine, APAP, and NSAIDs in reducing acute pain in the prehospital setting. Opioids may cause fewer total side effects than ketamine, but more than APAP or NSAIDs. Combining an opioid and ketamine may reduce acute pain more than an opioid alone but comparative harms are uncertain. When initial morphine is inadequate, giving ketamine may provide greater and quicker acute pain relief than giving additional morphine, although comparative harms are uncertain. Due to indirectness, strength of evidence is generally low, and future research in the prehospital setting is needed.
Key words:
Introduction
Appropriate management of acute pain is an integral part of patient management in the prehospital setting. The prevalence of pain specifically in the prehospital setting varies, with estimates ranging from 20 to 53% (Citation1). Adequate pain relief is known to minimize anxiety and cardiac complications associated with acute pain (Citation2). However, as many as 43% of adults and 83% of pediatric patients have insufficient prehospital pain relief (Citation3, Citation4).
For patients experiencing moderate to severe traumatic injury pain, current guidelines (based on moderate quality evidence) strongly recommend initial prehospital management with a weight-based opioid, either intravenous (IV) morphine or IV/intranasal (IN) fentanyl (Citation5). Further complicating the appropriate use of prehospital opioids is the fear of their abuse and the resulting epidemic in the United States (Citation6, Citation7). When combined with concerns of adverse events, such as vomiting and subsequent airway obstruction, respiratory depression, hypotension, and sedation (Citation8), alternative analgesics have been sought. Nonopioid analgesics, including ketamine, acetaminophen (APAP), nitrous oxide/oxygen, and nonsteroidal anti-inflammatory drugs (NSAIDs) have been used to provide adequate analgesia. This systematic review assessed the comparative effectiveness and harms of opioids compared to nonopioid analgesics for the prehospital management of moderate to severe acute pain.
Methods
The systematic review was performed at the University of Connecticut Evidence-based Practice Center (EPC) through a contract with the U.S. Agency for Health-Care Research and Quality (AHRQ). With input from the National Highway Traffic Safety Administration (NHTSA), the project technical expert panel (TEP), and AHRQ, we developed and followed a protocol (Citation9) in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (Citation10) (PROSPERO CRD42018114959). This manuscript presents the comparisons and outcomes with conclusions and graded strength of evidence (see Strength of Evidence Assessment section). Additional comparisons and outcomes are presented in the full report that is available on the AHRQ website (Citation11).
Search Strategy
We searched MEDLINE, MEDLINE In-Process and Other Nonindexed Citations, EMBASE and Cochrane Central Register of Controlled Trials via OVID from earliest date through May 9, 2019 (Supplemental Appendix A). We supplemented the bibliographic database searches with backward citation tracking of relevant citations. We searched http://www.clinicaltrials.gov and the International Controlled Trials Registry Platform for ongoing studies and completed studies that reported results. There were no limits placed on publication date or language.
Study Selection
We included randomized controlled trials (RCTs), prospective or retrospective controlled cohort studies and case-control studies of patients with moderate to severe acute pain, without restrictions on age. We determined pain intensity by reviewing 1) study inclusion criteria, 2) reported baseline pain scores, or 3) in the absence of 1 or 2, we assumed pain to have been at least moderate for trials studying opioids or ketamine. We did not exclude based on the specific tool or threshold used by the study to define moderate or severe pain. Studies that targeted patients with mild pain, non-zero pain or labor and delivery pain were excluded. We also included studies of patients with acute onset pain, moderate to severe in intensity, who had an inadequate responsive to the first analgesic. These studies were evaluated separately from those studying initial analgesia.
We included studies that compared two of the following analgesics, regardless of dose, route or frequency of administration: opioids (fentanyl or morphine) and nonopioids (acetaminophen, ketamine, nitrous oxide/oxygen, or nonsteroidal anti-inflammatory drugs [NSAIDs; ketorolac or ibuprofen]). The combination of an opioid with ketamine compared with an opioid alone was also included.
Outcomes included pain (continuous and dichotomous), time to analgesic effect, memory of pain, “any adverse event” (as in total number of subjects with an adverse event), hypotension, respiratory depression, mental status changes, blood pressure, heart rate, respiratory rate, oxygen saturation, nausea, vomiting, and emergence delirium.
Studies were required to be conducted in the prehospital setting and in the absence of sufficient prehospital data, we allowed inclusions of studies from the emergency department (ED) to provide indirect evidence. There were no restrictions based on timing aside from studies from the ED setting for which we included pain related outcomes through 60 minutes.
EPC reviewers screened titles and abstracts using two independent investigators to identify citations that met eligibility criteria. We reviewed full-text publications when both reviewers agreed that a citation was eligible; if needed, a third reviewer was consulted to resolve any disagreement until consensus was reached. Finally, we contacted corresponding authors, when needed, to assess the study for inclusion.
