Abstract
Objective
A thorough understanding of the epidemiology, patient characteristics, trauma mechanisms, and current outcomes among patients with severe traumatic brain injury (TBI) is important as it may inform potential strategies to improve prehospital emergency care. The aim of this study is to describe the prehospital epidemiology, characteristics and outcome of (suspected) severe TBI in the Netherlands.
Methods
The BRAIN-PROTECT study is a prospective observational study on prehospital management of patients with severe TBI in the Netherlands. The study population comprised all consecutive patients with clinical suspicion of TBI and a prehospital GCS score ≤ 8, who were managed by one of the 4 Helicopter Emergency Medical Services (HEMS). Patients were followed-up in 9 trauma centers until 1 year after injury. Planned sub-analyses were performed for patients with “confirmed” and “isolated” TBI.
Results
Data from 2,589 patients, of whom 2,117 (81.8%) were transferred to a participating trauma center, were analyzed. The incidence rate of prehospitally suspected and confirmed severe TBI were 3.2 (95% CI: 3.1;3.4) and 2.7 (95% CI: 2.5;2.8) per 100,000 inhabitants per year, respectively. Median patient age was 46 years, 58.4% were involved in traffic crashes, of which 37.4% were bicycle related. 47.6% presented with an initial GCS of 3. The median time from HEMS dispatch to hospital arrival was 54 minutes. The overall 30-day mortality was 39.0% (95% CI: 36.8;41.2).
Conclusion
This article summarizes the prehospital epidemiology, characteristics and outcome of severe TBI in the Netherlands, and highlights areas in which primary prevention and prehospital care can be improved.
Introduction
Traumatic Brain Injury (TBI) is associated with high mortality rates and limited functional recovery among survivors (Citation1–3). Treatment of TBI, long-term rehabilitation and costs associated with loss of productive life years impose a great financial burden on society (Citation4,Citation5). Reducing morbidity and mortality of the patients affected most heavily – those suffering from severe TBI – is of great benefit for individual patients as well as society.
Most of the previous studies on severe TBI focus on intra-hospital treatment and recovery after hospitalization (Citation6,Citation7). Patients with TBI are, however, at risk of developing secondary brain injury before they even reach the hospital, therefore management during the period immediately following the initial trauma, i.e., the prehospital period, is of great importance in the modification of patients’ outcome (Citation8).
There is broad consensus that prehospital treatment strategies should aim to prevent and treat factors that are known to trigger secondary brain injuries, such as hypoxia, hypotension, as well as hypo- and hyperventilation. However, it is less clear how this can best be achieved. Guidelines for prehospital treatment are rather vague and are based on low quality evidence (Citation9). The prehospital treatment of severe TBI is therefore often opinion based rather than evidence based and resembles a “black box”, in which the effects of treatments on outcomes are largely unknown.
To address these knowledge gaps, a large-scale prospective multicenter study on prehospital treatment of severe TBI was initiated in the Netherlands: the BRAIN-PROTECT study (Citation8). A first step in identifying beneficial treatment strategies, optimal prehospital care and effective primary prevention is a thorough understanding of the patient population being treated in the context of the involved Emergency Medical System. The aim of this study is to describe the epidemiology, patient characteristics, operational characteristics, trauma mechanisms, and outcomes of patients with (suspected) severe TBI in the Netherlands.
Methods
Study Design and Setting
The BRAIN-PROTECT study is a prospective observational study perfomed on the prehospital treatment of patients with suspected severe TBI in the Netherlands. A detailed description of the methodology has been published previously (Citation8). Briefly, in the Netherlands, prehospital trauma care is provided by regional ambulance services as well as 4 physician-staffed Helicopter Emergency Medical Services (HEMS) located in Amsterdam, Rotterdam, Nijmegen, and Groningen. HEMS have the option to respond to trauma incidents with a modified ambulance vehicle instead of helicopter if required due to operational, technical or meteorological reasons.
Commonly two ambulance vehicles and one HEMS team are dispatched to major trauma incidents including suspected severe TBI. Only in exceptional cases, e.g., unavailability of a HEMS or a quick transport of patients due to proximity of a nearby treatment hospital, the HEMS is not involved in the prehospital treatment of patients with severe TBI. Patients with suspected TBI were transferred to specialized traumacenters, of which 9 participated in this study (Amsterdam UMC, location VUmc (Amsterdam), Erasmus MC (Rotterdam), Radboud UMC (Nijmegen), UMC Groningen (Groningen), Isala (Zwolle), Medisch Spectrum Twente (Enschede), Elisabeth-TweeSteden Ziekenhuis (Tilburg), NoordWest Ziekenhuisgroep (Alkmaar), UMC Utrecht (Utrecht)). Ongoing care and follow-up was the responsibility of these centers.
