Abstract
Introduction
Emergency Medical Services (EMS) clinicians commonly encounter patients with acute pain. A new set of evidence-based guidelines (EBG) was developed to assist in the prehospital management of pain. Our objective was to describe the methods used to develop these evidence-based guidelines for prehospital pain management.
Methods
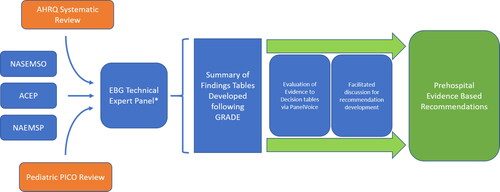
The EBG development process was supported by a previous systematic review conducted by the Agency for Healthcare Research and Quality (AHRQ) covering nine different population, intervention, comparison, and outcome (PICO) questions. A technical expert panel (TEP) was formed and added an additional pediatric-specific PICO question. Identified evidence was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework and tabulated into Summary of Findings tables. The TEP then utilized a rigorous systematic method, including the PanelVoice function, for recommendation development which was applied to generate Evidence to Decision Tables (EtD). This process involved review of the Summary of Findings tables, asynchronous member judging, and facilitated panel discussion to generate final consensus-based recommendations.
Results
The work product described above was completed by the TEP panel from September 2020 to April 2021. For these recommendations, the overall certainty of evidence was very low or low, data for decisions on cost effectiveness and equity were lacking, and feasibility was rated well across all categories. Based on the evidence, one strong and seven conditional recommendations were made, with two PICO questions lacking sufficient evidence to generate a recommendation.
Conclusion
We describe a protocol that leveraged established EBG development techniques, the GRADE framework in conjunction with a previous AHRQ systematic review to develop treatment recommendations for prehospital pain management. This process allowed for mitigation of many confounders due to the use of virtual and electronic communication. Our approach may inform future guideline development and increase transparency in the prehospital recommendations development processes.
Introduction
The presentation of pain in the prehospital setting is documented and treated inconsistently in the United States, necessitating development of up-to-date and evidence-based clinical guidelines (Citation1). Pain can be due to a myriad of conditions, including traumatic injuries or underlying illness (Citation2), but a core clinical priority in these cases is the management of the patient’s symptoms (Citation3). Previous research suggests that in many settings, and for diverse conditions, the management of pain in the prehospital setting is less than optimal, and evidence-based guidelines (EBG) may assist EMS clinicians in choosing the most appropriate pharmacologic intervention to manage pain (Citation1, Citation4). One of the first prehospital EBGs published using the National Prehospital EBG Model Process focused on analgesia in trauma (Citation5, Citation6). Emerging evidence related to analgesics in prehospital and in-hospital settings, the need for clinical guidance related to pain management for non-traumatic conditions, and the significant controversy over the use of opioids due to the public health impact of the recent opioid epidemic highlight the need for an updated prehospital pain EBG (Citation7). With this in mind, the development of a new EBG for prehospital pain managment was supported in part by the National Highway Traffic Safety Administration (NHTSA), Office of Emergency Medical Services (OEMS), and the Health Resources and Services Administration (HRSA), Maternal and Child Health Bureau’s EMS for Children (EMSC) Program leveraging a strong collaboration between the National Association of EMS Physicians (NAEMSP), the American College of Emergency Physicians (ACEP), and the National Association of State Emergency Medical Services Officials (NASEMSO).
The overall objective of the project was to develop evidence-based guidelines for prehospital pain management (Citation8). This manuscript describes the detailed methodology to develop these evidence-based recommendations. Included in this approach was leveraging the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system for rigorous evidence evaluation followed by a systematic approach for recommendation development.
Methods
Study Plan and Protocol
The objective of this study was to develop evidence-based recommendations and treatment guidelines for prehospital pain management. This was done by leveraging the previously completed work of a separately funded AHRQ systematic review and the expertise of a technical expert panel.
To facilitate the development of the evidence-based approach for prehospital pain management, NHTSA partnered with the Agency for Healthcare Research and Quality (AHRQ) to identify the evidence related to prehospital pain management (Citation8). A preceding systematic review was therefore funded by NHTSA through the Evidence Based Practices Center at AHRQ. The University of Connecticut Evidence Based Practice Center provided the systematic review through the AHRQ (PROSPERO ID# CRD42018114959), which has since been published (Citation9).