Data Extraction and Study Quality Assessment
One investigator abstracted data into a standardized online form and into evidence tables while a second investigator verified entries. Two independent investigators assessed the quality of each included study using the Cochrane Risk of Bias Tool (Citation12) for RCTs and the Newcastle Ottawa Scale (Citation13) for observational studies. The overall study risk of bias was classified for each included study as “low,” “moderate,” “high,” or “unclear.”
Data Synthesis and Statistical Analysis
Pain was classified as traumatic, nontraumatic or mixed. We synthesized all pain classifications together and when possible, we analyzed and reported results for traumatic pain separately. Additional subgroup analyses that were possible included subject age, pain location, analgesic route and timing of administration. We collected and analyzed three times points: 15 minutes (post-drug administration through 15 minutes), 30 minutes (20 to 30 minutes) and 60 minutes (40 to 60 minutes). We did not combine prehospital and ED study data together in meta-analyses and instead we reported results separately when applicable.
When there were two or more trials of similar pharmacologic comparisons and outcomes, we performed random-effects meta-analysis using inverse-variance weighting. Between-study variance was estimated using the Paule-Mandel estimator (Citation14). The Hartung-Knapp method was used to adjust 95% confidence intervals (CI) when three or more studies were meta-analyzed (Citation15, Citation16); otherwise, a traditional DerSimonian-Laird random-effects model was used (Citation17). Continuous outcomes are reported as mean differences and 95% CI. For continuous pain scales, we converted scores (e.g., 0–100 scale) to a 10-point scale using the methods of Thorlund et al. (Citation18). For binary outcomes, risk differences (RD) are reported with corresponding 95% CI. For outcomes with zero events in one or both study arms, continuity correction was used (Citation19, Citation20).
We assessed presence of statistical heterogeneity using the Cochrane p-value (p < 0.10 significant) and the I2 statistic which represents the percentage (0–100%) of variability in the treatment estimate that is attributable to heterogeneity (Citation21). Small study effects were evaluated for through visual inspection of funnel plots. Tests for funnel plot asymmetry were conducted when 10 or more studies reported a given outcome (Citation22). We conducted subgroup analyses to evaluate for the presence of effect modifiers. All analyses were performed using the “meta” package (version 4.9-4) in R (version 3.5.2; the R Project for Statistical Computing).
Strength of Evidence (SOE) Assessment
We prioritized comparisons and outcomes upon which to construct conclusions with input from NHTSA, the project TEP and AHRQ. These included the comparisons of opioid to nonopioid or combined administration of opioid and ketamine to opioid alone. The prioritized outcomes include changes in pain severity (continuous measures), presence of pain (dichotomous measures), time to analgesic effect, respiratory depression, hypotension, change in mental status, and “any adverse event.” Other comparisons and outcomes do not have an accompanying conclusion with SOE and are reported in the full report (Citation11).
Conclusions were constructed with consideration of the absolute effect estimates and their corresponding confidence intervals compared to clinically important differences (CID) established for this review; details of the chosen thresholds and how conclusions were made are found in Supplemental Appendix B. We graded the SOE for each conclusion using established guidance (Citation23), two independent senior investigators evaluated SOE for each prioritized comparison and outcome. The SOE was judged to be one of four levels (high, moderate, low, or insufficient), in consideration of five domains: study limitations, consistency, directness (prehospital setting vs. ED setting, the latter which is indirect evidence), precision, and reporting bias.
Results
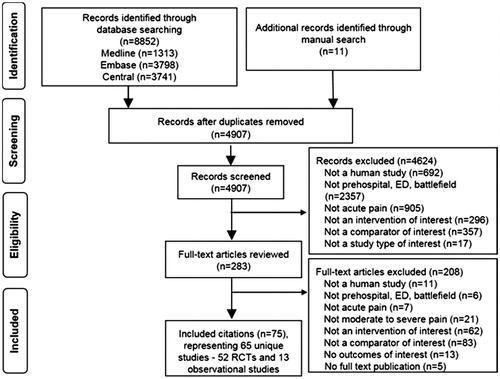
Our search identified 4,907 nonduplicate records, of which 283 required full-text review after title and abstract screening, resulting in 65 unique studies; 52 RCT and 13 observational studies (). Of these 65 studies, 37 RCTs (Citation24–60) and 4 observational studies (Citation61–64) contributed data to graded comparisons and are described in Supplemental Appendix C Tables 2–5. Most evidence was studying initial analgesia and comparing opioids with ketamine, followed by opioids with APAP and the combination of opioids and ketamine with opioids alone. The majority of studies were of adults with a mean age in the second to fourth decades. Source/etiology of pain was mixed; the most common cause for traumatic pain was extremity injuries/fractures. Most often, analgesics were administered intravenously (IV); there were also several trials studying intranasal (IN) administration of ketamine and fentanyl using an atomizer to deliver the IV solution IN. The majority of studies were conducted in the ED setting.
Figure 1. Identification and screening of citations. Abbreviations: ED = emergency department; OBS = observational; RCT = randomized controlled trial.