The Medical Research Ethics Committees of the Amsterdam University Medical Center, location VUmc (reference number 2012/041) and Erasmus MC Rotterdam (reference number MEC-2012-515) reviewed the study protocol and concluded that the research is not subject to the Dutch Medical Research Involving Human Subjects Act. Patient consent was waived as described previously (Citation8). This report adheres to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines (Citation10).
Selection of Participants
The four HEMS teams prospectively recorded data on all patients with a clinical suspicion of severe TBI, based on a trauma mechanism or clinical findings suggestive for TBI in combination with a prehospital Glasgow Coma Scale (GCS) score ≤ 8. Data from all consecutive patients meeting the predefined criteria and who were transported to a hospital for further treatment were included in the study. We included data from patients with suspected – rather than confirmed – TBI as the prehospital treatment was based on the clinical suspicion rather than on the definitive diagnosis. This study focused on severe TBI, because these patients are considered at high risk for impairments of vital functions and secondary brain injury, may markedly differ from patients with less severe TBI regarding benefit/harm of interventions, and might particularly profit from optimal prehospital treatment strategies (Citation8). The inclusion period began on February 12th 2012 with a phased approach across the participating HEMS and lasted until December 31st 2017. Patients were followed up in participating trauma centers for in-hospital data and outcome data until 1 year after inclusion, i.e., up to December 2018.
For planned subgroup analyses, patients were further stratified into “confirmed TBI” and “isolated TBI”. Patients were characterized as confirmed TBI if the Head Abbreviated Injury Score (H-AIS) was 3 or more. Isolated TBI was characterized as a H-AIS ≥3, with Neck, Spine, Thorax, Abdomen and Extremities AIS ≤2.
Measurements
Data collection was based on the Utstein template for uniform reporting of data following major trauma (Citation11). Prehospital data, including operational data, mechanism of injury, clinical findings, vital parameters and treatments, were prospectively collected by the four HEMS, using standardized forms. Follow up data, including in-hospital data and outcome data were collected by a trained data manager. The published study protocol contains a more detailed overview of the measured and collected variables (Citation8).
Outcomes
The primary outcome parameter was mortality at 30 days. Secondary outcomes included 1-year survival, and functional outcome assessed with the Glasgow Outcome Scale (GOS) at hospital discharge and the Glasgow Outcome Scale – Extended (GOS-E) at 1 year (10–14 months), as also described in detail in the study protocol (Citation8).
Analysis
Descriptive statistics were used for demographic data, injury characteristics, and outcome data. These included means and standard deviations, medians and quartiles, or numbers and percentages as appropriate (Citation12). Denominators were reported for the percentages whenever the calculation was based on less than the total number of patients (e.g., for subgroup analyses or due to missing data). Estimates of population parameters were accompanied by 95% confidence intervals (CI) (Citation13). A Kaplan-Meier survivor function was used to describe survival over time (Citation14). Three separate logistic regression models were fit to model the unadjusted relationship between age, initial GCS as well as Injury Severity Score (ISS) and the logit of 30-day mortality (Citation15). Restricted cubic splines were constructed to display the nonlinear association between these data. Adjusted analyses were not planned for this mainly descriptive epidemiologic study. The sample size was based on the targeted inclusion of 2500 patients as described in detail in the published study protocol (Citation8). Analyses were done in Stata/IC 16.0, StatCorp, College Station, TX.
Incidence Rate Estimation
Population data of the Dutch Central Bureau of Statistics (www.cbs.nl) were used as denominator for the estimation of the incidence rate of severe TBI (Citation16). Numerators were based on inclusion data from 2015 to 2017, as all 4 Dutch HEMS operators were including patients, and a nearly complete coverage of patients with suspected severe TBI can be assumed in these three years (Citation8). To estimate the incidence rate of confirmed TBI, we extrapolated the proportion of confirmed TBI from the patients who were followed-up for AIS scores to the total sample (i.e., 83.7% of patients with available AIS data had confirmed TBI, and we used this percentage as the best estimate for the percentage of confirmed TBI across the entire sample of included patients).
Results
Characteristics of Study Subjects
In total, data of 2,589 consecutive patients treated between February 2012 and December 2017 were recorded and analyzed. Among these patients 2,117 (81.8%) were transferred to a participating trauma center (Supplementary Figure 1).
Main Results
Incidence Rate
The incidence rate of suspected severe TBI was 3.2 (95% CI: 3.1; 3.4) per 100,000 inhabitants per year, based on a population average of 16,987,118 inhabitants and an average number of 543 patients per year (2015–2017). For confirmed severe TBI, the estimated annual incidence rate was 2.7 (95% CI: 2.5; 2.8) patients per 100,000 population.
Patient Characteristics
Included patients ranged in age between 1 and 97 years (median 46 (24, 65) years), and 1,827/2,580 were male (70.8%). Age distribution was bimodal with an early peak during adolescence (females) or young adulthood (males) and a second peak at around 50–80 years (both genders, Supplementary Figure 2).