This previous AHRQ systematic review was used as the first step in the development of the new EBG for prehospital pain management. In this previous review, a single robust search strategy, inclusive of various methods of prehospital pain management, was used to identify all available literature. Using a predefined analysis plan, 9 PICO (population, intervention, comparison, outcome) questions were generated and addressed with associated evidence collection and evidence comparison for commonly used prehospital pain medications (Citation9). Each question was based upon the population of patients in acute moderate-to-severe pain in the prehospital setting. Interventions consisted of opioids, non-opioids, and combinations of opioids and ketamine (Citation9). Comparisons were opioids to a non-opioid, combination of opioid plus ketamine to ketamine alone, a non-opioid to a different non-opioid, and opioids to a different opioid. Outcomes were coalesced into four key questions dichotomized into patient-centered outcomes (pain score, presence of pain, time to analgesic effect) and adverse events (hypotension, nausea, vomiting, respiratory depression) (Citation9).
The project plan for the current analysis () included the use of a technical expert panel (TEP) identified by NASEMSO to develop the recommendations using the previous work conducted by the AHRQ. This TEP consisted of pain management experts, EMS physicians, EMS clinicians, EMS researchers, a pharmacologist, a patient advocate, and an evidence-based guideline methodologist to provide a holistic approach to guideline development. This panel evaluated the full scope of prehospital pain management and provided context so that recommendations could be developed. The TEP evaluated each of the nine PICO questions included in the previous AHRQ review and identified a specific gap surrounding pediatric pain management. The TEP then proposed and evaluated a number of pediatric specific PICO questions, and ultimately included one for consideration. From these ten included PICO questions, the TEP developed Summary of Findings tables generated from the previous AHRQ systematic reviews and those from reviews conducted by the panel. Additionally, the TEP conducted literature reviews to provide prehospital care context with respect to the scope of the problem and cost-effectiveness, so that those considerations could be incorporated into the Summary of Findings tables. This process supported the formation of Evidence to Decision tables (EtD) leading to recommendations for implementation.
Developing Summary of Findings Tables from Previous AHRQ Review
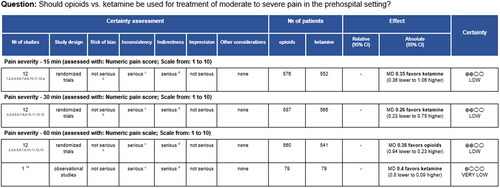
The methods and procedures of the previous AHRQ systematic review are described elsewhere including the selection of abstracts, the detailed screening, full text reviews, and the tables generated (Citation9). The previous AHRQ systematic review yielded 75 studies including 65 unique studies, with 52 of these being randomized controlled trials and 13 observational evaluations (Citation9). Furthermore, the previous AHRQ systematic review provided graded strength of evidence analysis for PICO questions comparing opioids to non-opioids and opioids to a combination of opioid and non-opioids. Non-graded strength of evidence analysis was provided for non-opioids to a different non-opioid and for opioids compared to a different opioid (Citation9). This grading process analyzed the included studies for inconsistency, imprecision, and indirectness, allowing for a certainty assessment to be generated.
To provide consistent data for the TEP members, strength of evidence grading was completed for all studies that informed PICO questions. This expanded on the previous AHRQ systematic review where not all PICO questions received grading of evidence. This process involved leveraging data from the previous AHRQ systematic review for graded PICO questions and conducting additional TEP grading for non graded questions. All Summary of Findings tables were generated, including using strength of evidence analysis, and checked by two authors (ARP, JRP) using the GRADEPro Guideline Development Tool (GRADEPro GDT). Tables, including individual and pooled analysis as provided by the previous AHRQ review, were then presented to the TEP panel for review.
Developing Additional Evidence Profile Tables for Pediatrics
After thorough review of the included PICO questions, the TEP determined the previous AHRQ systematic review provided insufficient evidence to generate pain management recommendations for the unique prehospital needs of pediatric patients. Due to behavioral, social, and clinical differences, children infrequently receive prehospital pain management interventions (Citation4, Citation10–12). The panel determined that pediatric-specific recommendations based on best-available pediatric-specific evidence were needed to improve the prehospital management of children with painful complaints.