Risk of Bias
Risk of bias of individual studies are shown in Supplemental Appendix C Tables 6 and 7. Most RCTs (31/37, 83.8%) were rated with low or unclear risk of bias. Two RCTs (5.4%) were rated with medium risk of bias due either to inadequate randomization procedures or an open label design. Three RCTs (8.1%) were rated as low risk of bias for objective outcomes but medium risk of bias for subjective outcomes due to an open-label design. Two (50%) observational studies were rated as low risk of bias. One RCT (1.9%) was rated with high risk of bias due to its open-label design, inappropriate sequence generation and allocation concealment, and high differential attrition between groups. One observational study (25%) was rated as medium risk of bias attributed to a select group of users (battlefield), controlling for a single factor and unknown follow-up. One (25%) observational studies was rated as high risk of bias attributed to a select group of users (battlefield), uncontrolled analyses and inadequate cohort follow-up.
Opioids versus Ketamine
Direct evidence from the prehospital setting is insufficient to conclude comparative effectiveness of opioids and ketamine in reducing pain (Citation25, Citation62). Using indirect evidence from the ED, we found no evidence of a clinically important difference in the reduction of pain scores when opioids are compared with ketamine at 15, 30, and 60 minutes (all low SOE) (, Supplemental Appendix D Figure 1). There is insufficient evidence for the outcome of pain presence and time to analgesic effect (Supplemental C Appendix Table 8).
Table 1. Conclusions and strength of evidence for the comparison of opioids vs. ketamine as initial analgesics
Based on indirect evidence from the ED, opioids may cause fewer total adverse events than ketamine (low SOE), less dizziness than ketamine (low SOE), and may cause more respiratory depression than ketamine (low SOE). Dizziness may be associated with an age less than 18 years old or with the intranasal route of administration (Supplemental Appendix D Figures 2 and 3) although this interaction is unclear because these two subgroups were represented by the same trials. Evidence is insufficient for the outcomes of hypotension and other measures of mental status changes.
In patients with an inadequate response to initial analgesia with IV morphine, giving IV ketamine may reduce pain more than giving additional opioids (low SOE) and may be quicker to reduce pain to a clinically important difference compared to giving additional opioids (low SOE) (, Supplemental Appendix D Figure 4). These conclusions are based on direct evidence from the prehospital setting. Evidence is insufficient for the outcomes of any adverse event, hypotension, and mental status changes (Supplemental Appendix C Table 9).
Table 2. Conclusions and strength of evidence for the comparison of opioids vs. ketamine in patients with inadequate response to initial analgesia
Opioids + Ketamine vs. Opioid
Combining an opioid and ketamine may reduce pain more than opioids alone, at 15 and 30 minutes (low SOE) (, Supplemental Appendix D Figure 5), but not at 60 minutes (low SOE). A single trial (Citation41) in the prehospital setting agreed that a clinically important difference favoring the combination of analgesics was possible at both 15 and 30 minutes. There is insufficient evidence for the outcomes of pain presence, any adverse events, hypotension, mental status changes, and respiratory depression (Supplemental Appendix C Table 10).
Table 3. Conclusions and strength of evidence for the comparison of combining an opioid and ketamine vs. an opioid
Opioids vs. Acetaminophen
Based on indirect evidence from the ED, we found no evidence of a clinically important difference in the reduction of pain scores with IV opioids compared to IV APAP at 15, 30, and 60 minutes (all low SOE) (, Supplemental Appendix D Figure 6). We found no evidence of a clinically important difference in the time to analgesia with IV opioids compared with IV APAP (low SOE) (Citation49). There is insufficient evidence for the outcome of pain presence (Supplemental Appendix C Table 11).
Table 4. Conclusions and strength of evidence for the comparison of opioids vs. acetaminophen
Opioids may cause more adverse events than APAP (low SOE). We found no evidence of a clinically important difference in hypotension with opioids compared to APAP (low SOE). No subjects had hypotension in the APAP group and 8 (2.6%) had hypotension in the opioid group. Opioids cause more dizziness than APAP (moderate SOE). Evidence is insufficient for the outcomes of “mild” sedation and respiratory depression.
Opioids versus Nitrous Oxide
A single RCT compared opioids to nitrous oxide/oxygen and evidence was insufficient to conclude comparative effects (Supplemental Appendix C Table 12) (Citation57).
Opioids versus Nonsteroidal Anti-Inflammatory Drugs
We found no evidence of a clinically important difference in the reduction of pain scores when opioids are compared with NSAIDs at 30 and 60 minutes (all moderate SOE) (, Supplemental Appendix D Figure 7). Evidence is insufficient to conclude effects at 15 minutes and for pain presence (Supplemental Appendix C Table 13). Opioids may cause more adverse events and may cause more drowsiness than NSAIDs (low SOE). Evidence is insufficient for the outcomes of hypotension and other measures of mental status changes.