Preinjury American Society of Anesthesiologists (ASA) physical status scores were available for 1,692 of the patients transported to one of the participating trauma centers. Of these patients, 1017 (60.1%) had an ASA score of I, indicating no pre-injury systemic disease, while the others had some degree of preexisting comorbidity, in particular arterial hypertension and cardiac disease (). The prevalence of preinjury anticoagulant drug use, including antiplatelet drugs, low-molecular weight heparin and vitamin K antagonists, was 14.2% (193/1,363).
Table 1. Preinjury condition of patients
Operational Characteristics
HEMS crews attended to the incident with the helicopter in 1,989/2,586 (76.9%) of cases, and by road due to operational considerations (short distance to incident location) or for technical and meteorological reasons in the remainder of cases.
Median time from dispatch to arrival of the HEMS team at the scene was 18 (13, 23) minutes (n = 2,268). Median on-scene time was 16.5 (11, 24) minutes (n = 1,798) and median transport time to the hospital was 18 (12, 24) minutes (n = 1,819). The total overall median time from HEMS dispatch to hospital arrival was 54 (43, 66) minutes (n = 1,729). This may underestimate the actual prehospital run time, because HEMS were secondarily summoned by the ambulance at the scene in 19% of cases, and the actual time of ambulance dispatch was not recorded in our dataset. A sensitivity analysis including only primary dispatches in which the HEMS was dispatched together with the ambulance services showed identical median total prehospital run time and identical quartiles. The median air and road distances between the scene of the incident and the trauma center were 22.3 (10.3, 39.1) km (n = 2,066) and 30.2 (15.9, 50.4) km (n = 2,069), respectively. A total of 329 patients (12.8%) were transported to the trauma center by helicopter, and the remaining patients were transported by ambulance (total n = 2,567).
Trauma and Injury Characteristics
and summarize the trauma mechanisms and injury characteristics.
Figure 1. Trauma mechanism. Pie chart of the distribution of trauma mechanisms (right panel) and percentages stratified by age (at median) and gender (left panel).
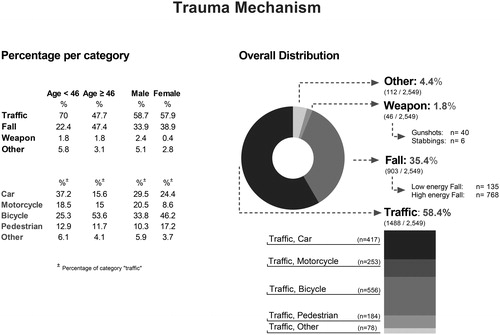
Table 2. Injury characteristics
More than half of the patients were involved in road traffic crashes (58.4%, n = 1,488/2,549). Bicycle crashes comprised 21.8% of all incidents, and 37.4% of all traffic crashes. The use of a helmet was observed in only 7.2% (40 cases) of bicycle crashes. Falls from height accounted for 35.4% cases. Violence and assaults were uncommon in our patient population (<2%). Results stratified by age (at median) and gender are shown in .
The first GCS scores recorded on arrival of the EMS are presented in . Slightly less than half of the patients (47.6%) presented with an initial GCS of 3. In the remaining patients the GCS scores were approximately uniformly distributed between 4 and 8, except for 13 patients who initially had a GCS >8 on first EMS contact but subsequently deteriorated to 8 or less during the prehospital phase.
Figure 2. Distribution of first GCS. Bar chart presenting the distribution of first measured GCS scores on scene, stratified by subgroup (“all patients”, “confirmed TBI” and “isolated TBI”). Actual numbers (n) of cases per category of GCS and per group are presented at the bottom.
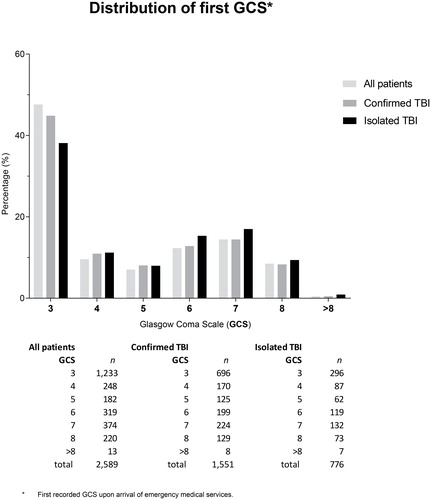
Injury Severity Score data were available from 1853 patients transported to participating trauma centers. Of these, 1,551 patients (83.7%, 95% CI: 81.9; 85.4%) had confirmed TBI (i.e., Head AIS ≥3), and 776 patients (50.0% of patients with confirmed TBI, 95% CI: 47.5; 52.6%) had an isolated TBI without relevant extracranial injuries. The median Head AIS score was 4 (3, 5), and the median ISS score was 26 (20,36), (Supplementary Figure 3). The Rotterdam and Marshall CT scores of the first CT after presentation at the Emergency Department are shown in Supplementary Figure 4.