All the pain management interventions considered by the TEP in the adult population have indications for use in children. The TEP had significiant discussion on the differences in pain management between adult and pediatric patients and attempted to identify gaps that may require further evaluation. A number of topics were identified but the TEP chose to focus on the use of intranasal fentanyl in prehospital care since there was previous research that established the safety and efficacy of intranasal administration of fentanyl in children (Citation13–16). After thorough discussion, the TEP determined that there was a need to obtain and evaluate the quality of evidence regarding “the comparative effectiveness (Outcome, defined key questions 1 − 4 in the previous AHRQ systematic review) of intranasal fentanyl (Intervention) versus intravenous morphine (Comparison) in the management of acute moderate-to-severe prehospital pain in children (Population).”
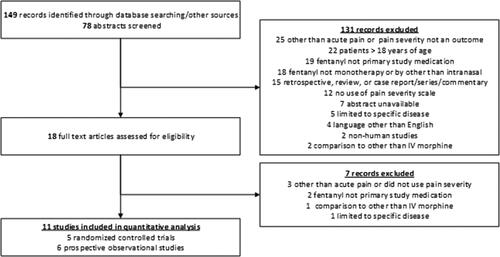
To achieve the development of evidence necessary to address this new PICO question, a single study author (LRB) concurrently reviewed, evaluated, and graded existing published literature outside of the previous AHRQ review. To identify relevant literature, PubMed was searched (1966 to March 2021) for study titles that contained search terms for only those in the ‘Child: birth-18 years’ category. Keywords included: ‘intranasal, fentanyl, and EMS’, ‘intranasal, fentanyl, and prehospital’, ‘fentanyl, pain, and EMS’, ‘fentanyl, pain, and prehospital’, ‘intranasal, fentanyl, and emergency’ and ‘intranasal and fentanyl.’ Included studies were those that were: conducted in humans, written in English, involved patients ≤18 years of age being managed for acute pain, used a pain severity scale, included pain severity as an outcome, involved monotherapy with intranasal fentanyl, and were either randomized controlled trials, prospective studies, or observational studies. Exclusion criteria included: case reports/series/commentaries, comparison to interventions other than IV morphine, and studies that were limited to specific diseases (i.e. sickle cell disease). Finally, to ensure complete capture of relevant studies, studies in available systematic reviews of pediatric intranasal fentanyl were reviewed (Citation14, Citation17–20). Preference was given to studies that occurred in the acute care setting (i.e. prehospital or emergency department) but did not exclude in-hospital studies if they otherwise met inclusion criteria. Though this methodology did not have similar rigor to that conducted in the previous systematic review, the TEP placed high importance on the question and recognized the need for an evaluation within the bounds of the NHTSA work plan.
Identified studies were screened and the abstracts reviewed of literature that were without obvious exclusion criteria based on initial review. Studies listed under the “Similar Articles” heading on the PubMed site for each individual abstract were reviewed for inclusion. Ultimately, we included 11 published studies that met inclusion and exclusion criteria for the pediatric fentanyl question ().
Following GRADE methodology, each of the included studies were reviewed based on study design, sample size, and study setting for risk of bias, inconsistency, indirectness, imprecision, and effect (Citation21). Outcomes graded included pain severity across multiple time points, overall pain reduction, and adverse events associated with administration of study medications. Results of this evaluation were populated into a Summary of Finding table for review by the TEP.
Applying a Systematic Method to Developing Recommendations
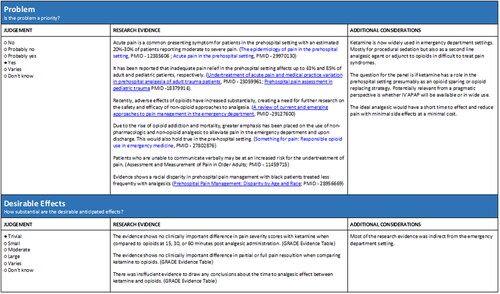
Evidence to decision (EtD) tables were generated for each PICO question following the review of the Summary of Findings tables. This process involved review of the Summary of Findings tables, asynchronous member judging, and facilitated panel discussions to generate final consensus-based recommendations. EtD tables provided a structured approach to generating recommendations from evaluated PICO questions by making the criteria, evidence used to inform judgements, and the judgements themselves more explicit (Citation22). The EtD frameworks were prepared using GRADEPro GDT. In accordance with the GRADE methodology, twelve criteria were used to inform each of the recommendations and included: problem, desirable effects, undesirable effects, certainty of evidence, values, balance of effects, resources required, certainty of evidence of required resources, cost effectiveness, equity, acceptability, and feasibility (Citation22). Detailed explanations for each of the criteria can be found in the GRADE handbook (Citation23).