Table 5. Conclusions and strength of evidence for the comparison of opioids vs. nonsteroidal anti-inflammatory drugs
Discussion
As initial analgesics for acute pain in the prehospital setting, we found no evidence of clinically important differences of pain reduction when opioids are compared with ketamine, APAP or NSAIDs. Combined administration of an opioid and ketamine may be more effective in reducing pain that opioids alone. These conclusions are all graded with a low strength of evidence, primarily due to inconsistency and reliance on indirect evidence from the ED setting. There are also important considerations for applicability of this evidence. Studies comparing efficacy of opioids with ketamine mostly compared weight-based IV morphine 0.1 mg/kg with IV ketamine (variable weight-based dosing). Some studies evaluated IN fentanyl and IN ketamine by administering the IV solution via an atomizer. The doses of ketamine were primarily sub-dissociative, although too few studies were available to conduct dose-related subgroup analysis. When ketamine was studied in combination with opioids, a single IV dose was added to the opioid regimen; how administration of multiple ketamine dose impacts outcomes is unknown. Nine of the 10 trials that compared opioids with APAP compared IV morphine 0.1 mg/kg with IV APAP 1 gm, thus results cannot be extrapolated to other routes or doses. There were only three studies comparing opioids with NSAIDs with a mixed representation of oral and IV dosage forms.
Although we found opioids may cause fewer total adverse events than ketamine, the cumulative frequency of events in both groups was at least 50%, suggesting most patients will experience an event regardless. These trials studied primarily IN analgesic administration and based on our subgroup analyses, the lower overall adverse event risk with opioids may be associated with the IN route or with younger age (<18 years). In contrast, we founds opioids may cause more adverse events than IV APAP or NSAIDs when used as initial analgesics. Evidence to evaluate specific harms was often insufficient but importantly, we found opioids to be associated with more respiratory depression than ketamine. This is a potentially fatal complication of opioid use for either acute or chronic pain management. Findings of dizziness and drowsiness with ketamine and opioids, although bothersome to patients, are less concerning and are expected side effects of these analgesics.
Current guidelines strongly recommend (based on moderate quality evidence) initial prehospital management of moderate to severe traumatic pain with a weight-based opioid, either intravenous (IV) morphine or IV/intranasal (IN) fentanyl (Citation5). National model guidelines for pain management in the prehospital setting recommend either opioid or nonopioid analgesics but the specific drug and route of administration differs based on whether treatment is for moderate or severe pain (Citation65). Our results are in support of the option of both opioid and nonopioid analgesics for patients with moderate to severe pain. Importantly, we found no evidence that opioids are better at reducing pain in this setting but are associated with more side effects than APAP or NSAIDs. Although we do not make clinical recommendations, we encourage the application of this evidence in future guideline development.
With the current opioid overdose epidemic and concerns about potential misuse of and addiction to opioids, recent interest in nonopioid alternatives has grown. Support for the use of sub-dissociative doses of IV ketamine for acute pain management is growing nationally (Citation66, Citation67). Our conclusions support the efficacy of ketamine, and when compared to opioids there was no evidence of a clinically important differences in reducing pain. Elevations in blood pressure and heart rate with ketamine may be common (Citation68), but we did not formulate conclusions for outcomes concerning changes in blood pressure, heart rate or respiratory rate. Similarly, we did not grade SOE for emergence reactions and although reported to be uncommon with sub-dissociative ketamine doses (Citation66); cumulatively 8.4% (12 of 143 subjects) of ketamine treated subjects from the included studies experienced this effect.
Limitations
The major limitation of this review is the indirectness of evidence, which led to our downgrading of conclusions. We believe the single most important future research need is addressing this evidence gap with pain management studies set in the prehospital environment. In addition, research is needed to explore subgroups further, including patient and drug regimen characteristics and EMS personnel training and how these characteristics may modify comparative effectiveness and harms of analgesics. Use of ED data was associated with addition challenges. Pain, and usually cardiorespiratory monitoring parameters, were measured multiple times throughout the study period. We chose to evaluate these outcomes at 15, 30, and 60 minutes to balance clinical applicability and multiple hypothesis testing. Assessment of mental status changes was challenging because this outcome can be described in many ways. While we were quite liberal in what we allowed as a mental status change, we separately analyzed each distinct “symptom” since within a study these outcomes may not have been mutually exclusive. Similarly, the assessment of emergence delirium posed a challenge since several signs or symptoms may be associated with this phenomenon. We were strict in collecting data explicitly reported by the authors as emergence reactions, delirium or phenomenon rather than assuming a vaguely reported symptom may have been emergence delirium.
Conclusion
As initial analgesia, opioids are no different than ketamine, APAP and NSAIDs in reducing acute pain in the prehospital setting. Opioids may cause fewer total side effects than ketamine, but more than APAP or NSAIDs. Differences in specific side effects vary between analgesics and can further inform treatment decisions. Combined administration of an opioid and ketamine may reduce acute pain more than an opioid alone but comparative harms are uncertain. When initial morphine is inadequate in reducing pain, giving ketamine may provide greater and quicker acute pain relief than giving additional morphine, although comparative harms are uncertain. Due to indirectness, strength of evidence is generally low, and future research in the prehospital setting is needed.