Outcome
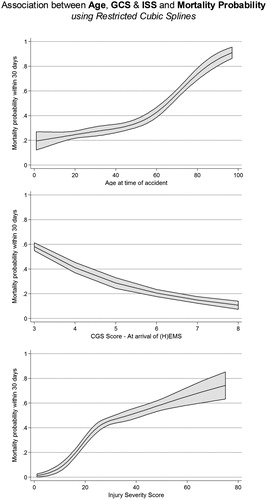
The overall 30-day mortality was 39.0% (95% CI: 36.8; 41.2, n = 1,985), for confirmed TBI 42.4% (95% CI: 39.9; 44.9, n = 1,547) and for isolated TBI 41.3% (95% CI: 37.8; 44.8, n = 775). displays the 30-day mortality per HEMS and per participating trauma center. A higher patient age as well as increasing injury severity (initial GCS score, ISS score) were associated with a higher 30-day mortality (all p-values for linear trend <0.001; restricted cubic splines shown in ).
Figure 3. Distribution of 30-day mortality. Proportions and 95% confidence intervals of mortality per anonymized trauma center and per HEMS, stratified by patient subgroup (“all patients”, “confirmed TBI” and “isolated TBI”).
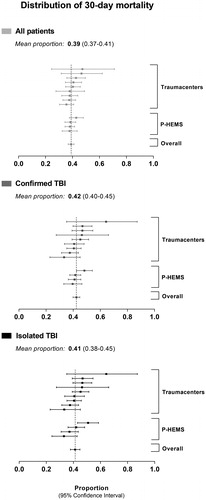
Figure 4. Association between Age, GCS & ISS and Mortality Probability. Unadjusted relationship between age, GCS, ISS and 30-day mortality.
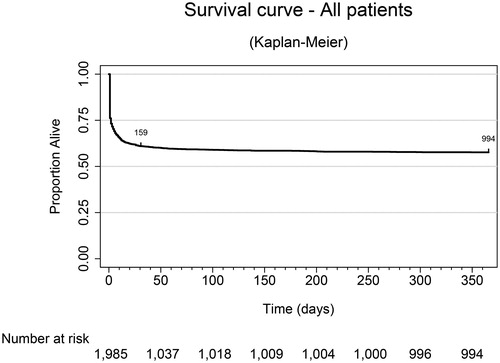
Overall mortality was 42.1% (95% CI: 39.8; 44.5, n = 1,717) at 1 year. shows the Kaplan-Meier curve of survival during the 12-month follow up period. As can be seen, mortality mostly occured before day 10, and the curve flattened out after 30 days.
Figure 5. Survival curve. Kaplan Meier curve presenting the survival time up to one year after the accident.
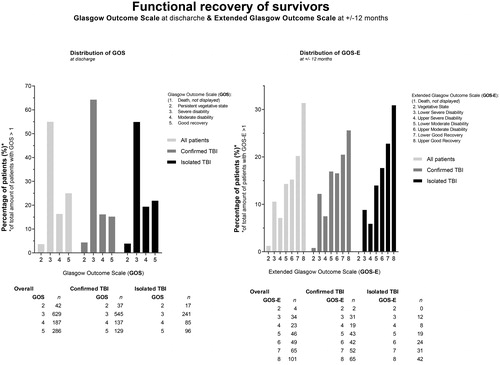
Functional recovery of survivors at hospital discharge and at 1 year are shown in . While most patients were discharged from hospital with severe disabilities (of those discharged alive and with available GOS data, 59% had a GOS of ≤3 at discharge), more than half of survivors had a good recovery at 1 year (52% with a GOS-E of 7 or 8).
Figure 6. Functional recovery of survivors. Bar chart presenting the distribution of the Glasgow Outcome Scale (GOS) scores at discharge and the Extended Glasgow Outcome Scale (GOS-E) scores at +/- 12 months. Data are presented for the three subgroups, i.e., “all patients”, “confirmed TBI” and “isolated TBI”. Actual numbers (n) of cases per category of GOS & GOS-E and per group are presented at the bottom.
Discussion
This article presents the first results of the BRAIN-PROTECT study, focusing on the epidemiology, prehospital characteristics, and outcomes of severe TBI in the Netherlands.