To assist the TEP recommendation development, the EtD tables were populated with critical contextual information through a nonsystematic review of the relevant research evidence for each of the twelve criteria using PubMed. To supplement the literature search, the reference list of the previous AHRQ systematic review was also examined for relevant studies. If no evidence was found, it was noted, and any relevant information or assumptions used to make a judgment were included in the additional considerations section (Citation22). The EtD tables were also populated with key messages for each of the twelve criteria with links containing more detailed information. The key messages were based on the research evidence and additional considerations found from the literature search. These messages were used to inform each of the judgements and provide structure to the facilitated discussion. Additional considerations were meant to augment, not lead, discussion regarding each PICO question.
Each TEP member asynchronously evaluated the generated Summary of Findings tables and contextual data in the EtD tables for each PICO. Specifically, each member of the TEP was asked to use both the research evidence and additional considerations to make a judgment about each of the 12 criteria. Any additional evidence used to inform the judgment was also recorded. This asynchronous data evaluation was conducted using the PanelVoice function of GRADEPro GDT.
After the asynchronous evaluation of evidence for each PICO question, a recommendation development meeting was conducted consisting of the full TEP and GRADE methodologist. During the meeting, a facilitated discussion led by the GRADE methodologist (author ESL) occurred for each of the twelve criteria until a unified judgment for each criterion was reached. Dissenting views about the judgment were respected and recorded in the EtD table. The discussion included considerations around the importance and implications of each of the criteria and their respective judgements. The panel then used these judgements to develop a draft recommendation with unanimous agreement for each PICO question. Any areas that required further review were identified and completed by a member of the panel.
Results
The work product described above was completed by the TEP panel from September 2020 to April 2021, supported by the staff of NASEMSO along with representation from NAEMSP and ACEP. A detailed description of the TEP and their conflicts of interest statements are included in the Recommendations manuscript. All TEP work was conducted electronically with regularly scheduled virtual meetings for Summary of Findings table review and EtD table generation.
The Summary of Findings tables were generated after review of the studies identified in the previous AHRQ systematic review. However, in the previous AHRQ review, five PICO questions were graded while four PICO questions were ungraded, requiring the TEP to grade these studies. As an example, is an abbreviated Summary of Findings table for the outcome of pain severity for the comparison of IV opioids to IV ketamine. The rest of the outcomes for this PICO question, and all the other Summary of Findings tables, are available in Appendix 1. The evidence review and the associated Summary of Findings table for the pediatric review are also included in Appendix 1.
From the generated Summary of Findings tables, the TEP panel conducted asynchronous evaluations followed by a facilitated discussion with the methodologist. From these, the EtD tables were generated. is an example of one EtD table for the problem and desirable effects of the comparison of IV opioids to IV ketamine. All EtD tables are reported in Appendix 2.
All PICO questions were identified as being a problem worth investigating, with both desirable and undesirable effects spread out among questions. Certainty of evidence was primarily very low or low, leading to increased use of the selection “don’t know” among balance of effects and resources required. Uncertainty, via lack of studies found, impacted certainty of evidence of required resources and thereby cost effectiveness. Lack of data for decisions on equity also led to an increased judegement of “don’t know”. Feasibility was well represented, with the majority of judgements consisting of “yes” or “probably yes”. One strong and seven conditional recommendations were made, with two PICO questions lacking sufficient evidence to generate a recommendation. A separate manuscript contains the details of each recommendation (Citation24).
Discussion
In this manuscript, we describe the rigorous methodology used to summarize the best available evidence for prehospital pain management, followed by generation of evidence based recomendations. We leveraged established EBG development techniques using the GRADE system, in conjunction with a rigorous systematic approach to developing treatment recommendations for prehospital pain management. Specifically, we utilized the PanelVoice function of the GRADEpro GDT for development of each treatment recommendation. This started with asynchronous review and judging by TEP members followed by a facilitated virtual group discussion for final treatment recommendation development. Through this process, we were able to minimize the TEP’s overall cognitive burden in managing each PICO question. This novel reduction was important because members were tasked with developing recommendations for 10 PICO questions, each with four key subquestions, and reviewing results of many detailed evidence evaluations over their time on the panel. This approach is a good model for the development of other prehospital EBGs for which the cognitive burden for the expert panel may be significant or face to face meetings impossible.