Supplemental Material
Download MS Word (4.4 MB)Additional information
Funding
References
- McLean SA, Maio RF, Domeier RM. The epidemiology of pain in the prehospital setting. Prehosp Emerg Care. 2002;6:402–5. PMID: 12385606.
- Thomas SH, Shewakramani S. Prehospital trauma analgesia. J Emerg Med. 2008;35:47–57. PMID: 17997072. doi:10.1016/j.jemermed.2007.05.041.
- Albrecht E, Taffe P, Yersin B, Schoettker P, Decosterd I, Hugli O. Undertreatment of acute pain (oligoanalgesia) and medical practice variation in prehospital analgesia of adult trauma patients: a 10 yr retrospective study. Br J Anaesth. 2013;110:96–106. PMID: 23059961. doi:10.1093/bja/aes355.
- Izsak E, Moore JL, Stringfellow K, Oswanski MF, Lindstrom DA, Stombaugh HA. Prehospital pain assessment in pediatric trauma. Prehosp Emerg Care. 2008;12:182–6. PMID 18379914
- Gausche-Hill M, Brown KM, Oliver ZJ, Sasson C, Dayan PS, Eschmann NM, Weik TS, Lawner BJ, Sahni R, Falck-Ytter Y, et al. An evidence-based guideline for prehospital analgesia in trauma. Prehosp Emerg Care. 2014;18:25–34. PMID: 24279813. doi:10.3109/10903127.2013.844873.
- Clark DJ, Schumacher MA. America’s opioid epidemic: supply and demand considerations. Anesth Analg. 2017;125:1667–74. PMID: 29049112. doi:10.1213/ANE.0000000000002388.
- Hoppe JA, Nelson LS, Perrone J, Weiner SG, Rathlev NK, Sanchez LD, Babineau M, Griggs CA, Mitchell PM, Ma J, et al. Prescribing Opioids Safely in the Emergency Department (POSED) study investigators. Opioid prescribing in the cross section of US emergency departments. Ann Emerg Med. 2015;66:253–259. PMID: 25952503.
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Phys. 2008;11:S105–S20. PMID: 18443635.
- Pharmacologic management of acute pain by EMS in the prehospital setting. Effective Health Care Program, AHRQ [accessed 2019 Jul 11]. https://effectivehealthcare.ahrq.gov/topics/acute-pain-ems/protocol.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLos Med. 2009;6:e1000097. PMID: 19621072.
- Sobieraj DM, Baker WL, Martinez BK, Miao B, Hernandez AV, Coleman CI, Cicero MX, Kamin RA. Comparative effectiveness of analgesics to reduce acute pain in the prehospital setting. Comparative Effectiveness Review No. 220. (Prepared by the University of Connecticut Evidence-based Practice Center under Contract No. 290-2015-00012-I.) AHRQ Publication No. 19-EHC021-EF. Rockville, MD: Agency for Healthcare Research and Quality; August 2019. doi:10.23970/AHRQEPCCER220. [Accessed 2019 Sep 9].
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. PMID: 22008217. doi:10.1136/bmj.d5928.
- Wells GA, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute.
- Paule RC, Mandel J. Consensus values, regressions, and weighting factors. J Res Natl Bur Stan. 1989;94:197–203. PMID: 28053410. doi:10.6028/jres.094.020.
- Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–701. SepPMID: 12939780. doi:10.1002/sim.1482.
- Seide SE, Rover C, Friede T. Likelihood-based random-effects meta-analysis with few studies: empirical and simulation studies. BMC Med Res Methodol. 2019;19:16. PMID: 30634920. doi:10.1186/s12874-018-0618-3.
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–45. PMID: 26343745. doi:10.1016/j.cct.2015.09.002.
- Thorlund K, Walter SD, Johnston BC, Furukawa TA, Guyatt GH. Pooling health-related quality of life outcomes in meta-analysis—a tutorial and review of methods for enhancing interpretability. Res Syn Meth. 2011;2:188–203. PMID: 26061786. doi:10.1002/jrsm.46.
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Statist Med. 2007;26:53–77. PMID: 16596572. doi:10.1002/sim.2528.
- Cheng J, Pullenayegum E, Marshall JK, Iorio A, Thabane L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open. 2016;6:e010983. PMID: 27531725. doi:10.1136/bmjopen-2015-010983.
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. PMID: 12958120. doi:10.1136/bmj.327.7414.557.
- Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;342:d4002. PMID: 21784880. doi:10.1136/bmj.d4002.