During the study period, a total of 2,589 patients with prehospital suspicion of severe TBI were managed by one of the four HEMS. This corresponds to an annual incidence rate of 3.2 per 100,000 inhabitants. The incidence rate of confirmed severe TBI was estimated as 2.7 per 100,000 per year. In Europe, a wide variability of incidence rates has previously been reported. Across all TBI severities and ages, the reported incidence rates ranged from 47.3 to 694 per 100,000 inhabitants per year (Citation17), with an estimated pooled incidence rate of 262 per 100,000 per year (Citation18). Previous studies in the Netherlands reported an annual TBI incidence rate of 213.6/100,000 (Citation4) and observed that only approximately 1% of TBI patients have severe TBI (Citation19). Taken together, this suggests that the expected annual incidence rate of severe TBI should be somewhere around 2.1 (previous Dutch studies) to 2.6 (pooled European data)/100.000 in the Netherlands, which is very close to and consistent with the estimate in our study. Only few other studies have specifically investigated the incidence rate of severe TBI. Masson et al reported an annual incidence rate of 17.3/100,000 in France in 1996 (Citation20), and Andelic et al observed incidence rates of 5.2/100,000 in 2009 and 4.1/100,000 in 2010 in Norway (Citation21). It is unclear whether the differences to our data reflect true differences between countries, whether developments in primary prevention may have led to a general decrease of the incidence rate of severe TBI over time, or whether it was simply attributable to different methods to define TBI and to identify patients.
The patient characteristics of our population were similar to those reported in previous studies. The majority of patients with severe TBI were male, which is likely to be at least partially due to a higher risk propensity in men compared to women (Citation22,Citation23). Also consistent with previous literature (Citation24), the median age was around 45 years in our patients, indicating that many patients are quite young. In fact, 74% were younger than 65 years – which is the current but dynamically changing retirement age in the Netherlands – indicating that severe TBI often affects the economically most relevant part of society. Costs of TBI have previously been estimated to be as high as €314.6 (currently about USD $370.8) million per year in the Netherlands (Citation4). Especially the rather young, healthy and working adults contributed significantly to this number, not only by direct short-term healthcare costs associated with the trauma, but also in particular due to long term and indirect costs due to loss of productive life years, inability to work, as well as lifetime care. Hence, our data support that severe TBI is not only a serious medical condition but also a relevant economic factor in the Netherlands.
Previous studies including all TBI severities often described a trimodal age distribution with peaks at early childhood, late adolescence/early adulthood and in the elderly (Citation25). In our population of severe TBI patients, the age distribution was bimodal and without a peak in early childhood. Less than 4% of patients were below age 10, suggesting that severe TBI is rare among infants, toddlers and young children in the Netherlands. Interestingly, the location of the early age peak differed between females (at adolescence) and males (at young adulthood). This may suggest that measures targeted at primary prevention of TBI of certain age groups may also need to take into account the gender of those to be protected.
Slightly more than half of the patients had no relevant comorbidities, which can be explained by the fact that many patients were relatively young. However, this also means that a relevant proportion of patients – especially those at advanced age - did have more or less severe comorbidity: of those older than 65 years, almost 80% had comorbidities such as chronic hypertension, which in turn, may shift the cerebral autoregulation curve and affect cerebral perfusion after TBI, with adverse effects on eventual outcome. Likewise, patients with cardiac disease may have limited reserve to cope with hypovolemia and tachycardia after trauma. Importantly, the overall prevalence of anticoagulant drug use – which can significantly aggravate intra- and extracranial hemorrhage – was 14%, and this increased to about 50% in patients older than 65 years. While emergency healthcare providers – from our experience – may have the tendency to focus on the trauma itself, these observations reinforce that treatment often also needs to consider chronic comorbidities and medications, in particular in the elderly.
Similar to previous reports (Citation25), road traffic crashes were a major mechanism of injury in our patients, followed by falls. Cycling is popular among all ages in the Netherlands, and so not surprisingly, cyclists were involved in a major proportion of traffic related injuries, in up to 53.6% of traffic related cases in patients older than 45 years. In contrast to other European countries, commuter cyclists in the Netherlands rarely use cycling helmets (in our sample only 7.2% of bicyclists used a helmet). The high proportion of cycling related TBI which we observed suggest that not only general attempts to improve traffic safety but also attempts in particular to increase bicycle helmet use might play a pivotal role in the primary prevention of severe TBI in the Netherlands.
Among the falls, about 7% were because of a suicide attempt, and a few due to assault, but the vast majority were trauma incidents occurring at home or at work. This suggests the potential for improvements in private and occupational safety with respect to fall prevention. In particular, stairs tend to be very steep in Dutch buildings, and especially elderly patients may be at risk of falling. In fact, falls accounted for about 50% of TBI cases in patients older than 65 years. While we did not explicitly record the percentage of patients who have fallen down stairs, we are aware that this is a major contributor. All other trauma mechanisms were of far lesser importance, suggesting that resources for primary prevention of TBI should primarily be directed at traffic and fall prevention. In particular, our data hightlighted differences in trauma mechanisms depending on patient age, which should be considered for primary prevention strategies.
The Netherlands is a rather small, densely populated and well-developed country, with relative short distances to trauma centers (mean distance over the road of 30 km). This differs from many other geographical regions and may explain the low rate of helicopter transported patients (12.8%) versus ground ambulance patients. HEMS response times and prehospital treatment times seemed reasonable, with a median total prehospital run time of less than 1 hour for primary HEMS missions. Nonetheless, further improvements may be possible and (H)EMS operators as well as prehospital healthcare providers should critically reevaluate their procedures and determine whether time can be saved in the prehospital trajectory.