Many of the techniques used in this process are similar to other previously developed evidence-based guidelines. The GRADE system has been used by many other organizations including the International Liaison Committee on Resuscitation, the American Heart Association, and the American College of Chest Physicians (Citation25–27). This has also been applied in prehospital care guidelines development (Citation6, Citation28–31). In these previous guidelines, GRADE methodology was applied and Summary of Findings tables were generated by the expert panels assembled. This allowed for development of recommendations from these tables. However, none of these evaluations leveraged a systematic approach to recommendation development utilized in this study.
Specific to prehospital pain management, the GRADE methodology has been previously applied to a 2014 analyis of analgesia for trauma (Citation6). In this evidence based guideline process, the authors assembled a panel of experts who then identified evidence related to their chosen PICO question. Contrasting this approach to the methodology used to generate our recommendations, the presence of a previous AHRQ systematic review and generation of evidence to decision tables increases the methodological rigor as well as improving process transparency.
This method has not been used in prehospital care but has demonstrated success in recommendation development in other areas (Citation32). This technique allows for a level of guideline transparency which previously has not been available and allows for managing any competing interests that may exist (Citation23). Further, it allows for future decision making in this area to understand the perspective, mindset, and motivations of this TEP with clear documentation of how the decisions were derived. Another additional benefit is that the use of asynchronous judging by panel members, with the ability to describe their position, enabled them to express views which may be in the minority with safety. This also helped the facilitator ensure that any minority voices were heard and were not lost by the impact of other influential members or ideas.
Limitations
There were several challenges which were faced in the process of developing these guidelines. First, all guideline activities due to the SARS-CoV-2 pandemic were done virtually and electronically. The TEP did not convene in-person and working relationships for this project were formed only through virtual meetings. It is possible that with a lack of familiarity or technological expertise with virtual meetings, panel members' opinions or voices may not have been heard. However, the use of PanelVoice in this process may have mitigated this effect. Second, due to the design of the previous AHRQ systematic review, the TEP was required to grade all the ungraded PICO questions evaluated. This was important since the TEP then had similar data to develop EtD tables for recommendations. It is also important to note that the previous AHRQ systematic review provided the original 9 PICO questions utilizing a highly inclusive single systematic review. This approach would mitigate any issues of potentially missing landmark articles. However, the data collection process for the 10th PICO question was a limited systematic review, potentially impacting the totality of included research. In addition, while the TEP was comprised of subject matter experts, a differently assembled TEP may have reached different conclusions based on the available data presented.
Through the evaluation done by the group, we discovered that there was a limited body of literature of high quality that directly addressed the prehospital questions evaluated by the TEP. Of the ten PICO questions addressed, only one had literature which had “moderate” certainty of evidence, while the rest were “low” or “very low” (Appendix 1). Much of what drove this level of certainty of evidence was the indirectness of the literature to prehospital care (e.g., studies conducted in the emergency department), and precision of the data. Further, in this process, pain was classified as moderate to severe without additional inclusion or exclusion criteria. This was necessary due to the mentioned indirectness and precision of data available in the prehospital setting but does place increased limitations on the recommendations offered. Finally, the age of 18 was used as a cutoff for the pediatric age range, which may fail to capture the nuances present in pain response for the range of pediatric populations.
Conclusion
In this evaluation, we have leveraged established EBG development techniques, the GRADE framework, in conjunction with a systematic process to develop treatment recommendations for prehospital pain management. This process included asyncronous review and voting, facilitated discussion, and consideration of 12 contextual criteria areas that allowed for mitigation of many confounders and bias due to the use of virtual and electronic communication. Future guideline processes should consider this approach to increase transparency in the recommendation development processes.
Author Contributions
GL, ESL, SB, MS, and ARP conceived, designed, and collected the data. All authors analyzed the data. JRP, LRB, KG, and ARP drafted the manuscript. All authors contributed substantially to the interpretation of the data and revision of the manuscript. JRP takes responsibility for the paper as a whole.
Supplemental Material
Download MS Word (93.1 KB)Acknowledgments
The authors would like to thank National Highway Traffic Safety Administration (NHTSA), Office of Emergency Medical Services (OEMS), and the Health Resources and Services Administration (HRSA), and Maternal and Child Health Bureau’s EMS for Children (EMSC) Program for their support of this work. Of note, the contents of these documents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by the U.S. Government. For more information, please visit EMS.gov and HRSA.gov.