- Berkman ND, Lohr KN, Ansari MT, Balk EM, Kane R, McDonagh M, Morton SC, Viswanathan M, Bass EB, Butler M, Bartlehner G, Hartling L, McPheeters M, Morgan LC, Reston J, Sista P, Whitlock E, Chang S. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol. 2015;68:1312–24. PMID: 25721570. doi:10.1016/j.clinepi.2014.11.023.
- Frey TM, Florin TA, Caruso M, Zhang N, Zhang Y, Mittiga MR. Effect of intranasal ketamine vs fentanyl on pain reduction for extremity injuries in children: the PRIME randomized clinical trial. JAMA Pediatr. 2019;173:140–6. PMID: 30592476. doi:10.1001/jamapediatrics.2018.4582.
- Tran KP, Nguyen Q, Truong XN, Le V, Le VP, Mai N, Husum H, Losvik OK. A comparison of ketamine and morphine analgesia in prehospital trauma care: a cluster randomized clinical trial in rural Quang Tri province, Vietnam. Prehosp Emerg Care. 2014;18:257–64. PMID: 24400915. doi:10.3109/10903127.2013.851307.
- Sub-dissociative ketamine for the management of acute pediatric pain. NCT01951963. HealthPartners Institute. [accessed 2019 Mar 11]. https://clinicaltrials.gov/ct2/show/NCT01951963?term=NCT01951963&rank=1.
- Reynolds SL, Bryant KK, Studnek JR, Hogg M, Dunn C, Templin MA, Moore CG, Young JR, Walker KR, Runyon MS. Randomized controlled feasibility trial of intranasal ketamine compared to intranasal fentanyl for analgesia in children with suspected extremity fractures. Acad Emerg Med. 2017;24:1430–40. PMID: 28926159. doi:10.1111/acem.13313.
- Graudins A, Meek R, Egerton-Warburton D, Oakley E, Seith R. The PICHFORK (Pain in Children Fentanyl or Ketamine) trial: a randomized controlled trial comparing intranasal ketamine and fentanyl in children with limb injuries. Ann Emerg Med. 2015;65:248–54. PMID: 25447557
- Verki MM, Mozafari J, Tirandaz F, Motamed H, Khazaeli A. Efficacy of nebulized fentanyl and low dose ketamine for pain control of patients with long bone fractures: a randomized, double-blind, clinical trial. African J Emerg Med. 2019;9:119–22. doi:10.1016/j.afjem.2019.02.003.
- Quinn K, Kriss S, Drapkin J, Likourezos A, Pushkar I, Brady J, Yasavolian M, Chitnis SS, Motov S, Fromm C. Analgesic efficacy of intranasal ketamine versus intranasal fentanyl for moderate to severe pain in children: a prospective, randomized, double-blind study. Pediatr Emerg Care. 2018;1. 0000000000001556. [Epub ahead of print]. PMID: 30045355. doi:10.1097/PEC.
- Shimonovich S, Gigi R, Shapira A, Sarig-Meth T, Nadav D, Rozenek M, West D, Halpern P. Intranasal ketamine for acute traumatic pain in the emergency department: a prospective, randomized clinical trial of efficacy and safety. BMC Emerg Med. 2016;16:43. PMID: 27829367. doi:10.1186/s12873-016-0107-0.
- Farnia MR, Jalali A, Vahidi E, Momeni M, Seyedhosseini J, Saeedi M. Comparison of intranasal ketamine versus IV morphine in reducing pain in patients with renal colic. Am J Emerg Med. 2017;35:434–7. PMID: 27931762. doi:10.1016/j.ajem.2016.11.043.
- Motov S, Mann S, Drapkin J, Butt M, Likourezos A, Yetter E, Brady J, Rothberger N, Gohel A, Flom P, et al. Intravenous subdissociative-dose ketamine versus morphine for acute geriatric pain in the emergency department: a randomized controlled trial. Am J Emerg Med. 2019;37:220–7. PMID: 29807629. doi:10.1016/j.ajem.2018.05.030.
- Mahshidfar B, Mofidi M, Fattahi M, Farsi D, Hafezi Moghadam P, Abbasi S, Rezai M. Acute pain management in emergency department, low dose ketamine versus morphine, a randomized clinical trial. Anesth Pain Med. 2017;7:e60561. PMID: 29696126. doi:10.5812/aapm.60561.
- Motov S, Rockoff B, Cohen V, Pushkar I, Likourezos A, McKay C, Soleyman-Zomalan E, Homel P, Terentiev V, Fromm C. Intravenous subdissociative-dose ketamine versus morphine for analgesia in the emergency department: a randomized controlled trial. Ann Emerg Med. 2015;66:222–9. PMID: 25817884. doi:10.1016/j.annemergmed.2015.03.004.
- Miller JP, Schauer SG, Ganem VJ, Bebarta VS. Low-dose ketamine vs morphine for acute pain in the ED: a randomized controlled trial. Am J Emerg Med. 2015;33:402–8. PMID: 25624076. doi:10.1016/j.ajem.2014.12.058.