Our data show that most mortality after severe TBI occurred within 10 days and only few patients died after 30 days, suggesting that once patients survived up to this time point, they had a good chance of becoming long-term survivors. This may not only be prognostically reassuring for patients and relatives but can also have implications for TBI research. We are aware from own experience that long-term follow up of a large number of patients is quite challenging, and the data demonstrated that 30-day mortality is a useful surrogate for long term mortality in TBI research. Of those who survived until one year, more than 50% experience good recovery as indicated by a score of 7 (lower good recovery) or 8 (upper good recovery) in the GOS-E score obtained during a structured interview (Citation26). These scores indicate that a majority of survivors can function independently and resume normal life activities with either no (GOS-E = 8) or only minor problems such as concentration or memory deficits (GOS-E = 7) affecting daily life. Telephonic assessment of the GOS-E by a well-trained datamanager has been demonstrated as a valid alternative for face-to-face assessment (Citation27).
Similar to reported incidence estimates, the reported mortality after TBI varies widely across literature (Citation28–30). The 30-day mortality of 39% and 1-year mortality of 42% which we observed was lower than the 47% mortality for HEMS treated patients reported by Franschman et al in a previous Dutch study with comparable patients (GCS <9, but only patients ≥16 years were included) using data from 2008–2009 (Citation31). It is possible that this difference reflects a general improvement in TBI survival within the last decade, but sampling variability and differences in patient selection and follow-up cannot be excluded. In this study, we only did a cursory analysis of associations of factors and mortality, in particular, to determine the validity of the data and to test whether the previously described and well-known associations between age, initial CGS, and injury severity with mortality would also hold in our dataset (Citation1,Citation24,Citation32,Citation33). As expected, significant relationships were observed. Future studies using the BRAIN-PROTECT dataset will look with much more detail into the adjusted relationships between demographic factors, prehospital characteristics, treatments and outcomes.
The BRAIN-PROTECT study is subject to the inherent limitations of observational studies and does not allow causal inferences on relationships between treatment related factors and outcomes (Citation8). This is less of a concern in this present study, which primarily aimed to describe prehospital characteristics and the epidemiology of severe TBI in the Netherlands.
To minimize selection bias, all consecutive patients with prehospital suspicion of severe TBI were included. As severe TBI is a primary dispatch criterion for HEMS in the Netherlands (Citation34), we expected that we would have included the vast majority of patients, allowing a useful estimate of the incidence rate of potentially treatable severe TBI. Nonetheless, we may have missed a few patients, e.g., due to unavailability of HEMS or due to a quick transport of patients when the trauma occurred in close proximity of a treatment hospital.
We did not include patients who were declared dead at the incident scene but only patients admitted to a trauma center, and the reported incidence rate therefore underestimates the true incidence rate of severe TBI. However, previous studies reporting epidemiologic data also commonly estimated the incidence rate from patients admitted to the emergency department (Citation17,Citation18,Citation25), which facilitates a comparison of our data to previous literature. Importantly, while our data could not estimate the overall incidence rate, the incidence rate that we reported actually represents the clinically more relevant estimate of the incidence rate of potentially treatable severe TBI – i.e., the number of patients that may have benefit from optimal prehospital treatment.
In summary, this article reports the prehospital characteristics and epidemiology of severe TBI in the Netherlands. The data allowed the most recent estimate of the incidence of severe TBI and set the framework for future BRAIN-PROTECT studies aiming to identify beneficial treatment strategies for this patient population. Moreover, the data may have implications for resource allocation in primary prevention, for the treatment of patients with comorbidities, and for future TBI research.
Additional information
Funding
References
- Majdan M, Plancikova D, Brazinova A, Rusnak M, Nieboer D, Feigin V, Maas A. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health. 2016;1(2):e76–e83. doi:10.1016/S2468-2667(16)30017-2.
- Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. doi:10.15585/mmwr.ss6609a1.
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8. doi:10.1097/00001199-200609000-00001.
- Scholten AC, Haagsma JA, Panneman MJ, van Beeck EF, Polinder S. Traumatic brain injury in the Netherlands: incidence, costs and disability-adjusted life years. PLoS One. 2014;9(10):e110905. doi:10.1371/journal.pone.0110905.
- van Dijck J, Dijkman MD, Ophuis RH, de Ruiter GCW, Peul WC, Polinder S. In-hospital costs after severe traumatic brain injury: A systematic review and quality assessment. PLoS One. 2019;14(5):e0216743. doi:10.1371/journal.pone.0216743.
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–41. doi:10.1016/S1474-4422(08)70164-9.
- The CRASH-3 Trial Collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. The Lancet. 2019;394:1713–23.