Disclosure Statement
Sabina Braithwaite, Lorin Browne, Remle Crowe, Kyle Guild, Eddy Lang, Ashish Panchal, Jonathan Powell, and Manish Shah report no conflicts of interest. George Lindbeck reports receiving an honorarium from NASEMSO for his work on the project.
References
- Hewes HA, Dai M, Mann NC, Baca T, Taillac P. Prehospital pain management: Disparity by age and race. Prehosp Emerg Care. 2018;22(2):189–97. doi:10.1080/10903127.2017.1367444.
- Panchal AR, Rivard MK, Cash RE, Corley JP, Jr., Jean-Baptiste M, Chrzan K, Gugiu MR. Methods and implementation of the 2019 EMS practice analysis. Prehosp Emerg Care. 2021:1–18. doi:10.1080/10903127.2020.1856985.
- Alonso-Serra HM, Wesley K, National Association of EMS Physicians Standards and Clinical Practices Committee. Prehospital pain management. Prehosp Emerg Care. 2003;7(4):482–88. doi:10.1080/312703002260.
- Browne LR, Shah MI, Studnek JR, Ostermayer DG, Reynolds S, Guse CE, Brousseau DC, Lerner EB. Multicenter evaluation of prehospital opioid pain management in injured children. Prehosp Emerg Care. 2016;20(6):759–67. doi:10.1080/10903127.2016.1194931.
- Lang ES, Spaite DW, Oliver ZJ, Gotschall CS, Swor RA, Dawson DE, Hunt RC. A national model for developing, implementing, and evaluating evidence-based guidelines for prehospital care. Acad Emerg Med. 2012;19(2):201–9. doi:10.1111/j.1553-2712.2011.01281.x.
- Gausche-Hill M, Brown KM, Oliver ZJ, Sasson C, Dayan PS, Eschmann NM, Weik TS, Lawner BJ, Sahni R, Falck-Ytter Y, et al. An evidence-based guideline for prehospital analgesia in trauma. Prehosp Emerg Care. 2014;18(Suppl 1):25–34. doi:10.3109/10903127.2013.844873.
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315(15):1624–45. doi:10.1001/jama.2016.1464.
- Developing an evidence-based approach to prehospital pain management; 2019 [accessed 2021 Apr 4]. https://www.ems.gov/newsletter/january2019/pain-management-ebg.html.
- Sobieraj DM, Martinez BK, Miao B, Cicero MX, Kamin RA, Hernandez AV, Coleman CI, Baker WL. Comparative effectiveness of analgesics to reduce acute pain in the prehospital setting. Prehosp Emerg Care. 2020;24(2):163–74. doi:10.1080/10903127.2019.1657213.
- Samuel N, Steiner IP, Shavit I. Prehospital pain management of injured children: a systematic review of current evidence. Am J Emerg Med. 2015;33(3):451–54. doi:10.1016/j.ajem.2014.12.012.
- Rutkowska A, Skotnicka-Klonowicz G. Prehospital pain management in children with traumatic injuries. Pediatr Emerg Care. 2015;31(5):317–20. doi:10.1097/PEC.0000000000000313.
- Browne LR, Studnek JR, Shah MI, Brousseau DC, Guse CE, Lerner EB. Prehospital opioid administration in the emergency care of injured children. Prehosp Emerg Care. 2016;20(1):59–65. doi:10.3109/10903127.2015.1056897.
- Borland ML, Jacobs I, Geelhoed G. Intranasal fentanyl reduces acute pain in children in the emergency department: a safety and efficacy study. Emerg Med (Fremantle). 2002;14(3):275–80. doi:10.1046/j.1442-2026.2002.00344.x.
- Setlur A, Friedland H. Treatment of pain with intranasal fentanyl in pediatric patients in an acute care setting: a systematic review. Pain Manag. 2018;8(5):341–52. doi:10.2217/pmt-2018-0016.
- Saunders M, Adelgais K, Nelson D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad Emerg Med. 2010;17(11):1155–61. doi:10.1111/j.1553-2712.2010.00905.x.
- Murphy AP, Hughes M, Mccoy S, Crispino G, Wakai A, O’Sullivan R. Intranasal fentanyl for the prehospital management of acute pain in children. Eur J Emerg Med. 2017;24(6):450–4. doi:10.1097/MEJ.0000000000000389.