- Majidinejad S, Esmailian M, Emadi M. Comparison of intravenous ketamine with morphine in pain relief of long bones fractures: a double blind randomized clinical trial. Emerg (Tehran). 2014;2:77–80. PMID: 26495351.
- Jahanian F, Hosseininejad SM, Amini Ahidashti H, Bozorgi F, Goli Khatir I, Montazar SH, Azarfar V. Efficacy and safety of morphine and low dose ketamine for pain control of patients with long bone fractures: a randomized, double-blind, clinical trial. Bull Emerg Trauma. 2018;6:31–6. PMID: 29379807. doi:10.29252/beat-060105.
- Jennings PA, Cameron P, Bernard S, Walker T, Jolley D, Fitzgerald M, Masci K. Morphine and ketamine is superior to morphine alone for out-of-hospital trauma analgesia: a randomized controlled trial. Ann Emerg Med. 2012;59:497–503. PMID: 22243959. doi:10.1016/j.annemergmed.2011.11.012.
- Johansson P, Kongstad P, Johansson A. The effect of combined treatment with morphine sulphate and low-dose ketamine in a prehospital setting. Scand J Trauma Resusc Emerg Med. 2009;17:61. NovPMID: 19943920. doi:10.1186/1757-7241-17-61.
- Galinski M, Dolveck F, Combes X, Limoges V, Smail N, Pommier V, Templier F, Catineau J, Lapostolle F, Adnet F. Management of severe acute pain in emergency settings: ketamine reduces morphine consumption. Am J Emerg Med. 2007;25:385–90. PMID: 17499654. doi:10.1016/j.ajem.2006.11.016.
- Hosseininejad SM, Jahanian F, Erfanian Irankar S, Moosazadeh M, Hosseini SA, Shahbakhti N, Bozorgi F. Comparing the analgesic efficacy of morphine plus ketamine versus morphine plus placebo in patients with acute renal colic: a double-blinded randomized controlled trial. Am J Emerg Med. 2019;37:1118–23. PMID: 30201237. doi:10.1016/j.ajem.2018.09.004.
- Sin B, Tatunchak T, Paryavi M, Olivo M, Mian U, Ruiz J, Shah B, de Souza S. The use of ketamine for acute treatment of pain: a randomized, double-blind, placebo-controlled trial. J Emerg Med. 2017;52:601–8. PMID: 28279542. doi:10.1016/j.jemermed.2016.12.039.
- Beaudoin FL, Lin C, Guan W, Merchant RC. Low-dose ketamine improves pain relief in patients receiving intravenous opioids for acute pain in the emergency department: results of a randomized, double-blind, clinical trial. Acad Emerg Med. 2014;21:1193–202. PMID: 25377395. doi:10.1111/acem.12510.
- Abbasi S, Bidi N, Mahshidfar B, Hafezimoghadam P, Rezai M, Mofidi M, Farsi D. Can low-dose of ketamine reduce the need for morphine in renal colic? A double-blind randomized clinical trial. Am J Emerg Med. 2018;36:376–9. PMID: 28821365. doi:10.1016/j.ajem.2017.08.026.
- Mohammadshahi A, Abdolrazaghnejad A, Nikzamir H, Safaie A. Intranasal ketamine administration for narcotic dose decrement in patients suffering from acute limb trauma in emergency department: a double-blind randomized placebo-controlled trial. Adv J Emerg Med. 2018;2:e30.
- Vahdati S, Morteza Baghi HR, Ghobadi J, Rajaei Ghafouri R, Habibollahi P. Comparison of paracetamol (Apotel®) and morphine in reducing post pure head trauma headache. Anesth Pain Med. 2014;4:e14903. PMID: 25237630. doi:10.5812/aapm.14903.
- Jalili M, Mozaffarpour Noori A, Sedaghat M, Safaie A. Efficacy of intravenous paracetamol versus intravenous morphine in acute limb trauma. Trauma Mon. 2016;21:e19649. doi:10.5812/traumamon.19649.
- Pathan SA, Mitra B, Straney LD, Afzal MS, Anjum S, Shukla D, Morley K, Al Hilli SA, Al Rumaihi K, Thomas SH, et al. Delivering safe and effective analgesia for management of renal colic in the emergency department: a double-blind, multigroup, randomised controlled trial. Lancet. 2016;387:1999–2007. PMID: 26993881. doi:10.1016/S0140-6736(16)00652-8.
- Serinken M, Eken C, Gungor F, Emet M, Al B. Comparison of intravenous morphine versus paracetamol in sciatica: a randomized placebo controlled trial. Acad Emerg Med. 2016;23:674–8. PMID: 26938140. doi:10.1111/acem.12956.
- Eken C, Serinken M, Elicabuk H, Uyanik E, Erdal M. Intravenous paracetamol versus dexketoprofen versus morphine in acute mechanical low back pain in the emergency department: a randomised double-blind controlled trial. Emerg Med J. 2014;31:177–81. PMID: 23407378. doi:10.1136/emermed-2012-201670.