- Bossers SM, Boer C, Greuters S, Bloemers FW, Den Hartog D, Van Lieshout EMM, Hoogerwerf N, Innemee G, van der Naalt J, Absalom AR, et al. Dutch prospective observational study on prehospital treatment of severe traumatic brain injury: the BRAIN-PROTECT study protocol. Prehosp Emerg Care. 2019;23(6):820–27. doi:10.1080/10903127.2019.1587126.
- Badjatia N, Carney N, Crocco TJ, Fallat ME, Hennes HM, Jagoda AS, Jernigan S, Letarte PB, Lerner EB, Moriarty TM, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12(Suppl 1):S1–S52. doi:10.1080/10903120701732052.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–57. doi:10.1016/S0140-6736(07)61602-X.
- Ringdal KG, Coats TJ, Lefering R, Di Bartolomeo S, Steen PA, Roise O, Handolin L, Lossius HM, Utstein TCD Expert Panel. The Utstein template for uniform reporting of data following major trauma: a joint revision by SCANTEM, TARN, DGU-TR and RITG. Scand J Trauma Resusc Emerg Med. 2008;16:7. doi:10.1186/1757-7241-16-7.
- Schober P, Vetter TR. Descriptive statistics in medical research. Anesth Analog. 2019;129(6):1445. doi:10.1213/ANE.0000000000004480.
- Schober P, Bossers SM, Schwarte LA. Statistical significance versus clinical importance of observed effect sizes: what do p values and confidence intervals really represent? Anesth Analg. 2018;126(3):1068–72. doi:10.1213/ANE.0000000000002798.
- Schober P, Vetter TR. Survival analysis and interpretation of time-to-event data: the tortoise and the hare. Anesth Analg. 2018;127(3):792–8. doi:10.1213/ANE.0000000000003653.
- Vetter TR, Schober P. Regression: the apple does not fall far from the tree. Anesth Analg. 2018;127(1):277–83. doi:10.1213/ANE.0000000000003424.
- CBS. Dutch population density statistics by Central Bureau for Statistics (CBS). 2019. Available from: http://statline.cbs.nl/Statweb/.
- Brazinova A, Rehorcikova V, Taylor MS, Buckova V, Majdan M, Psota M, Peeters W, Feigin V, Theadom A, Holkovic L, et al. Epidemiology of traumatic brain injury in Europe: a living systematic review. J Neurotrauma. 2018;33:1–30.
- Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF, Maas AI. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien). 2015;157(10):1683–96. doi:10.1007/s00701-015-2512-7.
- Meerhoff SR, de Kruijk JR, Rutten J, Leffers P, Twijnstra A. [Incidence of traumatic head or brain injuries in catchment area of Academic Hospital Maastricht in 1997]. Ned Tijdschr Geneeskd. 2000;144(40):1915–8.
- Masson F, Thicoipe M, Aye P, Mokni T, Senjean P, Schmitt V, Dessalles PH, Cazaugade M, Labadens P. Epidemiology of severe brain injuries: a prospective population-based study. J Trauma. 2001;51(3):481–9.
- Andelic N, Anke A, Skandsen T, Sigurdardottir S, Sandhaug M, Ader T, Roe C. Incidence of hospital-admitted severe traumatic brain injury and in-hospital fatality in Norway: a national cohort study. Neuroepidemiology. 2012;38(4):259–67. doi:10.1159/000338032.
- Byrnes JP, Miller DC, Schafer WD. Gender differences in risk taking: a meta-analysis. Psychol Bull. 1999;125(3):367–83. doi:10.1037/0033-2909.125.3.367.
- Lenartova L, Janciak I, Wilbacher I, Rusnak M, Mauritz W. Severe traumatic brain injury in Austria III: prehospital status and treatment. Wien Klin Wochenschr. 2007;119(1–2):35–45. doi:10.1007/s00508-006-0762-3.
- Spaite DW, Bobrow BJ, Keim SM, Barnhart B, Chikani V, Gaither JB, Sherrill D, Denninghoff KR, Mullins T, Adelson PD, et al. Association of statewide implementation of the prehospital traumatic brain injury treatment guidelines with patient survival following traumatic brain injury: the Excellence in Prehospital Injury Care (EPIC) study. JAMA Surg. 2019;154(7):e191152. doi:10.1001/jamasurg.2019.1152.
- Bruns J, Jr., Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(s10):2–10. doi:10.1046/j.1528-1157.44.s10.3.x.
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85. doi:10.1089/neu.1998.15.573.
- Bossers SM, van der Naalt J, Jacobs B, Schwarte LA, Verheul R, Schober P. Face-to-face versus telephonic extended Glasgow outcome score testing after traumatic brain injury. J Head Trauma Rehabilit. 2020;in print.
- Dawes AJ, Sacks GD, Cryer HG, Gruen JP, Preston C, Gorospe D, Cohen M, McArthur DL, Russell MM, Maggard-Gibbons M, et al. Compliance with evidence-based guidelines and interhospital variation in mortality for patients with severe traumatic brain injury. JAMA Surg. 2015;150(10):965–72. doi:10.1001/jamasurg.2015.1678.