- Mudd S. Intranasal fentanyl for pain management in children: a systematic review of the literature. J Pediatr Health Care. 2011;25(5):316–22. doi:10.1016/j.pedhc.2010.04.011.
- Hansen MS, Dahl JB. Limited evidence for intranasal fentanyl in the emergency department and the prehospital setting–a systematic review. Dan Med J. 2013;60(1):A4563.
- Hansen MS, Mathiesen O, Trautner S, Dahl JB. Intranasal fentanyl in the treatment of acute pain-a systematic review. Acta Anaesthesiol Scand. 2012;56(4):407–19. doi:10.1111/j.1399-6576.2011.02613.x.
- Murphy A, O'Sullivan R, Wakai A, Grant TS, Barrett MJ, Cronin J, McCoy SC, Hom J, Kandamany N. Intranasal fentanyl for the management of acute pain in children. Cochrane Database Syst Rev. 2014;(10):CD009942. doi:10.1002/14651858.CD009942.pub2.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. doi:10.1016/j.jclinepi.2010.04.026.
- Alonso-Coello P, Schunemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. doi:10.1136/bmj.i2016.
- Schünemann H, Brożek J, Guyatt GH, Oxman AD. Grade handbook; 2013 [accessed 2021 Apr 4]. https://gdt.gradepro.org/app/handbook/handbook.html
- Lindbeck G, Shah M, Braithwaite S, Powell JR, Panchal AR, Browne LR, Lang ES. Evidence-based guidelines for prehospital pain management: recommendations. Preshosp Emerg Care. 2021. doi:10.1080/10903127.2021.2018073.
- Magid DJ, Aziz K, Cheng A, Hazinski MF, Hoover AV, Mahgoub M, Panchal AR, Sasson C, Topjian AA, Rodriguez AJ, et al. Part 2: evidence evaluation and guidelines development: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16_suppl_2):S358–S65. doi:10.1161/CIR.0000000000000898.
- Nolan JP, Maconochie I, Soar J, Olasveengen TM, Greif R, Wyckoff MH, Singletary EM, Aickin R, Berg KM, Mancini ME, et al. Executive summary: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142(16_suppl_1):S2–S27. doi:10.1161/cir.0000000000000890.
- Smith MP, Lown M, Singh S, Ireland B, Hill AT, Linder JA, Irwin RS, CHEST Expert Cough Panel. Acute cough due to acute bronchitis in immunocompetent adult outpatients: CHEST expert panel report. Chest. 2020;157(5):1256–65. doi:10.1016/j.chest.2020.01.044.
- Lipp C, Dhaliwal R, Lang E. Analgesia in the emergency department: a GRADE-based evaluation of research evidence and recommendations for practice. Crit Care. 2013;17(2):1–8. doi:10.1186/cc12521.
- Shah MI, Macias CG, Dayan PS, Weik TS, Brown KM, Fuchs SM, Fallat ME, Wright JL, Lang ES. An evidence-based guideline for pediatric prehospital seizure management using GRADE methodology. Prehosp Emerg Care. 2014;18(sup1):15–24. doi:10.3109/10903127.2013.844874.
- Thomas SH, Brown KM, Oliver ZJ, Spaite DW, Lawner BJ, Sahni R, Weik RS, Falck-Ytter Y, Wright JL, Lang ES. An evidence-based guideline for the air medical transportation of prehospital trauma patients. Prehosp Emerg Care. 2014;18(Suppl 1):35–44. doi:10.3109/10903127.2013.844872.
- Patterson PD, Higgins JS, Van Dongen HPA, Buysse DJ, Thackery RW, Kupas DF, Becker DS, Dean BE, Lindbeck GH, Guyette FX, et al. Evidence-based guidelines for fatigue risk management in emergency medical services. Prehosp Emerg Care. 2018;22(sup1):89–101. doi:10.1080/10903127.2017.1376137.
- Morgano GP, Fulceri F, Nardocci F, Barbui C, Ostuzzi G, Papola D, Fatta LM, Fauci AJ, Coclite D, Napoletano A, et al. Italian National Institute of Health guigeline working group on Autism Spectrum Disorder. Introduction and methods of the evidence-based guidelines for the diagnosis and management of autism spectrum disorder by the Italian National Institute of Health. Health Qual Life Outcomes. 2020;18(1):81. doi:10.1186/s12955-020-01320-4.