- Serinken M, Eken C, Turkcuer I, Elicabuk H, Uyanik E, Schultz CH. Intravenous paracetamol versus morphine for renal colic in the emergency department: a randomised double-blind controlled trial. Emerg Med J. 2012;29:902–5. PMID: 22186009. doi:10.1136/emermed-2011-200165.
- Craig M, Jeavons R, Probert J, Benger J. Randomised comparison of intravenous paracetamol and intravenous morphine for acute traumatic limb pain in the emergency department. Emerg Med J. 2012;29:37–9. PMID: 21362724. doi:10.1136/emj.2010.104687.
- Al B, Sunar MM, Zengin S, Sabak M, Bogan M, Can B, Kul S, Murat Oktay M, Eren SH. Comparison of IV dexketoprofen trometamol, fentanyl, and paracetamol in the treatment of renal colic in the ED: a randomized controlled trial. Am J Emerg Med. 2018;36:571–6. PMID: 29029797. doi:10.1016/j.ajem.2017.09.019.
- Mollaei M, Esmailian M, Heydari F. Comparing the effect of intravenous acetaminophen (Apotel®) and intravenous morphine in controlling the pain of forearm and leg fractures in adults. J Isfahan Med Sch. 2016;34:293–8.
- Masoumi K, Forouzan A, Asgari Darian A, Feli M, Barzegari H, Khavanin A. Comparison of clinical efficacy of intravenous acetaminophen with intravenous morphine in acute renal colic: a randomized, double-blind, controlled trial. Emerg Med Int. 2014;2014:571326. PMID: 25197573. doi:10.1155/2014/571326.
- Kariman H, Majidi A, Amini A, Arhami Dolatabadi A, Derakhshanfar H, Hatamabadi H, Shahrami A, Yaseri M, Sheibani K. Nitrous oxide/oxygen compared with fentanyl in reducing pain among adults with isolated extremity trauma: a randomized trial. Emerg Med Australas. 2011;23:761–8. PMID: 22151676
- Masoumi B, Farzaneh B, Ahmadi O, Heidari F. Effect of intravenous morphine and ketorolac on pain control in long bones fractures. Adv Biomed Res. 2017;6:91. PMID: 28828342. doi:10.4103/2277-9175.211832.
- Safdar B, Degutis LC, Landry K, Vedere SR, Moscovitz HC, D’Onofrio G. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Ann Emerg Med. 2006;48:173–81. PMID: 16953530. doi:10.1016/j.annemergmed.2006.03.013.
- Le May S, Ali S, Plint AC, Masse B, Neto G, Auclair MC, Drendel AL, Ballard A, Khadra C, Villeneuve E, et al. Oral analgesics utilization for children with musculoskeletal injury (OUCH Trial): an RCT. Pediatrics. 2017;140:e20170186. PMID: 29021235. doi:10.1542/peds.2017-0186.
- Oberholzer N, Kaserer A, Albrecht R, Seifert B, Tissi M, Spahn DR, Maurer K, Stein P. Factors influencing quality of pain management in a physician staffed helicopter emergency medical service. Anesth Analg. 2017;125:200–9. PMID: 28489643. doi:10.1213/ANE.0000000000002016.
- Bronsky ES, Koola C, Orlando A, Redmond D, D’Huyvetter C, Sieracki H, Tanner A, 2nd, Fowler R, Mains C, Bar-Or D. Intravenous low-dose ketamine provides greater pain control compared to fentanyl in a civilian prehospital trauma system: a propensity matched analysis. Prehosp Emerg Care. 2018;1–8. [Epub ahead of print]. PMID: 29775117. doi:10.1080/10903127.2018.1469704.
- Schauer SG, Mora AG, Maddry JK, Bebarta VS. Multicenter, prospective study of prehospital administration of analgesia in the U.S. combat theater of Afghanistan. Prehosp Emerg Care. 2017;21:744–9. PMID: 28829661. doi:10.1080/10903127.2017.1335814.
- Shackelford SA, Fowler M, Schultz K, Summers A, Galvagno SM, Gross KR, Mabry RL, Bailey JA, Kotwal RS, Butler FK. Prehospital pain medication use by U.S. forces in Afghanistan. Mil Med. 2015;180:304–9. PMID: 25735021. doi:10.7205/MILMED-D-14-00257.
- National Model EMS Clinical Guidelines. Version 2.1 June 2018. NASEMSO Medical Directors Council.
- American College of Emergency Physicians. Sub-dissociative dose ketamine for analgesia [accessed 2018 Feb 28]. https://www.acep.org/patient-care/policy-statements/sub-dissociative-dose-ketamine-for-analgesia/.
- Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S, Bhatia A, Davis FN, Hooten WM, Cohen SP. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43:456–66. PMID: 29870457. doi:10.1097/AAP.000000000000806.
- Ketalar [package insert]. Par Pharmaceuticals, Inc. Chestnut Ridge, NY. August 2018.