- Bossers SM, Schwarte LA, Loer SA, Twisk JW, Boer C, Schober P. Experience in prehospital endotracheal intubation significantly influences mortality of patients with severe traumatic brain injury: a systematic review and meta-analysis. PLoS One. 2015;10(10):e0141034. doi:10.1371/journal.pone.0141034.
- Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SS, Ponsford J. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291(11):1350–7. doi:10.1001/jama.291.11.1350.
- Franschman G, Andriessen TM, Boer C, Van der Naalt J, Horn J, Haitsma I, Vos PE. Physician-based emergency medical service deployment characteristics in severe traumatic brain injury: a Dutch multicenter study. Injury. 2013;44(9):1232–6. doi:10.1016/j.injury.2013.06.002.
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Buki A, Chesnut RM, InTBIR Participants and Investigators, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi:10.1016/S1474-4422(17)30371-X.
- Timmons SD, Bee T, Webb S, Diaz-Arrastia RR, Hesdorffer D. Using the abbreviated injury severity and Glasgow Coma Scale scores to predict 2-week mortality after traumatic brain injury. J Trauma. 2011;71(5):1172–78.
- Franschman G, Verburg N, Brens-Heldens V, Andriessen TM, Van der Naalt J, Peerdeman SM, Valk JP, Hoogerwerf N, Greuters S, Schober P, Vos PE, et al. Effects of physician-based emergency medical service dispatch in severe traumatic brain injury on prehospital run time. Injury. 2012;43(11):1838–42. doi:10.1016/j.injury.2012.05.020.
Appendix.
BRAIN-PROTECT Collaborators Group Authorship
- Anne de Boer, SpoedZorgNet, Amsterdam University Medical Center, location AMC, Amsterdam, the Netherlands
- J. Carel Goslings, Dept. of Surgery, OLVG, Amsterdam, the Netherlands
- Sven H. van Helden, Dept. of Surgery, Isala Hospital, Zwolle, the Netherlands
- Danique Hesselink, Netwerk Acute Zorg Zwolle, Zwolle, the Netherlands
- Gijs van Aken, Netwerk Acute Zorg Zwolle, Zwolle, the Netherlands
- Albertus Beishuizen, Intensive Care Center, Medisch Spectrum Twente, Enschede, the Netherlands
- Rolf E. Egberink, Acute Zorg Euregio, Enschede, the Netherlands
- Nancy ter Bogt, Acute Zorg Euregio, Enschede, the Netherlands
- Mariska A.C. de Jongh, Netwerk Acute Zorg Brabant, Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands
- Koen Lansink, Dept. of Surgery, Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands
- Gerwin Roks , Dept. of Neurology, Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands
- Pieter Joosse, Dept. of Surgery, Noordwest Ziekenhuisgroep, Alkmaar, the Netherlands
- Kees J. Ponsen, Dept. of Surgery, Noordwest Ziekenhuisgroep, Alkmaar, the Netherlands
- Lukas L. van Spengler, Traumazorgnetwerk Midden-Nederland, Utrecht, the Netherlands
- Stasja Aspers, Traumazorgnetwerk Midden-Nederland, Utrecht, the Netherlands
- Marcel A. de Leeuw, Dept. of Anesthesiology and HEMS Lifeliner 1, Amsterdam University Medical Center, location VUmc, Amsterdam, the Netherlands
- Lothar A. Schwarte, Dept. of Anesthesiology and HEMS Lifeliner 1, Amsterdam University Medical Center, location VUmc, Amsterdam, the Netherlands
- Annelies Toor, Netwerk Acute Zorg Noordwest, Amsterdam, the Netherlands
- Robert J. Houmes, Dept. of Anesthesiology and HEMS Lifeliner 2, Erasmus MC, Rotterdam, the Netherlands
- Jan van Ditshuizen, Trauma Center Southwest Netherlands, Erasmus MC, Rotterdam, the Netherlands
- Tea van Voorden, Trauma Center Southwest Netherlands, Erasmus MC, Rotterdam, the Netherlands
- Michael J.R. Edwards, Dept. of Surgery, Radboud Unversity Medical Center, Nijmegen, the Netherlands
- Bert Dercksen, Dept. of Anesthesiology and HEMS Lifeliner 4, University Medical Center Groningen, Groningen, the Netherlands
- Rob Spanjersberg, Dept. of Anesthesiology, University Medical Center Groningen, Groningen, the Netherlands
- Lieneke Venema, Dept. of Anesthesiology and HEMS Lifeliner 4, University Medical Center Groningen, Groningen, the Netherlands
- Ellen Weelink, Dept. of Anesthesiology and HEMS Lifeliner 4, University Medical Center Groningen, Groningen, the Netherlands
- Inge H.F. Reininga, Emergency Care Network Northern Netherlands (AZNN), Groningen, the Netherlands
